Understanding Critical Quality Attributes for Nanocrystals from Preparation to Delivery
Abstract
:1. Introduction
2. Properties of Nanocrystals
3. Solid State Properties
| Category | Characterisation Method | Detection Principle | Information | Data Type | Variations | Sample Requirements | Considerations | References |
|---|---|---|---|---|---|---|---|---|
| Solid state form | X-ray powder diffraction (XRPD) | Diffraction of x-rays from lattice planes | Polymorphic form (unique diffraction peaks), amorphous form (no peaks) | Diffractogram, qualitative and quantitative (degree of crystallinity) | Hot stage XRPD to analyse solid state form as a function of temperature | Powder, paste or slurry form, several sample presentation setups possible, amount required depends on setup | Anisotropic particle shape leads to preferred orientation effects (change in relative intensities of diffraction peaks) | [17] |
| Peak broadening can occur as crystal lattice size decreases within nanoscale range | ||||||||
| Differential scanning calorimetry (DSC) | Change in heat flow due to sample changes during heat/cooling | Polymorphic form (melting temperature, crystallisation temperature) amorphous form (glass transition temperature), crystallinity (enthalpy of fusion, enthalpy of crystallisation, heat capacity change at glass transition temperature) | Thermogram, qualitative and quantitative | Modulated temperature DSC to separate overlapping irreversible and reversible thermal events, ultrafast heating | Powder form, few milligrams | Destructive. Results will be different with open or closed (hermetically sealed) pans | [18] | |
| Infrared (IR) spectroscopy (mid-IR spectroscopy) | Change in dipole moment during molecular vibrations | Polymorphic form (peak shifts and relative intensities), crystallinity (broadening of bands, peak shifts and relative intensities) | Spectrum, qualitative and quantitative, suitable for multivariate analysis | Diffuse reflectance IR (DRIFTS), attenuated total reflection (ATR), microscope | Powder or tablet form, depends on sampling setup, few milligrams. Wet samples usually problematic. | Sample preparation/measurement can involve pressure which can induce solid state transformations | [19,20] | |
| Raman spectroscopy | Change in polarisability during molecular vibrations | Polymorphic form, crystallinity | Spectrum, qualitative and quantitative, suitable for multivariate analysis | Various sample holders (within spectrometer, sampling probes, microscope) | Powder or suspension, few milligrams (usually). Fluorescent samples are problematic. | Sample heating can be problematic. Samples can be in aqueous medium. | [20,21,22] | |
| Size and morphology | Dynamic light scattering (photon correlation spectroscopy) | Fluctuation of Rayleigh scattering of light associated with Brownian motion of nanoparticles | Particle size, particle size distribution | Particle size distribution (number based mean particle (hydrodynamic) size (Z-average), polydispersity index), quantitative | Suspension with suitable concentration | Suitable only for particles in nanometre size range | [23] | |
