Development of an Analytical Method Based on Temperature Controlled Solid-Liquid Extraction Using an Ionic Liquid as Solid Solvent
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of TC-SLE of Fe2+ with [BPy]PF6-PT
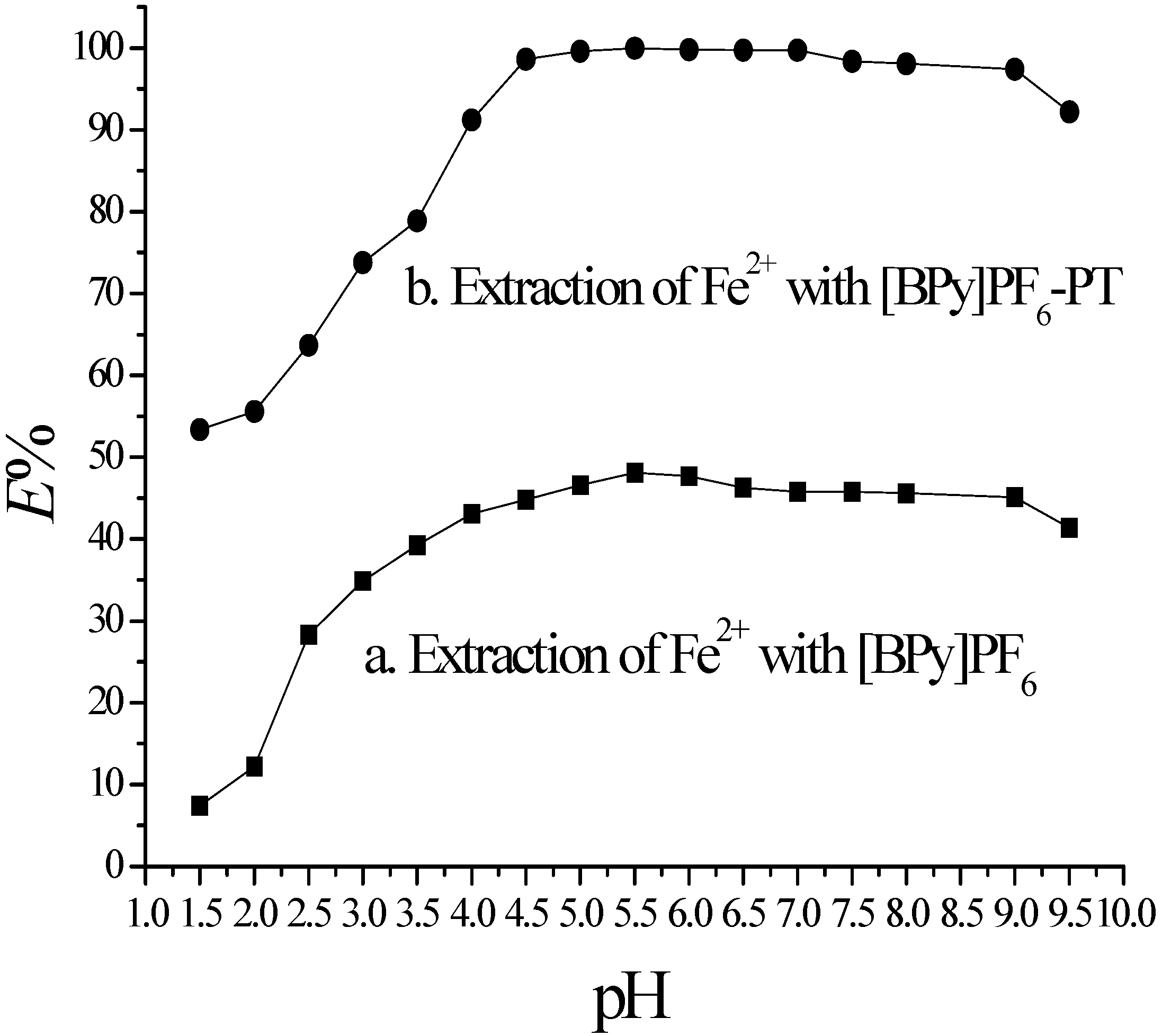
2.2. Effect of pH on TC-SLE of Fe2+
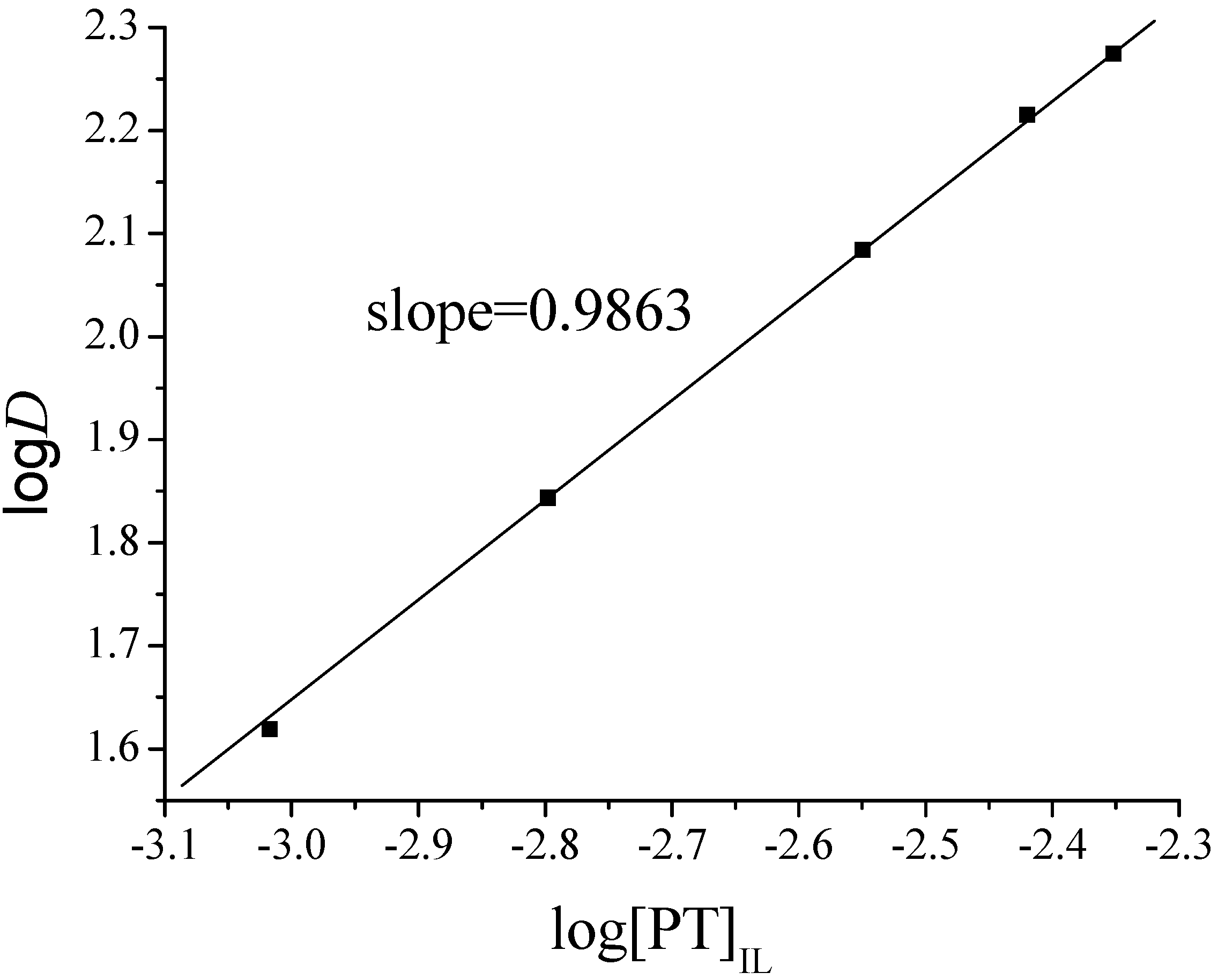
2.3. Effect of PT Concentration on TC-SLE of Fe2+

2.4. Composition of the TC-SLE Extracted Species
2.5. Back-Extraction of Fe2+

2.6. Determination of Fe2+ in Tea
| Sample | Determinate Concentration (μg/mL) | Average Concentration (μg/mL) | RSD | Contents of Fe2+/g Dried Tea (μg/g) | Average Contents of Fe2+/g Dried Tea(μg/g) | Reference Value [25] (μg/g) |
|---|---|---|---|---|---|---|
| 1 | 1.5294 | 1.4968 | 3.05% | 609.68 | 594.68 | 19.77~797.47 |
| 2 | 1.5161 | 575.28 | ||||
| 3 | 1.4481 | 603.48 | ||||
| 4 | 1.5429 | 610.74 | ||||
| 5 | 1.4475 | 574.22 |
| Sample | Fe2+ Content in the Sample (μg/mL) | Amount of Standard Fe2+ Added (μg/mL) | Measured Amount (μg/mL) | Percent Recovery (%) |
|---|---|---|---|---|
| 1 | 1.4865 | 0.7430 | 2.2471 | 102.4 |
| 1.4885 | 3.2142 | 90.6 | ||
| 2.2255 | 3.9046 | 108.6 | ||
| 2 | 1.5021 | 0.7430 | 2.2427 | 99.7 |
| 1.4885 | 3.0153 | 101.7 | ||
| 2.2255 | 3.7689 | 101.9 |
3. Experimental Section
3.1. General Information
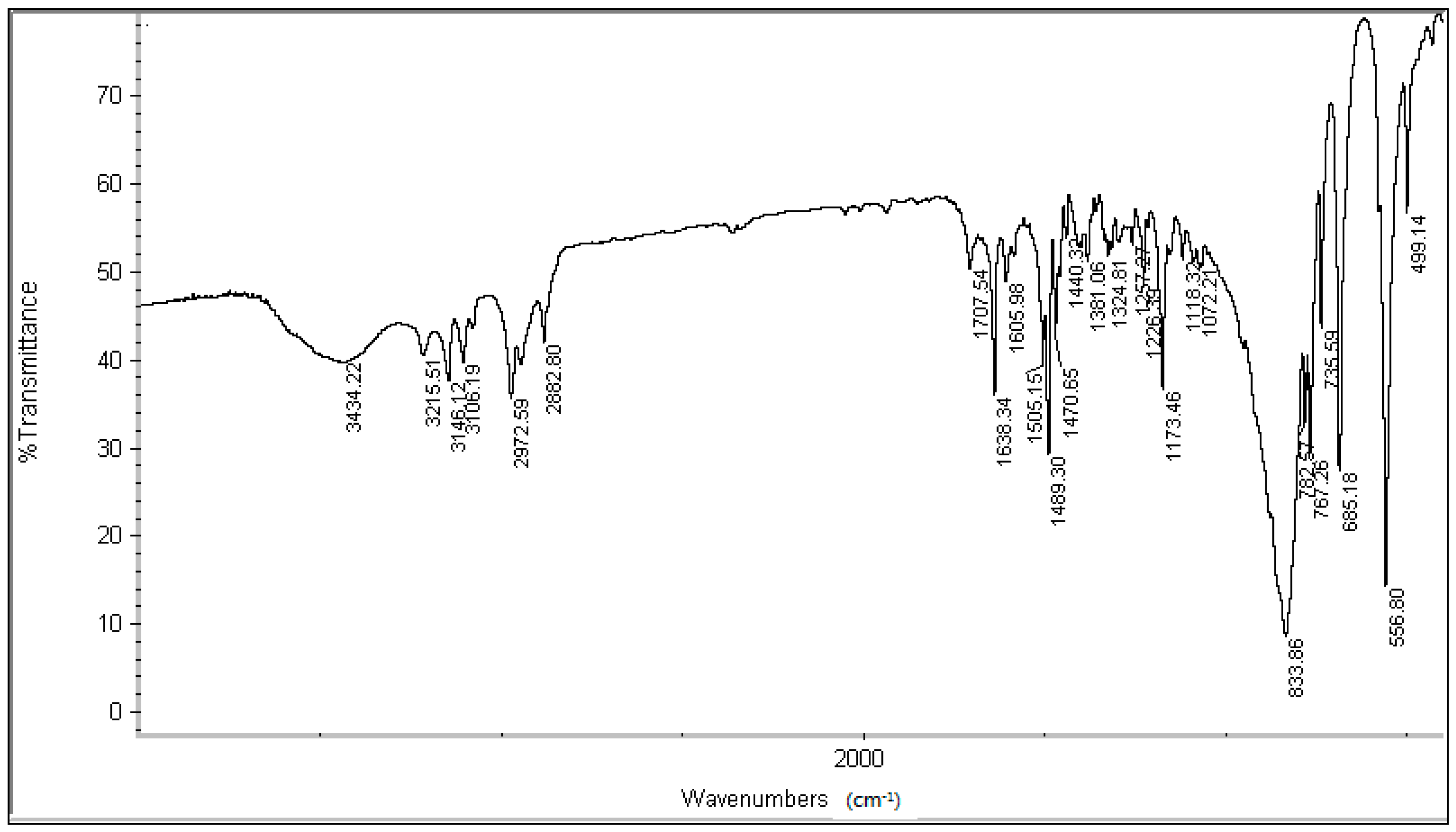
3.2. Synthesis and Characterization

3.3. Fe2+TC-SLE Procedure
3.4. Tea Sample Preparation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fan, Z.Y.; Hong, D.C.; Li, N. A variety of life elements in tea components. J. Jiangxi Coll. Tradit. Chin. Med. 1996, 8, 27–28. (In Chinese) [Google Scholar]
- Mulye, R.R.; Khopkar, S.M. Extraction spectrophotometric determination of iron(II) with Thiodibenzoylmethane. Fresenius’ J. Anal. Chem. 1974, 272, 283. [Google Scholar] [CrossRef]
