Adsorption Kinetics at Silica Gel/Ionic Liquid Solution Interface
Abstract
:1. Introduction
2. Results and Discussion
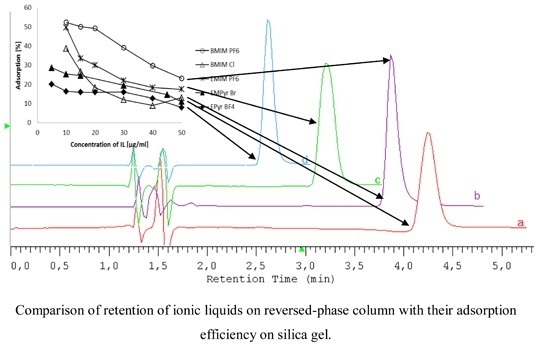
2.1. HPLC Conditions for Ionic Liquids Determination
| BMIM PF6 |  | [PF6]− |
| BMIM Cl |  | [Cl]− |
| EMIM PF6 |  | [PF6]− |
| EMPyr Br |  | [Br]− |
| EPyr BF4 |  | [BF4]− |
| Ionic Liquid | The Mobile Phase Composition | RT (min) | k | As | N (EUP) | λmax |
|---|---|---|---|---|---|---|
| BMIM PF6 | 15%MeOH, 30 mM phosphate buffer, 30 mM NaBF4 | 3.87 | 1.98 | 1.73 | 38,480 | 220 |
| BMIM Cl | 15%MeOH, 30 mM phosphate buffer, 30 mM NaBF4 | 3.92 | 2.02 | 1.11 | 26,233 | 220 |
| EMIM PF6 | 5%MeOH, 50 mM phosphate buffer, 30 mM NaPF6 | 3.20 | 1.46 | 1.32 | 12,673 | 220 |
| EMPyr Br | 8%MeOH, 30 mM phosphate buffer, 30 mM NaPF6 | 4.24 | 2.26 | 1.36 | 21,626 | 255 |
| EPyr BF4 | 5%MeOH, 50 mM phosphate buffer, 30 mM NaPF6 | 2.61 | 1.01 | 1.34 | 20,300 | 255 |
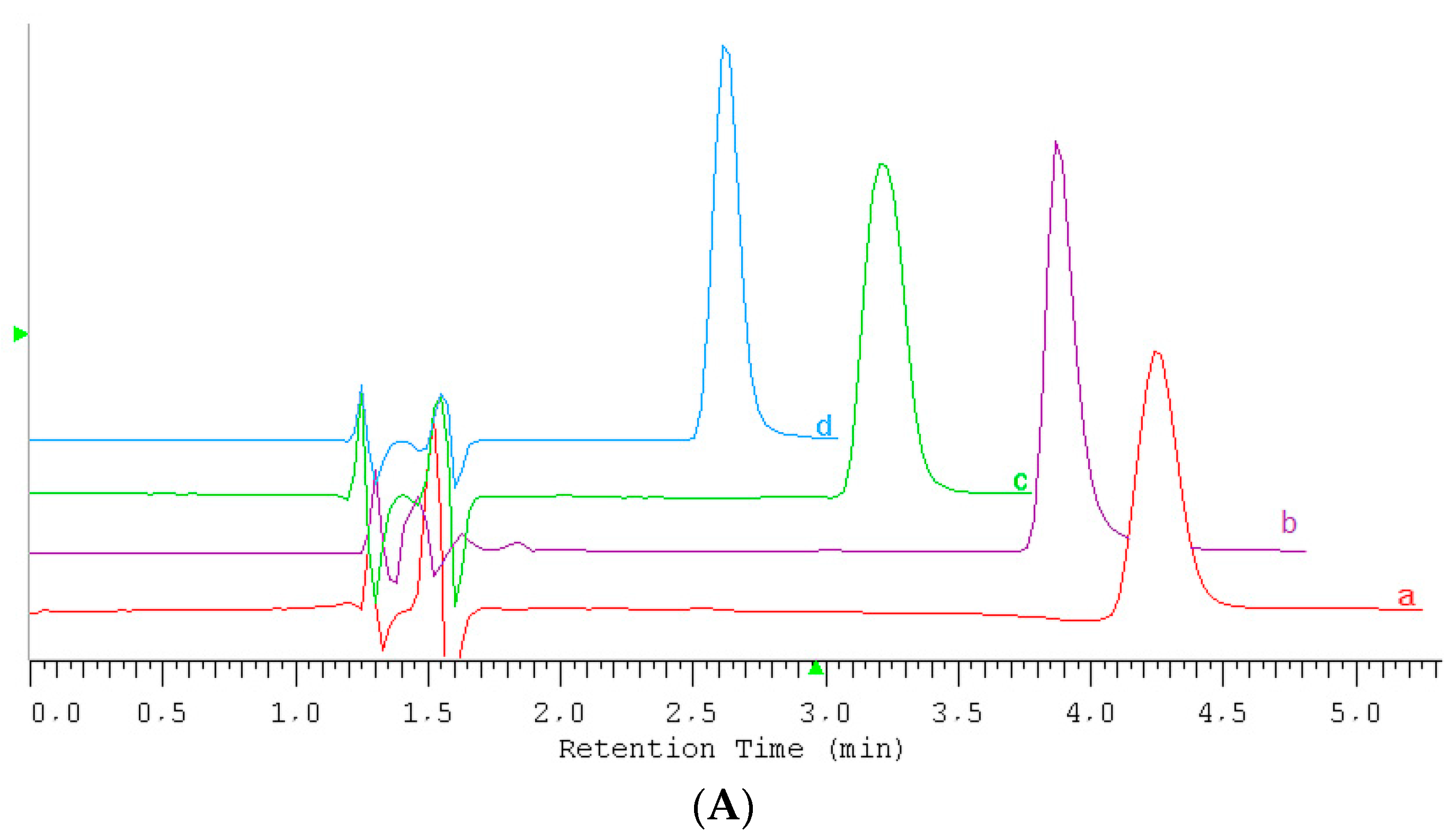
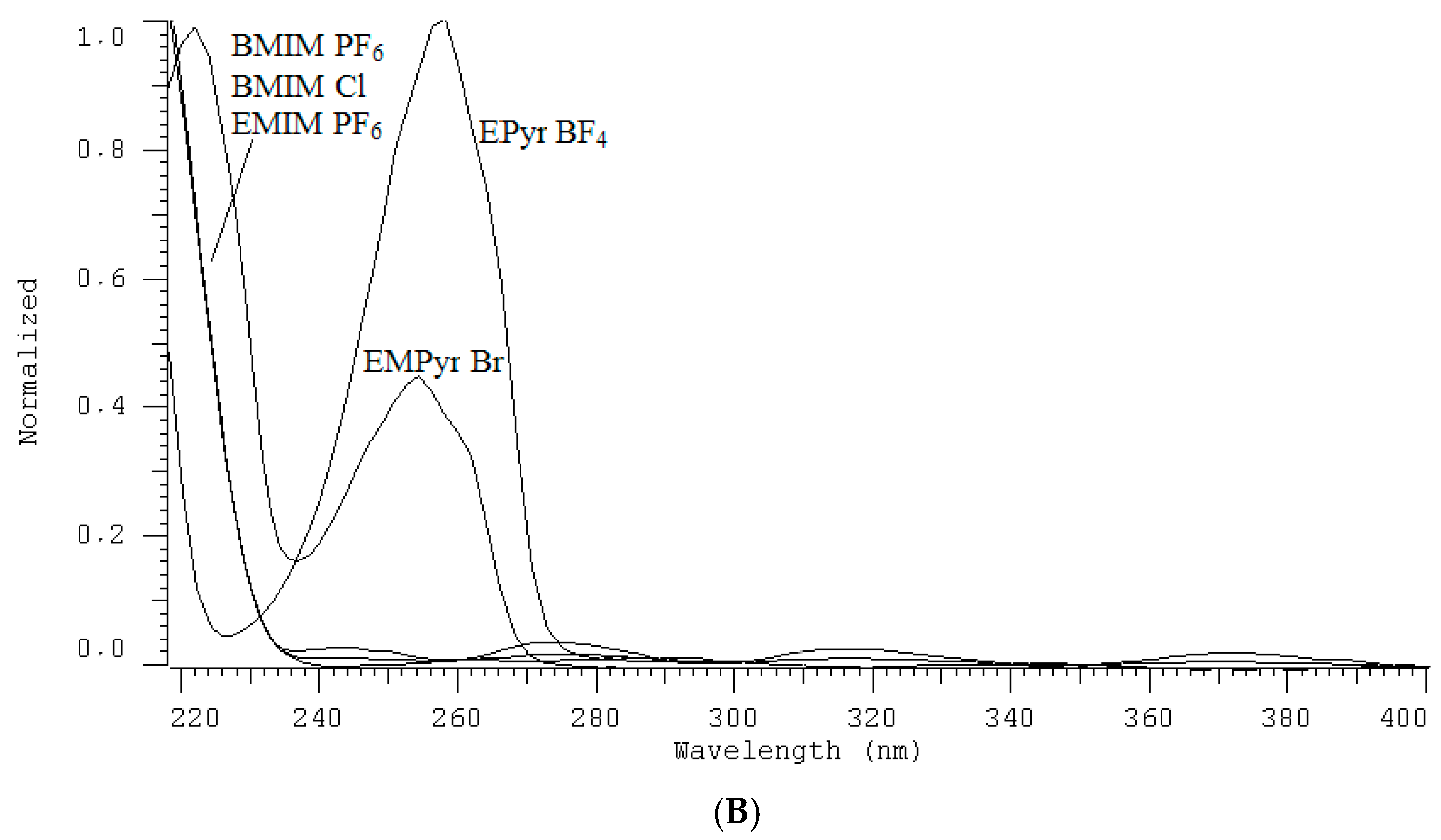
2.2. Conditions for IL Quantification
| Ionic Liquid | Conc. Range: (µg·mL−1) | a ± SD | b ± SD | R2 | s | F | LOD (µg·mL−1) | LOQ (µg·mL−1) | n |
|---|---|---|---|---|---|---|---|---|---|
| BMIM PF6 | 0.5–50 | 6824.04 (±98.07) | 8521.04 (±2735.60) | 0.9984 | 5397.32 | 4842.17 | 0.0474 | 0.1436 | 8 |
| BMIM Cl | 2.5–50 | 8110.84 (±145.70) | 18027.91 (±3908.27) | 0.9981 | 6575.45 | 3098.93 | 0.0593 | 0.1796 | 6 |
| EMIM PF6 | 5–50 | 6482.15 (±108.17) | 2386.59 (±3372.33) | 0.9983 | 4833.93 | 3590.78 | 0.0551 | 0.1669 | 6 |
| EMPyr Br | 5–50 | 17059.98 (±333.56) | 10599.09 (±10398.61) | 0.9977 | 14905.46 | 2615.88 | 0.0645 | 0.1954 | 6 |
| EPyr BF4 | 5–50 | 11531.70 (±126.90) | −4670.56 (±3956.14) | 0.9993 | 5670.77 | 8257.59 | 0.0363 | 0.1100 | 6 |