| Viscosity of suspension and temperature affect results | ||||||||
| Scanning electron microscopy (SEM) | Backscattering of electrons | Topographical information about particles | Scanning electron micrograph, particle morphology, size | Elemental analysis | Dry sample mounted on stage condition setup (vacuum), microgram requirement | Sample preparation destructive | [15] | |
| Transmission electron microscopy | Transmission of electrons | Density information | Transmission electron micrograph, morphology of cross sections, stabilizer- nanocrystal interaction | Embedded cross section preparation, microgram requirement | Sample preparation destructive | [24] | ||
| Surface properties | Zeta-potential | Dynamic electrophoretic mobility under electric field | Surface charge (zeta potential) | Zeta potential, quantitative | Suspension with suitable concentration | [25] | ||
| Surface plasmon resonance (SPR) | Changes in refractive index in the vicinity of a planar sensor surface | Surface adsorption | Spectrum, interaction between stabiliser drug crystals, qualitative and quantitative | Substrate on planar surface sensor required (not direct measurement of nanocrystals) | Careful sample preparation required | [26] | ||
| Drug delivery | Dissolution testing | Dissolved drug analysed over time, usually using UV spectroscopy or HPLC | Dissolution profile | Solution concentration vs time | Paddle, flow through cell (with/without membrane insert), pharmacopeial/non pharmacopeial | Separating nanocrystals from dissolution medium can be problematic | [14] | |
| Fluorescence microscopy | Fluorescence by endogenous or added fluorophores | Localization of nanocrystals in relation to cells and tissues | Fluorescence (and nanocrystal) image | One or two photon (two photon fluorescence offers inherent confocality, sub-micron spatial resolution, deeper penetration in tissues) fluorescence | Non-fluorescent nanocrystals require fluorphore to physically entrapped into nanocrystals | Entrapment and leakage of fluorophore can be difficult or problematic | [17] | |
| Non-linear Raman microscopy | Change in polarisability during molecular vibrations. | Label free localisation of particles | Intensity of CARS shift (narrow band) or spectrum, (multiplex or broad band). Most commonly qualitative. 2D or 3D images. | Can be dry or aqueous suspension, in cell cultures or tissue samples | Coloured and two-photon fluorescent samples can interfere with signal. Can be coupled with other nonlinear phenomena such as second harmonic generation or two photon electronic fluorescence | Label free. Optimal lateral spatial resolution approximately 300–400 nm. | [21,27] |
4. Particle Size and Surface Properties
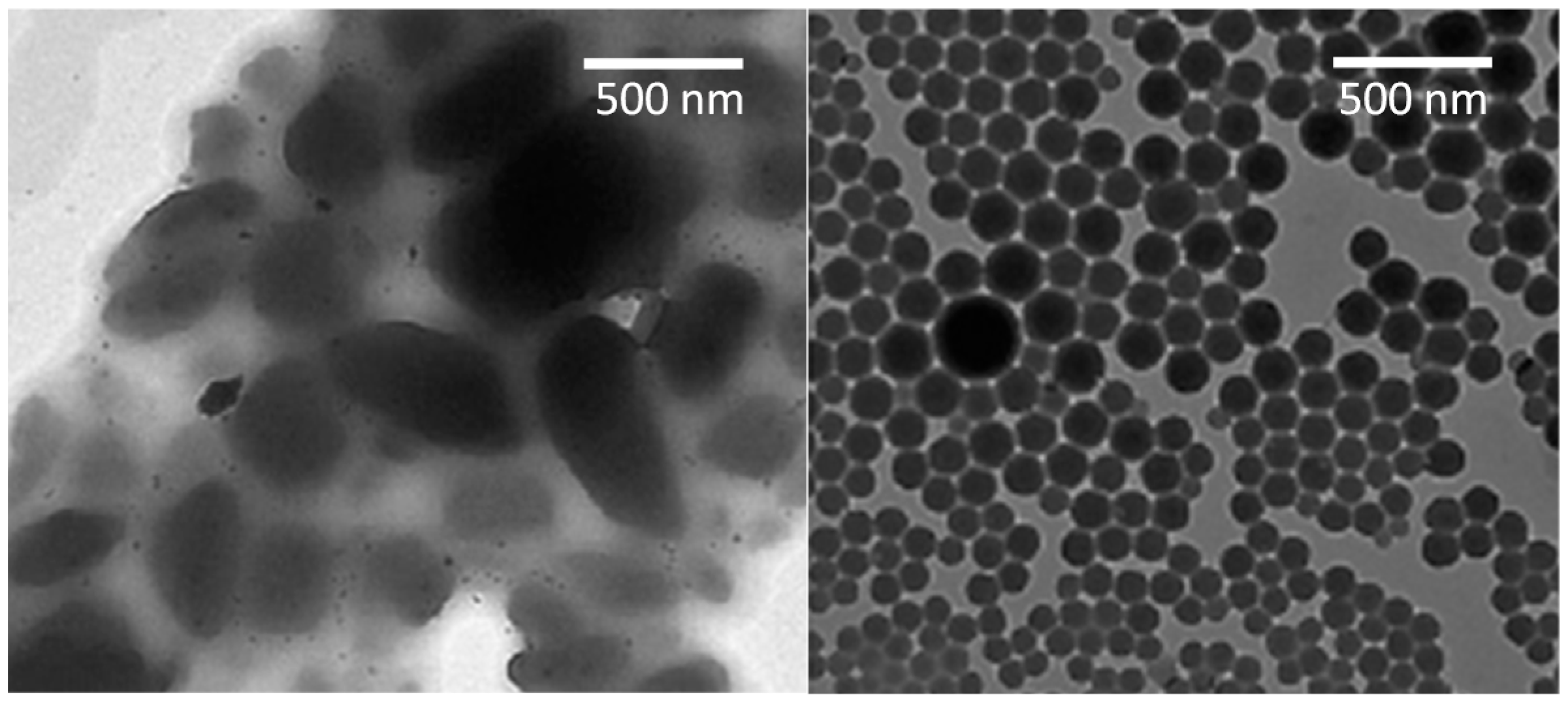

5. Dissolution of Nanocrystals: Apparent Solubility and Supersaturated State
| Sample | Concentration/mg/L | ||
|---|---|---|---|
| Distance from the Dissolution Surface | |||
| 0 mm | 2 mm | 3 mm | |
| Nanocrystals with Pluronic F 68, particle size 580 nm | 28.7 | 11.7 | 5.8 |
| Microcrystals with Pluronic F 68 | 11.4 | 4.0 | 2.6 |
| Nanocrystals with Pluronic F127, particle size 580 nm | 22.1 | 9.4 | 4.3 |