- Alexiev, A.A.; Bontchev, P.R.; Khristova, M. A simple and sensitive catalytic method for determination of the total iron-binding capacity of blood serum. Microchim. Acta 1979, 71, 165–174. [Google Scholar] [CrossRef]
- Endo, M.; Abe, S. Sequential flow-injection spectrophotometric determination of iron(II) and iron(III) by copper(II)-catalyzed reaction with Tiron. Fresenius’ J. Anal. Chem. 1997, 358, 546–547. [Google Scholar] [CrossRef]
- Jahres, C.M.G.; Yusty, M.A.L.; Lozano, J.S. Second derivative visible spectroscopic determination of iron and manganese in Galician wines. Fresenius’ J. Anal. Chem. 1990, 338, 703–706. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.; Dzyuba, S.V.; Bartsch, R.A. Influence of structural variation in room-temperature ionic liquids on the selectivity and efficiency of competitive alkali metal salt extraction by a crown ether. Anal. Chem. 2001, 73, 3737–3741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Deng, Y.; Li, C.; Chen, J. Separation of ternary systems of hydrophilic ionic liquid with miscible organic compounds by RPLC with refractive index detection. J. Sep. Sci. 2008, 31, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, A.J.; Seddon, K.R. Polarity study of some 1-alkyl-3-methylimidazolium ambient temperature ionic liquids with the solvatochromic dye, nile red. J. Phys. Org. Chem. 2000, 13, 591–595. [Google Scholar] [CrossRef]
- Kaar, J.L.; Jesionowski, A.M.; Berberich, J.A.; Moullton, R.; Russel, A.J. Impact of ionic liquid physical properties on lipase activity and stability. J. Am. Chem. Soc. 2003, 125, 4125–4131. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, S.B. The Hildebrand solubility parameters, cohesive energy densities and internal energies of 1-alkyl-3-methylimidazolium-based room temperature ionic liquids. Chem. Commun. 