2.3. Influence of Ionic Liquid Kind and Concentration on Adsorption Efficiency
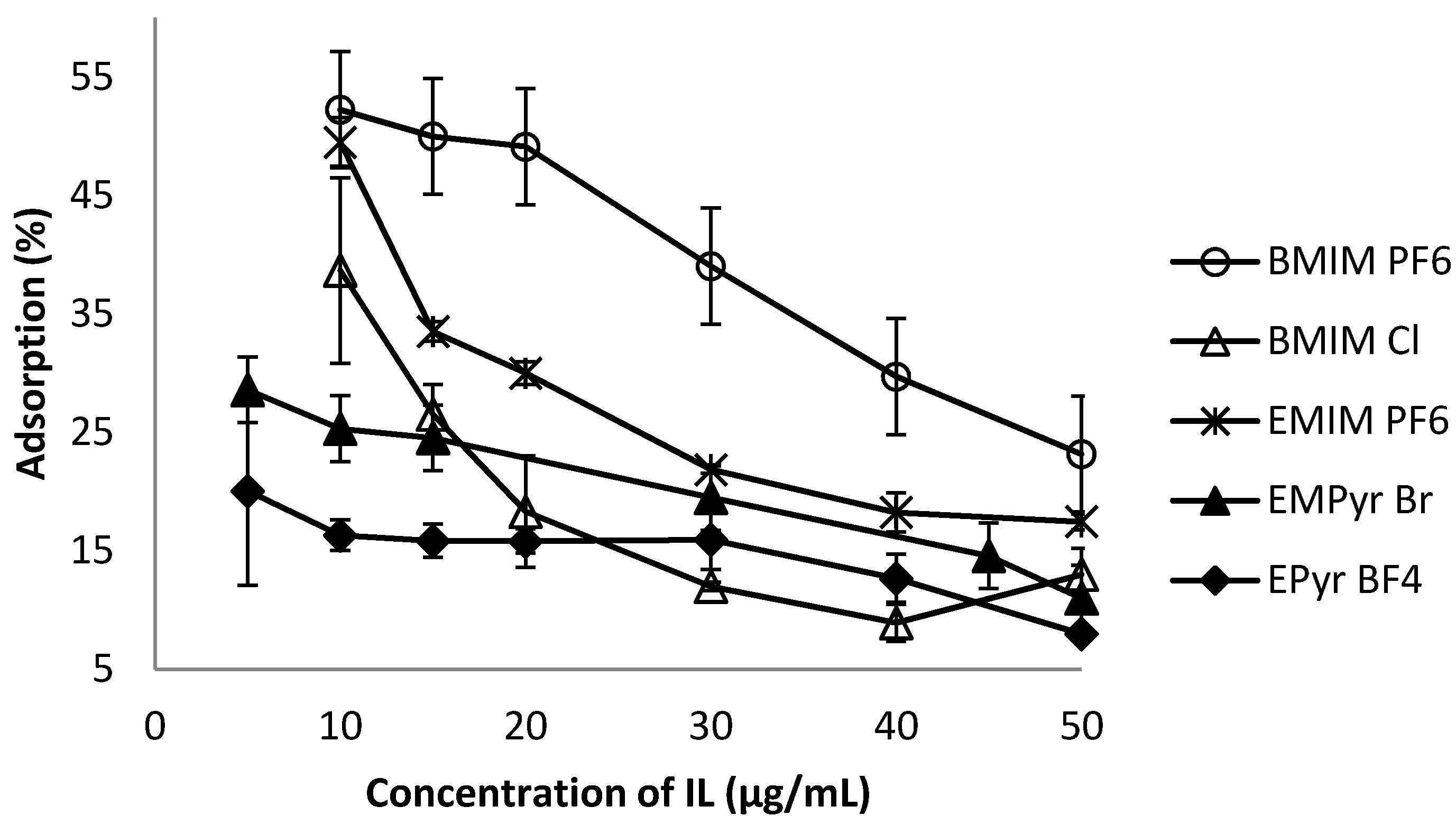
2.4. Influence of Solvent Kind and Concentration on Adsorption Efficiency

2.5. Influence of Temperature on Adsorption Efficiency

2.6. Kinetics of Adsorption Process

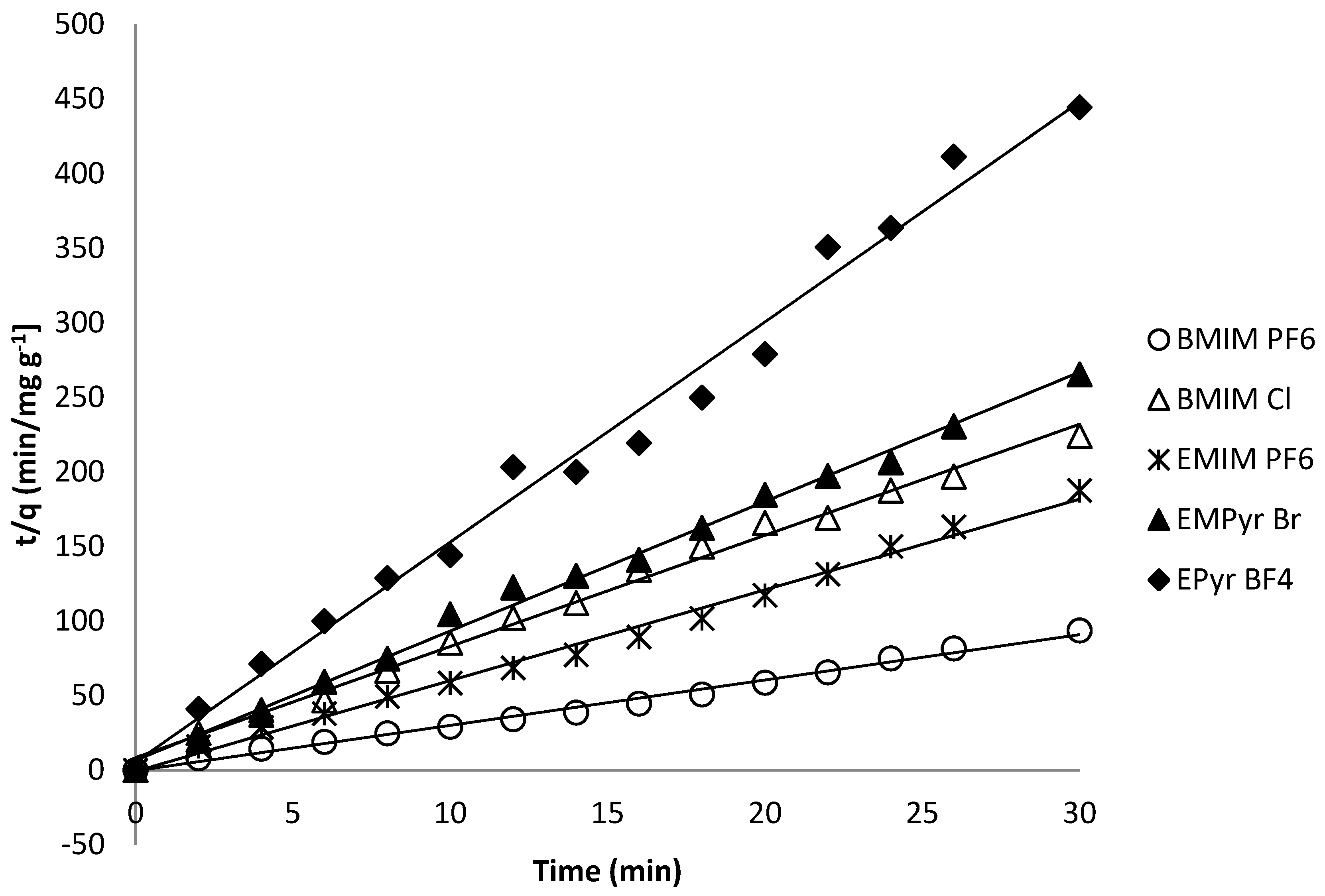
| Ionic Liquid | Slope | Intercept | R2 | qe | k2 | ∆q (%) 1 | er (%) 2 | qexp |
|---|---|---|---|---|---|---|---|---|
| BMIM PF6 | 2.1272 | 1.1717 | 0.9989 | 0.470 | 3.862 | 5.1 | 7.3 | 0.232 |
| BMIM Cl | 7.4047 | 7.3303 | 0.9950 | 0.135 | 7.479 | 7.0 | 9.9 | 0.121 |
| EMIM PF6 | 5.8594 | 1.1926 | 0.9905 | 0.170 | 28.788 | 6.2 | 9.3 | 0.160 |
| EMPyr Br | 8.7030 | 6.2926 | 0.9934 | 0.114 | 12.036 | 3.2 | 4.6 | 0.215 |
| EPyr BF4 | 15.0048 | 5.3759 | 0.9855 | 0.066 | 41.880 | 6.1 | 8.7 | 0.069 |
3. Materials and Methods
3.1. Reagents
3.2. Calibration Solutions
3.3. HPLC Quantification
3.4. Adsorption Experiments
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Lozinskaya, E.I.; Shaplov, A.S.; Kotseruba, M.V.; Komarova, L.I.; Lyssenko, K.A.; Antipin, M.Y.; Golovanov, D.G.; Vygodskii, Y.S. “One-pot” synthesis of aromatic Poly(1,3,4-oxadiazole)s in novel solvents—Ionic liquids. J. Polym. Sci. A1 2006, 44, 380–394. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Lozinskaya, E.I.; Odinets, I.L.; Lyssenko, K.A.; Kurtova, S.A.; Timofeeva, G.I.; Iojoiu, C.; Sanchez, J.Y.; Abadie, M.J.M.; Voytekunas, V.Y.; et al. Novel phosphonated Poly(1,3,4-oxadiazole)s: Synthesis in ionic liquid and characterization. React. Funct. Polym. 2008, 68, 208–224. [Google Scholar] [CrossRef]
- Matveeva, E.V.; Odinets, I.L.; Kozlov, V.A.; Shaplov, A.S.; Mastryukova, T.A. Ionic-liquid-promoted Michaelis-Arbuzov rearrangement. Tetrahedron Lett. 2006, 47, 7645–7648. [Google Scholar] [CrossRef]
