Eco-Friendly Synthesis of Some Thiosemicarbazones and Their Applications as Intermediates for 5-Arylazothiazole Disperse Dyes
Abstract
:1. Introduction
2. Results and Discussion
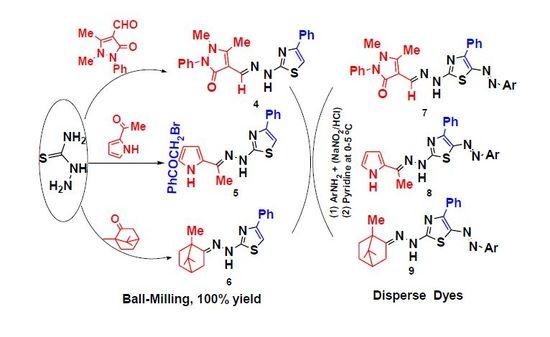
2.1. Chemistry
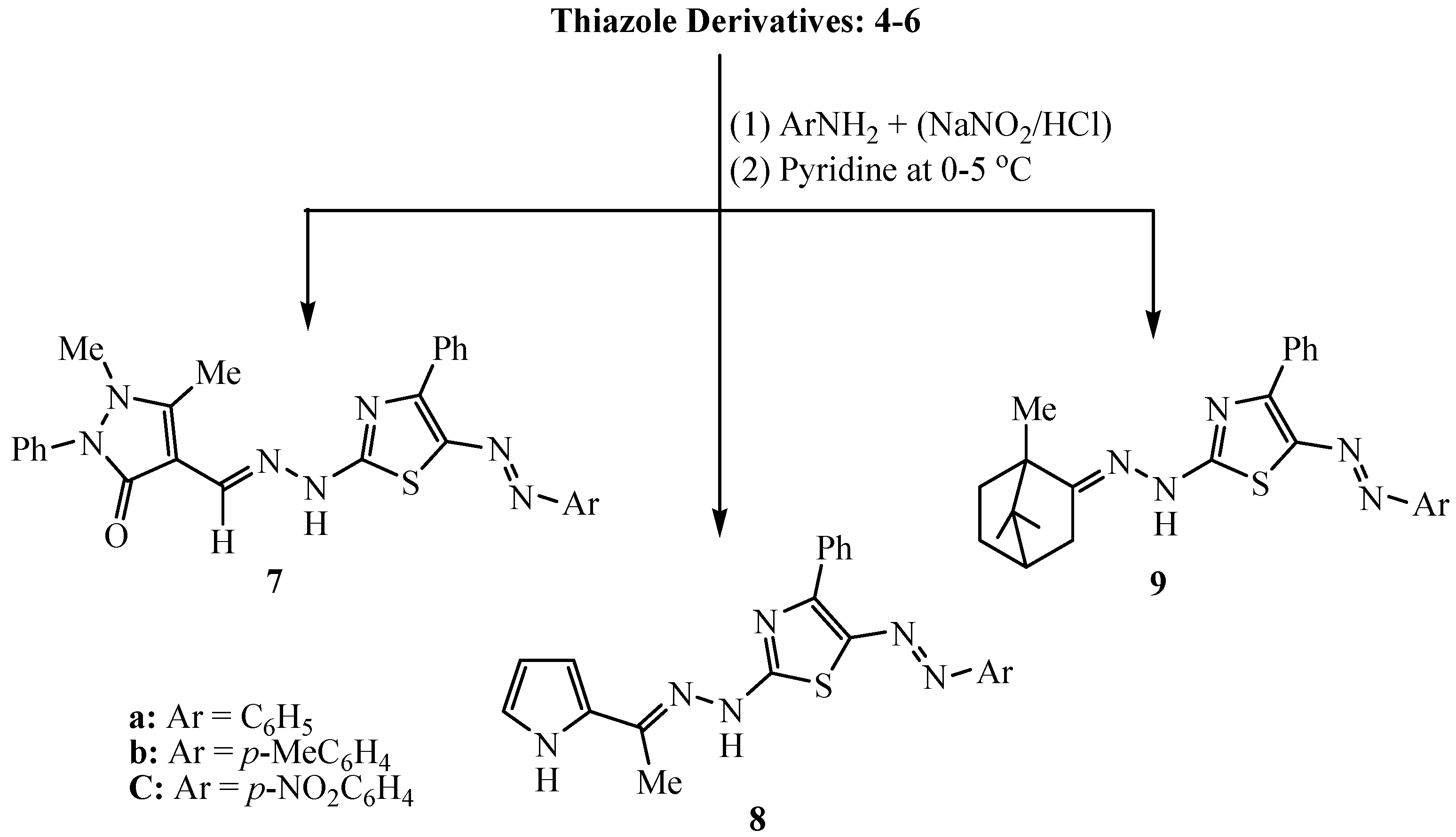
2.2. Application
2.2.1. Dyeing and Fastness Properties
| Dye | Washing | Perspiration | Rubbing | Sublimation | Light (60 h) | |||
|---|---|---|---|---|---|---|---|---|
| Acid | Alkali | Dry | Wet | Staining at 180 °C | Staining at 210 °C | |||
| 7a | 4–5 | 4 | 4–5 | 4–5 | 4–5 | 3–4 | 3 | 5–6 |
| 7b | 4–5 | 4–5 | 4–5 | 3 | 3 | 4 | 4 | 3–4 |
| 7c | 4–5 | 4 | 4–5 | 3–4 | 4–5 | 4 | 4 | 6 |
| 8a | 4–5 | 4 | 4 | 3–4 | 4 | 3–4 | 3 | 6 |