2005, 27, 3469–3471. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Sun, X.; Wang, X.; Sun, W.; Hou, W. Application of ionic liquid modified carbon ceramic electrode for the sensitive voltammetric detection of rutin. Talanta 2010, 82, 1853–1857. [Google Scholar] [CrossRef] [PubMed]
- Koel, M. Ionic liquids in chemical analysis. Crit. Rev. Anal. Chem. 2005, 35, 177–192. [Google Scholar] [CrossRef]
- Dai, S.; Ju, Y.H.; Barnes, C.E. Solvent extraction of strontium nitrate by a crown ether using room-temperature ionic liquids. J. Chem. Soc. Dalton Trans. 1999, 8, 1201–1202. [Google Scholar] [CrossRef]
- Luo, H.; Dai, S.; Bonnesen, P.V. Solvent extraction of Sr2+ and Cs+ based on room- temperature ionic liquids containing monoaza-substituted crown ethers. Anal. Chem. 2004, 76, 2773–2779. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.T.; Yang, Z.; Chen, C.J. Room temperature ionic liquid as a novel medium for liquid/liquid extraction of metal ions. Anal. Chim. Acta 2003, 488, 183–192. [Google Scholar] [CrossRef]
- Ajioka, T.; Oshima, S.; Hirayama, N. Use of 8-sulfonamidoquinoline derivatives as chelate extraction reagents in ionic liquid extraction system. Talanta 2007, 74, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Tsukatami, T.; Katano, H.; Tatsumi, H.; Deguchi, M.; Hiyarama, N. Halogen-free water- immiscible ionic liquids based on tetraoctylammonium cation and dodecylsulfate and dodecylbenzenesulfon ate anions, and their application as chelate extraction solvent. Anal. Sci. 2006, 22, 199–200. [Google Scholar] [CrossRef]