- Vygodskii, Y.S.; Shaplov, A.S.; Lozinskaya, E.I.; Filippov, O.A.; Shubina, E.S.; Bandari, R.; Buchmeiser, M.R. Ring-opening metathesis polymerization (ROMP) in ionic liquids: Scope and limitations. Macromolecules 2006, 39, 7821–7830. [Google Scholar] [CrossRef]
- Adams, D.J.; Dyson, P.J.; Taverner, S.J. Chemistry in Alternative Reaction Media; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Bharate, J.B.; Bharate, S.B.; Vishwakarma, R.A. Metal-Free, Ionic Liquid-Mediated Synthesis of Functionalized Quinolines. ACS Comb. Sci. 2014, 16, 624–630. [Google Scholar]
- Cui, X.; Cai, J.; Zhang, Y.; Li, R.; Feng, T. Kinetics of Transesterification of Methyl Acetate and n-Butanol Catalyzed by Ionic Liquid. Ind. Eng. Chem. Res. 2011, 50, 11521–11527. [Google Scholar] [CrossRef]
- Gu, Y.; Shi, F.; Deng, Y. Esterification of Aliphatic Acids with Olefin Promoted by Brønsted Acidic Ionic Liquids. J. Mol. Catal. A Chem. 2004, 212, 71–75. [Google Scholar] [CrossRef]
- Xing, H.; Wang, T.; Zhou, Z.; Dai, Y. Novel Brønsted-Acidic Ionic Liquids for Esterifications. Ind. Eng. Chem. Res. 2005, 44, 4147–4150. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, H.; Han, M.; Wang, D.; Wang, J. Transesterification of Cottonseed Oil Catalyzed by Brønsted Acidic Ionic Liquids. Ind. Eng. Chem. Res. 2007, 46, 7955–7960. [Google Scholar] [CrossRef]
- Qiao, K.; Yokoyama, C. Nitration of Aromatic Compounds with Nitric Acid Catalyzed by Ionic Liquids. Chem. Lett. 2004, 33, 808–809. [Google Scholar] [CrossRef]
- Xin, H.; Wu, Q.; Han, M.; Wang, D.; Jin, Y. Alkylation of Benzene with 1-Dodecene in Ionic Liquids [Rmim]+Al2Cl6X– (R = butyl, octyl and dodecyl; X = chlorine, bromine and iodine). Appl. Catal. A 2005, 292, 354–361. [Google Scholar] [CrossRef]