| 8b | 4–5 | 4–5 | 4 | 4 | 4 | 3–4 | 4 | 4 |
| 8c | 4–5 | 4–5 | 4–5 | 3 | 3 | 4 | 3–4 | 5–6 |
| 9a | 4–5 | 4 | 4 | 3 | 3 | 4 | 4 | 5–6 |
| 9b | 4–5 | 4–5 | 4 | 4 | 4–5 | 4 | 3–4 | 4 |
| 9c | 4–5 | 4–5 | 4–5 | 4 | 4–5 | 4 | 4 | 6 |
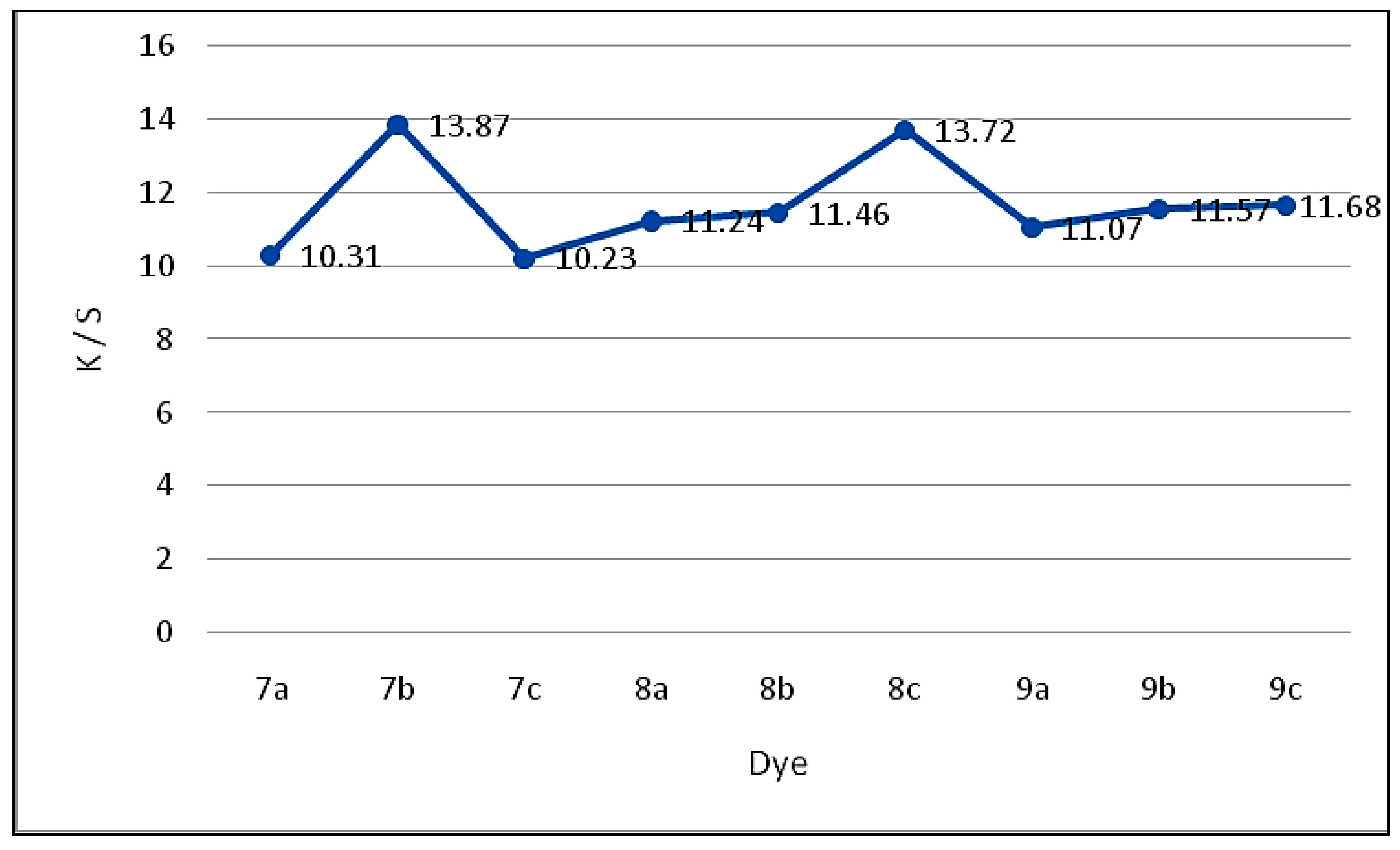
2.2.2. Color Assessment
| Dye | Exhaustion (%) | Molar Absorbitivity ɛ | Absorption λmax/nm | K/S | L* | a* | b* | C* | H |
|---|---|---|---|---|---|---|---|---|---|
| 7a | 61 | 1.42 × 105 | 451 | 10.31 | 81.07 | 3.34 | 20.41 | 51.03 | 67.49 |
| 7b | 64 | 1.18 × 105 | 417 | 13.87 | 40.99 | 3.32 | 41.81 | 44.88 | 66.24 |
| 7c | 61 | 1.89 × 104 | 447 | 10.23 | 74.23 | −1.23 | 37.20 | 55.34 | 80.62 |