| Microcrystals with Pluronic F127 | 17.1 | 7.6 | 4.0 |
| Bulk indomethacin | 2.1 | 0.3 | 0.0 |
6. Drug Absorption from Nanocrystalline Formulations
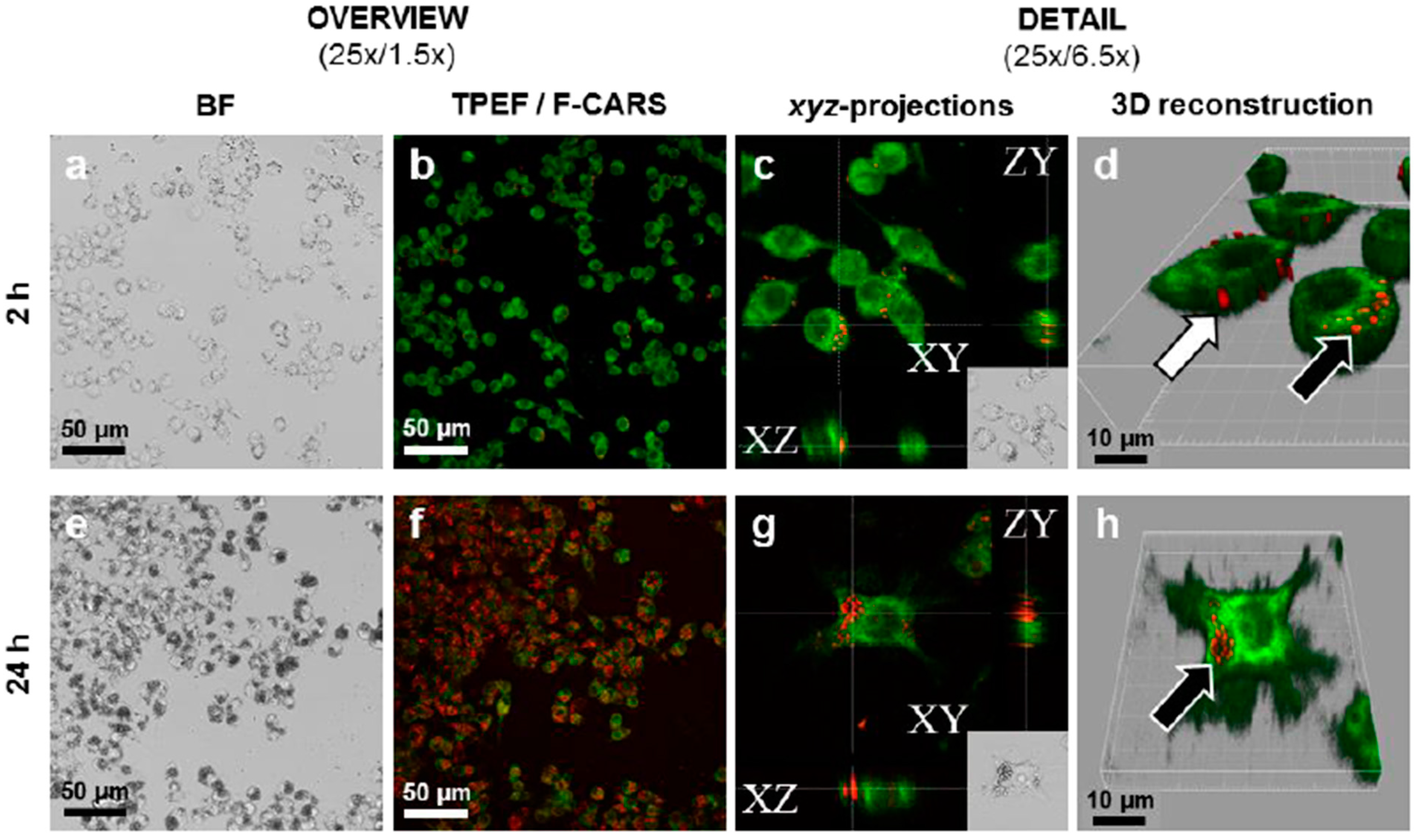
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Müller, R.H.; Keck, C.M. Twenty years of drug nanocrystals: Where are we, and where do we go? Eur. J. Pharm. Biopharm. 2012, 80, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Gohla, S.; Keck, C.M. State of the art of nanocrystals—Special features, production, nanotoxicology aspects and intracellular delivery. Eur. J. Pharm. Biopharm. 2011, 78, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.; Müller, R.H.; Möschwitzer, J.P. Bottom-up approaches for preparing drug nanocrystals: formulations and factors affecting particle size. Int. J. Pharm. 2013, 453, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, L.; Hirvonen, J.; Laaksonen, T. Drug Nanocrystals and Nanosuspension in Medicine. In Handbook of Nanobiomedical Research, Fundamentals, Applications and Recent Developments, 1 Materials for Nanomedicine; Torchilin, V., Ed.; World Scientific: Singapore, Singapore, 2014; Volume 3, pp. 169–197. [Google Scholar]
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A formulation approach for poorly-water-soluble compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef]
- Sarnes, A.; Østergaard, J.; Smedegaard Jensen, S.; Aaltonen, J.; Rantanen, J.; Hirvonen, J.; Peltonen, L. Dissolution study of nanocrystal powders of a poorly soluble drug by UV imaging and channel flow methods. Eur. J. Pharm. Sci. 2013, 50, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Rong, X.; Laru, J.; van Veen, B.; Kiesvaara, J.; Hirvonen, J.; Laaksonen, T.; Peltonen, L. Nanosuspensions of poorly soluble drugs: Preparation and development by wet milling. Int. J. Pharm. 2011, 411, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, I.; Bose, S.; Vippagunta, R.; Harmon, F. Nanosuspension for improving the bioavailability of a poorly soluble drug and screening of stabilizing agents to inhibit crystal growth. Int. J. Pharm. 2011, 409, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Tuomela, A.; Liu, P.; Puranen, J.; Rönkkö, S.; Laaksonen, T.; Kalesnykas, G.; Oksala, O.; Ilkka, J.; Laru, J.; Peltonen, L. Brinzolamide nanocrystal formulations for ophthalmic delivery: Reduction of elevated intraocular pressure in vivo. Int. J. Pharm. 2014, 467, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.; Afolabi, A.; Bhakay, A.; Leonardi, J.; Davé, R.; Bilgili, E. Enhanced physical stabilization of fenofibrate nanosuspensions via wet co-milling with a superdisintegrant and an adsorbing polymer. Eur. J. Pharm. Biopharm. 2015, 94, 372–385. [Google Scholar] [CrossRef] [PubMed]