- Yoo, K.; Burckle, E.C.; Smirniotis, P.G. Isobutane/2-Butene Alkylation Using Large-Pore Zeolites: Influence of Pore Structure on Activity and Selectivity. J. Catal. 2002, 211, 6–18. [Google Scholar] [CrossRef]
- Flieger, J.; el Blicharska, G.; Czajkowska-Zelazko, A. Ionic Liquids as Solvents in Separation Processes. Austin J. Anal. Pharm. Chem. 2014, 1, 1009. [Google Scholar]
- Flieger, J.; Czajkowska-Żelazko, A. Ionic Liquids in Separation Techniques in Ionic Liquids: Applications and Perspectives; Kokorin, A., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Han, D.; Row, K.H. Recent Applications of Ionic Liquids in Separation Technology. Molecules 2010, 15, 2405–2426. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Yuan, W.; Sheng, C.; Zhao, H.; Hu, H.; Baker, G.L. Optimizing the electrochemical performance of imidazolium-based polymeric ionic liquids by varying tethering groups. J. Polym. Sci. A1 2015, 53, 1339–1350. [Google Scholar] [CrossRef]
- Hu, H.; Yuan, W.; Jia, Z.; Baker, G.L. Ionic liquid-based random copolymers: A new type of polymer electrolyte with low glass transition temperature. RSC Adv. 2015, 5, 3135–3140. [Google Scholar] [CrossRef]
- Hu, H.; Yuan, W.; Lu, L.; Zhao, H.; Jia, Z.; Baker, G.L. Low glass transition temperature polymer electrolyte prepared from ionic liquid grafted polyethylene oxide. J. Polym. Sci. A1 2014, 52, 2104–2110. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Ponkratov, D.; Vlasov, P.; Lozinskaya, E.I.; Gumileva, L.V.; Surcin, C.; Morcrette, M.; Armand, M.; Aubert, P.H.; Vidal, F.; et al. Ionic semi-interpenetrating networks as new approach for highly conductive and stretchable polymer materials. J. Mater. Chem. A 2015, 3, 2188–2198. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Marcilla, R.; Mecerreyes, D. Recent Advances in Innovative Polymer Electrolytes based on Poly(ionic liquid)s. Electrochim. Acta 2015, 175, 18–34. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Ponkratov, D.O.; Aubert, P.; Lozinskaya, E.I.; Plesse, C.; Vidal, F.; Vygodskii, Y.S. A first truly all-solid state organic electrochromic device based on polymeric ionic liquids. Chem. Commun. 2014, 50, 3191–3193. [Google Scholar] [CrossRef] [PubMed]
- Shaplov, A.S.; Ponkratov, D.O.; Vlasov, P.S.; Lozinskaya, E.I.; Malyshkina, I.A.; Vidal, F.; Aubert, P.H.; Armand, M.; Vygodskii, Y.S. Solid-state electrolytes based on ionic network polymers. Polym. Sci. Ser. B 2014, 56, 164–177. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Ponkratov, D.O.; Aubert, P.; Lozinskaya, E.I.; Plesse, C.; Maziz, A.; Vlasov, P.S.; Vidal, F.; Vygodskii, Y.S. Truly solid state electrochromic devices constructed from polymeric ionic liquids as solid electrolytes and electrodes formulated by vapor phase polymerization of 3,4-ethylenedioxythiophene. Polymer 2014, 55, 3385–3396. [Google Scholar] [CrossRef]
- Vygodskii, Ya.S.; Mel’nik, O.A.; Shaplov, A.S.; Lozinskaya, E.I.; Malyshkina, I.A.; Gavrilova, N.D. Synthesis and ionic conductivity of polymer ionic liquids. Polym. Sci. Ser. A 2007, 49, 256–261. [Google Scholar] [CrossRef]
- Dadfarnia, S.; Shabani, A.M.; Bidabadi, M.S.; Jfari, A.A. A novel ionic liquid/micro-volume back extraction procedure combined with flame atomic absorption spectrometry for determination of trace nickel in sample of nutritional interest. J. Hazard. Mater. 2010, 173, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Siwek, A.; Pizoń, M.; Czajkowska-Żelazko, A. Ionic liquids as surfactants in micellar liquid chromatography. J. Sep. Sci. 2013, 36, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, S.; Salim, P.; Kragl, U. Driving forces of protein partitioning in an ionic liquid-based aqueous two-phase system. Biochem. Eng. J. 2009, 46, 176–185. [Google Scholar] [CrossRef]