| 8a | 62 | 1.17 × 105 | 453 | 11.24 | 89.36 | 5.35 | 3.14 | 53.62 | 76.96 |
| 8b | 62 | 1.22 × 105 | 444 | 11.46 | 72.91 | 5.22 | 47.57 | 48.46 | 82.52 |
| 8c | 65 | 2.11 × 105 | 452 | 13.72 | 73.22 | 0.37 | 23.11 | 47.56 | 84.88 |
| 9a | 62 | 1.08 × 105 | 464 | 11.07 | 65.51 | −1.44 | 30.77 | 44.39 | 80.63 |
| 9b | 63 | 1.17 × 105 | 451 | 11.57 | 80.93 | −1.05 | 14.02 | 51.74 | 74.65 |
| 9c | 65 | 1.41 × 105 | 443 | 11.68 | 80.34 | −0.56 | 16.23 | 55.23 | 66.09 |
3. Experimental Section
3.1. Materials and Methods
3.1.1. Chemicals and Reagents
3.1.2. Instrumentation
3.2. Synthesis
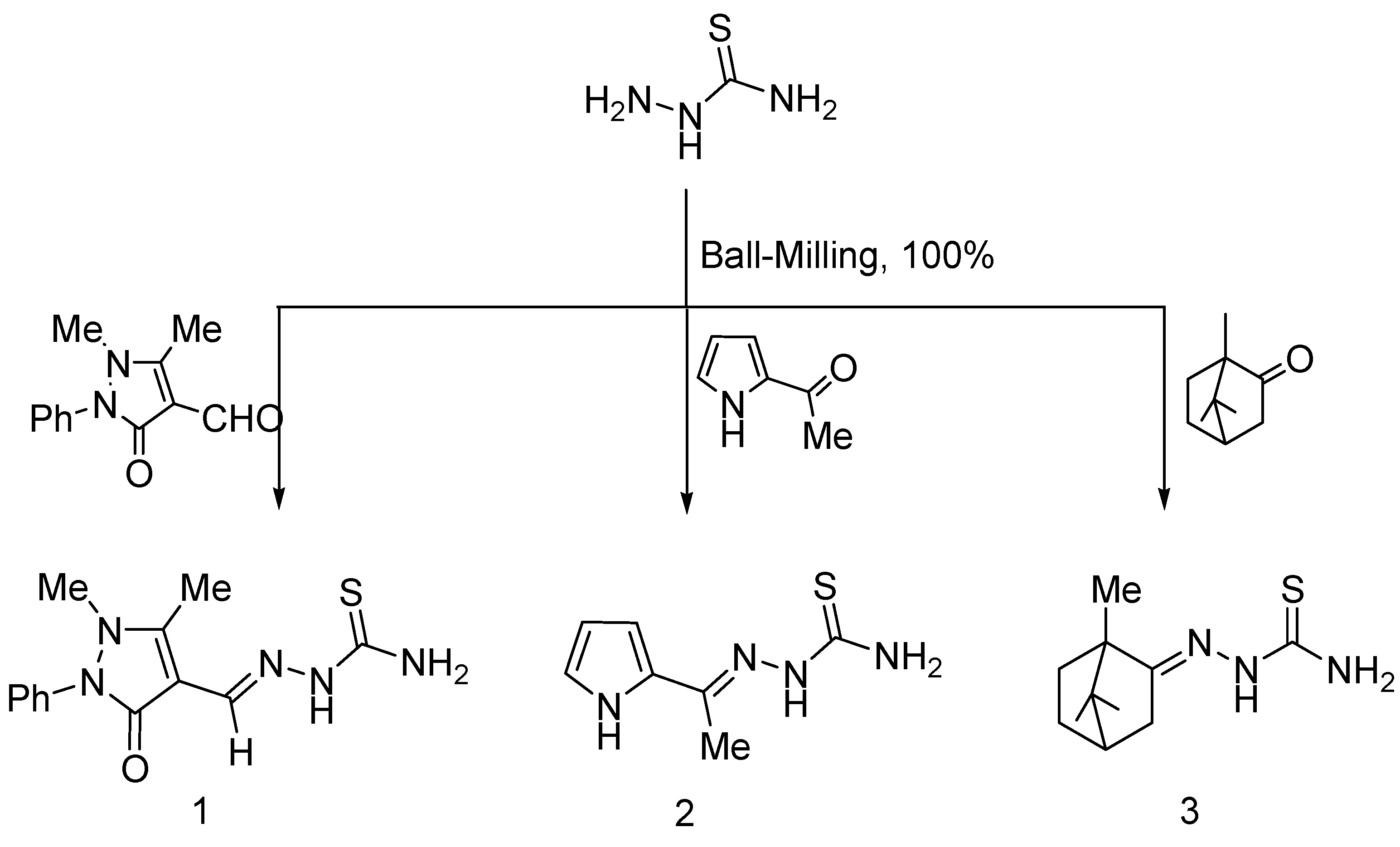
3.2.1. Solid-State Synthesis of Thiosemicarbazones 1–3
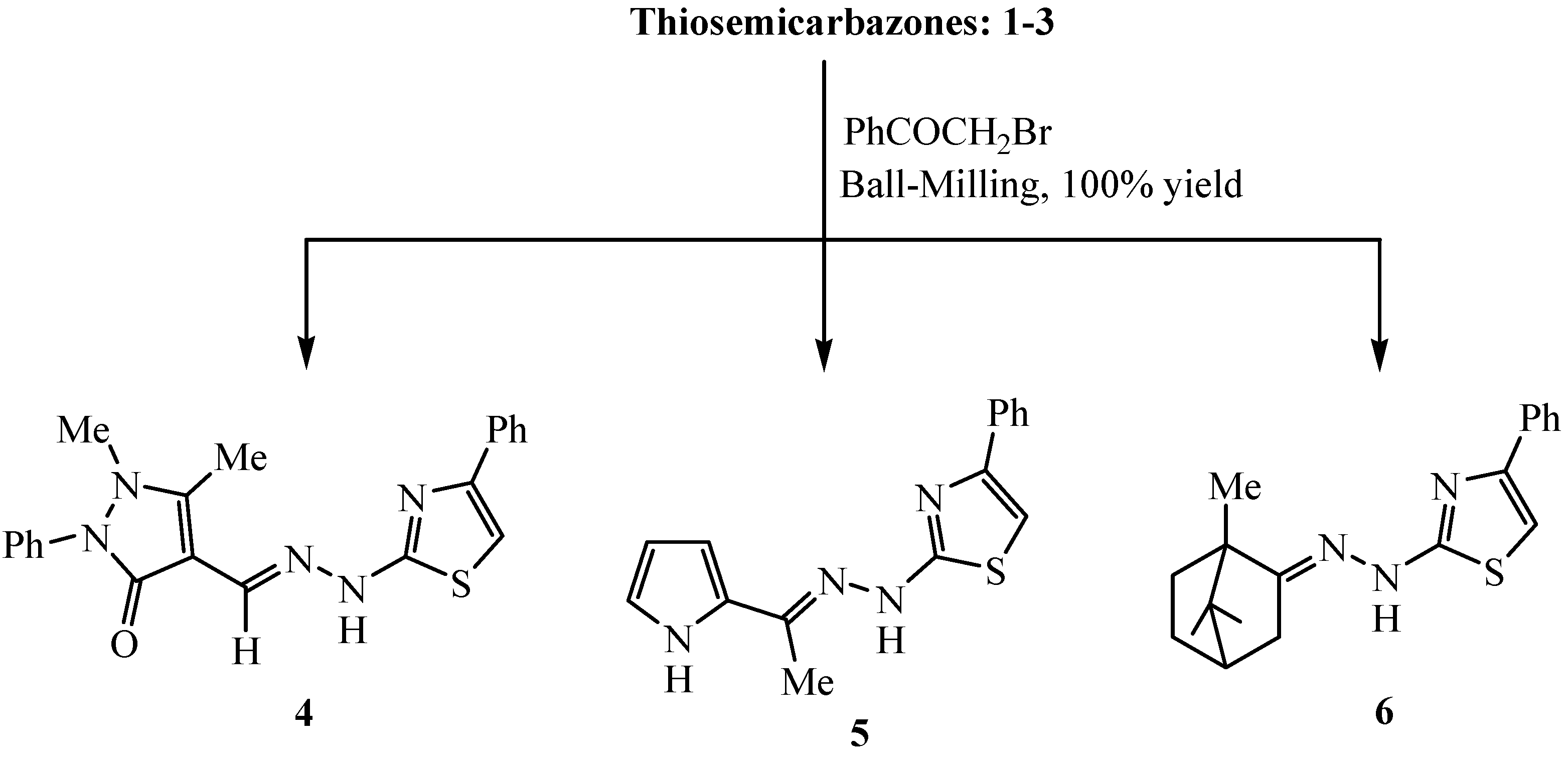