- Pan, Z.; Cai, Z.; Bian, M.; Lian, T.; Su, Z.; Zhang, Z.; Huang, D. Temperature dependent solid-liquid extraction behavior of rare earths using N-butyl pyridinium hexafluoropho- sphate with benzoyl acetone. Adv. Mater. Res. 2013, 734–737, 906–910. [Google Scholar]
- Pan, Z.; Weng, W.; Yan, H.; Yu, Y.; Wu, T.; Pan, J. Studies on temperature dependent ionic liquid solid-liquid extraction behavior of rare earth. App. Mech. Mater. 2013, 401–403, 817–821. [Google Scholar] [CrossRef]
- McKaveney, J.P.; Freiser, H. Solvent extraction of chromium with acetylacetone. Anal. Chem. 1958, 30, 1965–1968. [Google Scholar] [CrossRef]
- Brandstetr, J.; Vrestal, J. Photometrische bestimmung und trennung des rutheniums mittels acetylacetons. J. Collect. Czech. Commun. 1961, 26, 392–397. [Google Scholar] [CrossRef]
- Wuhan University. Experiment of Analytical Chemistry, 2nd ed.; Higher Education Press: Beijing, China, 1985; pp. 354–357. (In Chinese) [Google Scholar]
- Hu, Z.D.; Zhao, Z.F. Spectrophotometry, 1st ed.; Ningxia People’s Press: Ningxia, China, 1987; pp. 484–487. (In Chinese) [Google Scholar]
- Chen, L.; Luo, D.; Liang, Q.F.; Guo, Y.L.; Wang, G. Iron in the soil of Tieguanyin tea plantations in Fujian and its transfer into tea leaves. Fujian J. Agric. Sci. 2009, 24, 153–156. [Google Scholar]
- Abadia, J.; Monge, E.; Montanes, L. Extraction of iron from plant leaves by Fe (II) chelators. J. Plant Nutr. 2008, 7, 777–784. [Google Scholar] [CrossRef]
- Kassem, M.A.; Amin, A.S. Spectrophotometric determination of iron in environmental and food samples using solid phase extraction. Food Chem. 2013, 141, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Soylak, M. Ultrasound assisted-deep eutectic solvent extraction of iron from sheep, bovine and chicken liver samples. Talanta 2015, 136, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, S.; Huang, Y.; Yuan, D. Redox speciation