- Flieger, J.; Czajkowska-Żelazko, A. Aqueous two phase system based on ionic liquid for isolation of quinine from human plasma sample. Food Chem. 2015, 166, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Jiang, G.B.; Chi, Y.G.; Cai, Y.Q.; Zhou, Q.X.; Hu, J.T. Use of ionic liquids for liquid-phase microextraction of polycyclic aromatic hydrocarbons. Anal. Chem. 2003, 75, 5870–5876. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.G.; Neves, C.M.S.S.; Marrucho, I.M.; Lopes, J.N.C.; Rebelo, L.P.N.; Coutinho, J.A.P. High-performance extraction of alkaloids using aqueous two- phase systems with ionic liquids. Green Chem. 2010, 12, 1715–1718. [Google Scholar] [CrossRef]
- Berton, P.; Monasterio, R.P.; Wuilloud, R.G. Selective extraction and determination of vitamin B12 in urine by ionic liquid-based aqueous two-phase system prior to high-performance liquid chromatography. Talanta 2012, 97, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, Y.A.; Xie, X.Q.; Li, C.X. Extraction of trace acetylspiramycin in real aqueous environments using aqueous two-phase system of ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate and phosphate. Cent. Eur. J. Chem. 2010, 8, 1185–1191. [Google Scholar] [CrossRef]
- Li, C.X.; Han, J.; Wang, Y.; Yan, Y.S.; Xu, X.H.; Pan, J.M. Extraction and mechanism investigation of trace roxithromycin in real water samples by use of ionic liquid-salt aqueous two-phase system. Anal. Chim. Acta 2009, 653, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Lui, Q.; Xuesheng, H.; Wang, Y.; Yang, P.; Xia, H.; Yu, J.; Liu, H. Extraction of penicillin G by aqueous two-phase system of [Bmim]BF4/NaH2PO4. Chin. Sci. Bull. 2005, 50, 1582–1585. [Google Scholar]