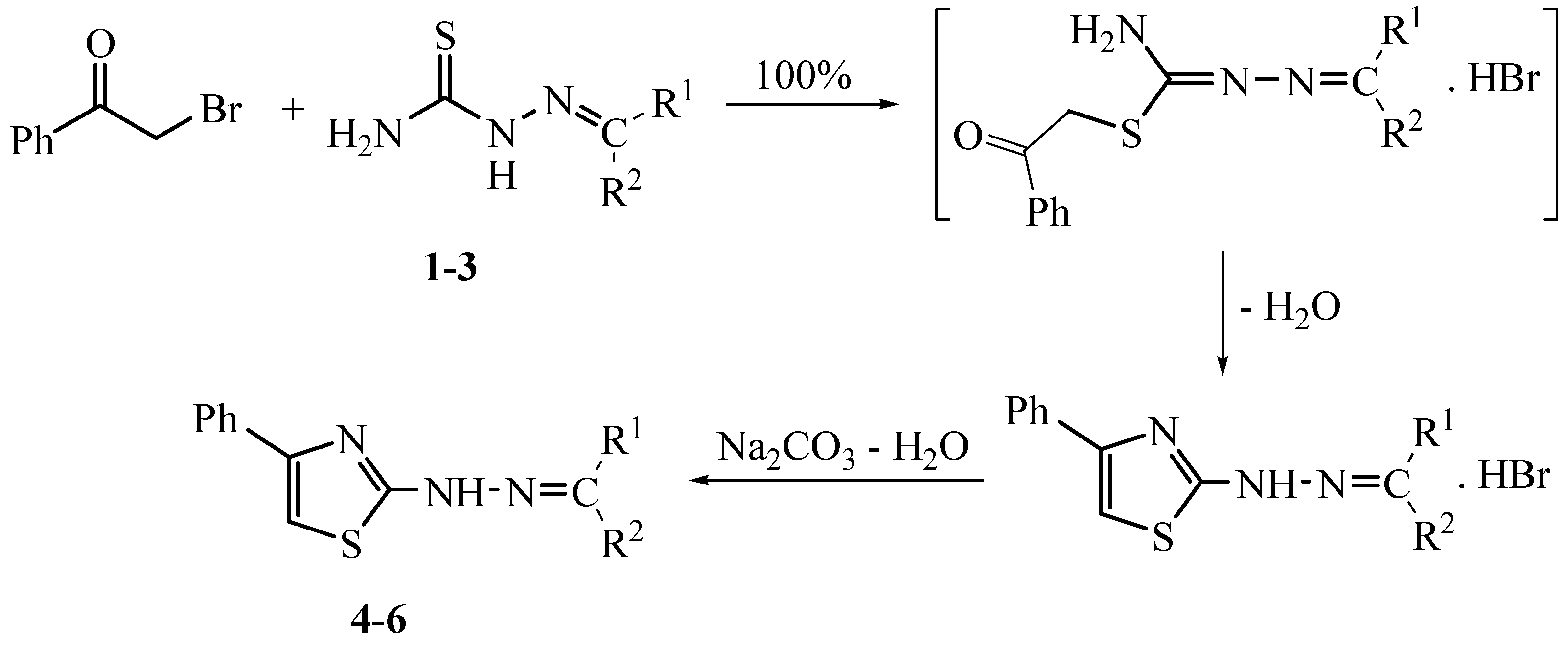
3.2.2. Solid-State Synthesis of Thiazole Derivatives 4–6
3.2.3. Synthesis of 5-Arylazo-2-(arylidenehydrazino)-4-phenyl-thiazole Dyes 7–9
3.3. Dyebath Preparation and Dyeing Procedure
3.4. Dyeing Characteristics on Polyester Fabrics
3.4.1. Color Fastness Tests
3.4.2. Color Properties of the Dyes on Polyester
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tanaka, K. Solvent-Free Organic Synthesis, 2nd ed.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2009. [Google Scholar]
- Kaupp, G. Organic solid-state reactions with 100% yield. In Organic Solid State Reactions; Toda, F., Ed.; Springer: Heidelberg, Germany, 2005; Volume 254, pp. 95–183. [Google Scholar]