- El-Setouhy, D.A.; Basalious, E.B.; Abdelmalak, N.S. Bioenhanced sublingual tablet of drug with limited permeability using novel surfactant binder and microencapsulated polysorbate: In vitro/in vivo evaluation. Eur. J. Pharm. Biopharm. 2015, 94, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Luo, J.; Tan, S.; Otieno, B.O.; Zhang, Z. The applications of vitamin E TPGS in drug delivery. Eur. J. Pharm. Sci. 2013, 49, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Pini, E.; Corrias, F.; Perricci, J.; Manconi, M.; Fadda, A.M.; Sinico, C. Formulation strategy and evaluation of nanocrystal piroxicam orally disintegrating tablets manufacturing by freeze-drying. Int. J. Pharm. 2014, 467, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; De Wulf, O.; Laru, J.; Heikkilä, T.; van Veen, B.; Kiesvaara, J.; Hirvonen, J.; Peltonen, L.; Laaksonen, T. Dissolution studies of poorly soluble drug nanosuspensions in non-sink conditions. AAPS PharmSciTech 2013, 14, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Sarnes, A.; Kovalainen, M.; Häkkinen, M.R.; Laaksonen, T.; Laru, J.; Kiesvaara, J.; Ilkka, J.; Oksala, O.; Rönkkö, S.; Järvinen, K.; et al. Nanocrystal-based per-oral itraconazole delivery: Superior in vitro dissolution enhancement versus Sporanox® is not realized in in vivo drug absorption. J. Control. Release 2014, 180, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Surwase, S.A.; Boetker, J.P.; Saville, D.; Boyd, B.J.; Gordon, K.C.; Peltonen, L.; Strachan, C.J. Indomethacin: New polymorphs of an old drug. Mol. Pharm. 2013, 10, 4472–4480. [Google Scholar] [CrossRef] [PubMed]
- Valo, H.K.; Laaksonen, P.H.; Peltonen, L.J.; Linder, M.B.; Hirvonen, J.T.; Laaksonen, T.J. Multifunctional hydrophobin: toward functional coatings for drug nanoparticles. ACS Nano 2010, 4, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Valo, H.; Kovalainen, M.; Laaksonen, P.; Häkkinen, M.; Auriola, S.; Peltonen, L.; Linder, M.; Järvinen, K.; Hirvonen, J.; Laaksonen, T. Immobilization of protein-coated drug nanoparticles in nanofibrillar cellulose matrices—Enhanced stability and release. J. Control. Release 2011, 156, 390–397. [Google Scholar] [CrossRef]
- Valo, H.; Arola, S.; Laaksonen, P.; Torkkeli, M.; Peltonen, L.; Linder, M.B.; Serimaa, R.; Kuga, S.; Hirvonen, J.; Laaksonen, T. Drug release from nanoparticles embedded in four different nanofibrillar cellulose aerogels. Eur. J. Pharm. Sci. 2013, 50, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Heinz, A.; Strachan, C.J.; Gordon, K.C.; Rades, T. Analysis of solid-state transformations of pharmaceutical compounds using vibrational spectroscopy. J. Pharm. Pharmacol. 2007, 61, 971–988. [Google Scholar] [CrossRef]
- Darville, N.; van Heerden, M.; Vynckier, A.; de Meulder, M.; Sterkens, P.; Annaert, P.; van den Mooter, G. Intramuscular administration of paliperidone palmitate extended-release injectable microsuspension induces a subclinical inflammatory reaction modulating the pharmacokinetics in rats. J. Pharm. Sci. 2014, 103, 2072–2087. [Google Scholar] [CrossRef] [PubMed]
- Strachan, C.J.; Rades, T.; Gordon, K.C.; Rantanen, J. Raman spectroscopy for quantitative analysis of pharmaceutical solids. J. Pharm. Pharmacol. 2007, 59, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Z.-H.; Li, T.; McNally, H.; Park, K.; Sturek, M. Development and evaluation of transferrin-stabilized paclitaxel nanocrystal formulation. J. Control. Release 2014, 176, 76–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laaksonen, T.; Liu, P.; Rahikkala, A.; Peltonen, L.; Kauppinen, E.I.; Hirvonen, J.; Järvinen, K.; Raula, J. Intact nanoparticulate indomethacin in fast-dissolving carrier particles by combined wet milling and aerosol flow reactor methods. Pharm. Res. 2011, 28, 2403–2411. [Google Scholar] [CrossRef] [PubMed]