analysis of dissolved iron in estuarine and coastal waters with on-line solid phase extraction and graphite furnace atomic absorption spectrometry detection. Talanta 2015, 137, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.W.; Zeng, H.; Yang, H.C.; Yu, M.L.; Wang, Z.Y.; Wu, W.J.; Huang, X.Q. Analytical application of pyridine type ionic liquid as solid solvent. Mater. Res. Innov. 2015, 19, s283–s287. [Google Scholar] [CrossRef]
- Experimental Curriculum Group of Physical Chemistry, Department of Chemistry, Beijing University. Experimentation of Physical Chemistry, 1st ed.; Beijing University Press: Beijing, China, 1981; pp. 238–240. (In Chinese) [Google Scholar]
- Menis, O.; Rains, T.C. Extration and flame photometric determination of iron. Anal. Chem. 1960, 32, 1837–1841. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Z.; Wang, Z.; Zhu, L.; Zhu, Z.; Cai, J.; Shen, X.; Fan, T.; Zhang, Y.; Chen, Z. Development of an Analytical Method Based on Temperature Controlled Solid-Liquid Extraction Using an Ionic Liquid as Solid Solvent. Molecules 2015, 20, 22137-22145. https://doi.org/10.3390/molecules201219842
Pan Z, Wang Z, Zhu L, Zhu Z, Cai J, Shen X, Fan T, Zhang Y, Chen Z. Development of an Analytical Method Based on Temperature Controlled Solid-Liquid Extraction Using an Ionic Liquid as Solid Solvent. Molecules. 2015; 20(12):22137-22145. https://doi.org/10.3390/molecules201219842
Chicago/Turabian StylePan, Zhongwei, Zhengquan Wang, Linna Zhu, Zhiming Zhu, Jinying Cai, Xiaoman Shen, Tingli Fan, Yingnan Zhang, and Zhixiu Chen. 2015. "Development of an Analytical Method Based on Temperature Controlled Solid-Liquid Extraction Using an Ionic Liquid as Solid Solvent" Molecules 20, no. 12: 22137-22145. https://doi.org/10.3390/molecules201219842