- Fontanals, N.; Pocurull, E.; Marcé, R.M.; Borrull, F. Handbook of Ionic Liquids. Properties, Applications and Hazards; Mun, J., Sim, H., Eds.; Nova Publisher: New York, NY, USA, 2012; p. 493. [Google Scholar]
- Vidal, M.-L.; Riekkola, A.; Canals, A. Ionic liquid-modified materials for solid-phase extraction and separation. Anal. Chim. Acta 2012, 715, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, N.; Jiang, G.; Liu, J.; Jönsson, J.Å.; Wen, M. Disposable ionic liquid coating for headspace solid-phase microextraction of benzene, toluene, ethylbenzene, and xylenes in paints followed by gas chromatography-flame ionization detection. J. Chromatogr. A 2005, 1066, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Meng, Y.; Anderson, J.L. Polymeric ionic liquids as selective coatings for the extraction of esters using solid-phase microextraction. J. Chromatogr. A 2008, 1208, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Peng, L. Ionic liquid-modified silica as sorbent for preconcentration of cadmium prior to its determination by flame atomic absorption spectrometry in water samples. Talanta 2010, 81, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Stepnowski, P.; Muller, A.; Behrend, P.; Ranke, J.; Hoffmann, J.; Jastorff, B. Reverse phase liquid chromatographic method for the determination of selected room temperature ionic liquids cations. J. Chromatogr. A 2003, 993, 173–178. [Google Scholar] [CrossRef]
- Kowalska, S.; Buszewski, B.; Stepnowski, P. The influence of stationary phase properties on ionic liquid cations separation in RP-HPLC. J. Sep. Sci. 2006, 29, 1116–1125. [Google Scholar]