- Van Eerdenbrugh, B.; Vermant, J.; Martens, J.A.; Froyen, L.; Van Humbeeck, J.; Augustijns, P.; van den Mooter, G. A screening study of surface stabilization during the production of drug nanocrystals. J. Pharm. Sci. 2009, 98, 2091–2103. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Viitala, T.; Kartal-Hodzig, A.; Liang, H.; Laaksonen, T.; Hirvonen, J.; Peltonen, L. Interaction studies between indomethacin nanocrystals and PEO/PPO copolymer stabilizers. Pharm. Res. 2015, 32, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Strachan, C.J.; Windbergs, M.; Offerhaus, H.L. Pharmaceutical applications of non-linear imaging. Int. J. Pharm. 2011, 417, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.S.M.; York, P.; Ali, A.M.A.; Blagden, N. Hydrocortisone nanosuspensions for ophthalmic delivery: a comparative study between microfluidic nanoprecipitation and wet milling. J. Control. Release 2011, 149, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Rutledge, G.C.; Myerson, A.S.; Trout, B.L. Production and characterization of carbamazepine nanocrystals by electrospraying for continuous pharmaceutical manufacturing. J. Pharm. Sci. 2012, 101, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Pireddu, R.; Sinico, C.; Ennas, G.; Marongiu, F.; Muzzalupo, R.; Lai, F.; Fadda, A.M. Novel nanosized formulations of two diclofenac acid polymorphs to improve topical bioavailability. Eur. J. Pharm Sci. 2015, 77, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Huey, B.D.; Burgess, D.J. Scanning probe microscopy method for nanosuspension stabilizer selection. Langmuir 2009, 25, 12481–12487. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, Y.; Shang, L.; Wang, Q.; Zhang, D. Stability of nanosuspensions in drug delivery. J. Control. Release 2013, 172, 1126–1141. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Pudavar, H.E.; Roy, I.; Ohulchansky, T.Y.; Chan, Y.; Pandery, R.K.; Prasad, P.N. New method for delivering a hydrophobic drug for photodynamic therapy using pure nanocrystal form of the drug. Mol. Pharm. 2007, 4, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, T. Cellular uptake mechanism of paclitaxel nanocrystals determined by confocal imaging and kinetic measurement. AAPS J. 2015, 17, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Ige, P.P.; Baria, R.K.; Gattani, S.G. Fabrication of fenofibrate nanocrystals by probe sonication method for enhancement of dissolution rate and oral bioavailability. Colloids Surf. B Biointerfaces 2013, 108, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Abu-Diak, O.A.; Jones, D.S.; Andrews, G.P. An investigation into the dissolution properties of celecoxib melt extrudates: Understanding the role of polymer type and concentration in stabilizing supersaturated drug concentrations. Mol. Pharm. 2011, 8, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Yoo, S.D.; Lee, S.H.; Kim, K.H.; Yoon, D.S.; Lee, K.H. Enhanced solubility and dissolution rate of itraconazole by a solid dispersion technique. Int. J. Pharm. 1999, 187, 209–218. [Google Scholar] [CrossRef]