- Flieger, J.; Czajkowska-Żelazko, A. Identification of ionic liquid components by RP-HPLC with diode array detector using chaotropic effect and perturbation technique. J. Sep. Sci. 2012, 35, 248–255. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhong, M.; Adzima, B.; Luebke, D.; Nulwala, H.; Matyjaszewski, K. A Simple and Universal Gel Permeation Chromatography Technique for Precise Molecular Weight Characterization of Well-Defined Poly(ionic liquid)s. J. Am. Chem. Soc. 2013, 135, 4227–4230. [Google Scholar] [CrossRef] [PubMed]
- Validation of Analytical Procedures: Text and Methodology. International Conference on Harmonization: Geneva, Switzerland, 2005.
- Marcus, Y. Thermodynamics of Solvation of Ions. J. Chem. Soc. Faraday Trans. 1991, 87, 2995–2999. [Google Scholar] [CrossRef]
- Ho, Y.-S.; Wase, D.; Forster, C. Kinetic studies of competitive heavy metal adsorption by sphagnum moss peat. Environ. Technol. 1996, 17, 71–77. [Google Scholar] [CrossRef]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Sample Availability: not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flieger, J.; Tatarczak-Michalewska, M.; Groszek, A.; Blicharska, E.; Kocjan, R. Adsorption Kinetics at Silica Gel/Ionic Liquid Solution Interface. Molecules 2015, 20, 22058-22068. https://doi.org/10.3390/molecules201219833
Flieger J, Tatarczak-Michalewska M, Groszek A, Blicharska E, Kocjan R. Adsorption Kinetics at Silica Gel/Ionic Liquid Solution Interface. Molecules. 2015; 20(12):22058-22068. https://doi.org/10.3390/molecules201219833
Chicago/Turabian StyleFlieger, Jolanta, Małgorzata Tatarczak-Michalewska, Anna Groszek, Eliza Blicharska, and Ryszard Kocjan. 2015. "Adsorption Kinetics at Silica Gel/Ionic Liquid Solution Interface" Molecules 20, no. 12: 22058-22068. https://doi.org/10.3390/molecules201219833