- Surwase, S.A.; Itkonen, L.; Aaltonen, J.; Saville, D.; Rades, T.; Peltonen, L.; Strachan, C.J. Polymer incorporation method affects the physical stability of amorphous indomethacin in aqueous suspension. Eur. J. Pharm. Biopharm. 2015, 96, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Frank, K.J.; Westedt, U.; Rosenblatt, K.M.; Hölig, P.; Rosenberg, J.; Mägerlein, M.; Fricker, G.; Brandl, M. What is the mechanism behind increased permeation rate of a poorly soluble drug from aqueous dispersions of an amorphous solid dispersion? J. Pharm. Sci. 2014, 103, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Murdande, S.B.; Pikal, M.J.; Shanker, R.M.; Bogner, R.H. Solubility advantage of amorphous pharmaceuticals, part 3: Is maximum solubility advantage experimentally attainable and sustainable? J. Pharm. Sci. 2011, 100, 4349–4356. [Google Scholar] [CrossRef] [PubMed]
- Sekikawa, H.; Nakano, M.; Arita, T. Inhibitory effect of polyvinylpyrrolidone on the crystallization of drugs. Chem. Pharm. Bull. 1978, 26, 118–126. [Google Scholar] [CrossRef]
- Raghavan, S.L.; Trividic, A.; Davis, A.F.; Hadgraft, J. Crystallization of hydrocortisone acetate: Influence of polymers. Int. J. Pharm. 2001, 212, 213–221. [Google Scholar] [CrossRef]
- Yokoi, Y.; Yonemochi, E.; Terada, K. Effects of sugar ester and hydroxypropyl methylcellulose on the physicochemical stability of amorphous cefditoren pivoxil in aqueous suspension. Int. J. Pharm. 2005, 290, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Warren, D.B.; Benameur, H.; Porter, C.J.H.; Pouton, C.W. Using polymeric precipitation inhibitors to improve the absorption of poorly water-soluble drugs: A mechanistic basis for utility. J. Drug Target. 2010, 18, 704–731. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Fri∂riksdóttir, H.; Gu∂mundsdóttir, T.K. The effect of water-soluble polymers on aqueous solubility of drugs. Int. J. Pharm. 1996, 127, 293–296. [Google Scholar] [CrossRef]
- Ueda, K.; Higashi, K.; Yamamoto, K.; Moribe, K. In situ molecular elucidation of drug supersaturation achieved by nano-sizing and amorphization of poorly water-soluble drug. Eur. J. Pharm. Sci. 2015, 77, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Quan, P.; Zhang, Y.; Cheng, J.; Liu, J.; Cun, D.; Xiang, R.; Fang, L. Influence of drug physicochemical properties on absorption of water insoluble drug nanosuspensions. Int. J. Pharm. 2014, 460, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Collnot, E.M.; Baldes, C.; Wempe, M.F.; Hyatt, J.; Navarro, L.; Edgar, K.J.; Schaefer, U.F.; Lehr, C.M. Influence of vitamin E TPGS poly(ethylene glycol) chain length on apical efflux transporters in Caco-2 cell monolayers. J. Control. Release 2006, 111, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhao, Y.; Liu, Y.; Chang, X.; Chen, C. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small 2011, 7, 1322–1337. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, G.; Ma, J.; Wang, X.; Wang, F.; Wang, H.; Sun, J. Paclitaxel nanosuspension coated with P-gp inhibitory surfactants: II. Ability to reverse the drug-resistance of H460 human lung cancer cells. Colloids Surf. B Biointerfaces 2014, 117, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.J.; Fu, Y.H.; Meng, Q.H.; Li, M.; Ying, X.Y.; Han, M.; He, Q.J.; Yang, B.; Zeng, S.; Hu, Y.Z.; et al. Evaluation of pluronic nanosuspensions loading a novel insoluble anticancer drug both in vitro and in vivo. Int. J. Pharm. 2013, 456, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, J.; Chen, Q.; Gao, Y.; Li, L.; Li, H.; Leng, D.; Wang, Y.; Sun, Y.; Jing, Y.; et al. Star-shape copolymer of lysine-linked dl-tocopherol polyethylene glycol 2000 succinate for doxorubicin delivery with reversal of multidrug resistance. Biomaterials 2012, 33, 6877–6888. [Google Scholar] [CrossRef] [PubMed]
- Varmal, M.V.; Panchagnula, R. Enhanced oral paclitaxel absorption with vitamin E-TPGS: Effect on solubility and permeability in vitro, in situ and in vivo. Eur. J. Pharm. Sci. 2005, 25, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.; Jones, A.T.; Stephens, D.J. Intracellular trafficking pathways and drug delivery: Fluorescence imaging of living and fixed cells. Adv. Drug Deliv. Rev. 2005, 57, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Tenuta, T.; Monopoli, M.P.; Kim, J.; Salvati, A.; Dawson, K.A.; Sandin, P.; Lynch, I. Elution of labile fluorescent dye from nanoparticles during biological use. PLoS ONE 2011, 6, e25556. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.S.; Gullotti, E.; Tong, L.; Highley, C.B.; Errabelli, D.R.; Hasan, T.; Cheng, J.X.; Kohane, D.S.; Yeo, Y. Intracellular drug delivery by poly(lactic-co-glycolic acid) nanoparticles, revisited. Mol. Pharm. 2009, 6, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Dorney, J.; Bonnier, F.; Garcia, A.; Casey, A.; Chambers, G.; Byrne, H.J. Identifying and localizing intracellular nanoparticles using Raman spectroscopy. Analyst 2012, 137, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Krafft, C.; Dietzek, B.; Popp, J. Raman and CARS microspectroscopy of cells and tissues. Analyst 2009, 134, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Samtani, M.N.; Vermeulen, A.; Stuyckens, K. Population pharmacokinetics of intramuscular paliperidone palmitate in patients with schizophrenia: A novel once-monthly, long-acting formulation of an atypical antipsychotic. Clin. Pharmacokinet. 2009, 48, 585–600. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peltonen, L.; Strachan, C. Understanding Critical Quality Attributes for Nanocrystals from Preparation to Delivery. Molecules 2015, 20, 22286-22300. https://doi.org/10.3390/molecules201219851
Peltonen L, Strachan C. Understanding Critical Quality Attributes for Nanocrystals from Preparation to Delivery. Molecules. 2015; 20(12):22286-22300. https://doi.org/10.3390/molecules201219851
Chicago/Turabian StylePeltonen, Leena, and Clare Strachan. 2015. "Understanding Critical Quality Attributes for Nanocrystals from Preparation to Delivery" Molecules 20, no. 12: 22286-22300. https://doi.org/10.3390/molecules201219851