- Beraldo, H.; Gambinob, D. The wide pharmacological versatility of semicarbazones, thiosemicarbazones and their metal complexes. Mini Rev. Med. Chem. 2004, 4, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Altun, A.; Kumru, M.; Dimoglo, A. Study of electronic and structural features of thiosemicarbazone and thiosemicarbazide derivatives demonstrating anti-HSV-1 activity. J. Mol. Struct. 2001, 535, 235–246. [Google Scholar] [CrossRef]
- Cory, J.G.; Cory, A.H.; Rappa, G.; Lorico, A.; Mao-Chin, L.; Tai-Shun, L.; Sartorelli, A.C. Inhibitors of ribonucleotide reductase: Comparative effects of amino- and hydroxy-substituted pyridine-2-carboxaldehydethiosemicarbazones. Biochem. Pharmacol. 1994, 48, 335–344. [Google Scholar] [CrossRef]
- Liu, M.C.; Lin, T-S.; Cory, J.G.; Cory, A.H.; Sartorelli, A.C. Synthesis and biological activity of 3- and 5-amino derivatives of pyridine-2-carboxaldehyde thiosemicarbazone. J. Med. Chem. 1996, 39, 2586–2593. [Google Scholar] [CrossRef] [PubMed]
- Finch, R.A.; Liu, M.-C.; Grill, S.P.; Rose, W.C.; Loomis, R.; Vasquez, K.M.; Cheng, Y.-C.; Sartorelli, A.C. Triapine (3-aminopyridine-2-carboxaldehyde-thiosemicarbazone): A potent inhibitor of ribonucleotide reductase activity with broad spectrum antitumor activity. Biochem. Pharmacol. 2000, 59, 983–991. [Google Scholar] [CrossRef]
- Borges, R.H.; Paniago, E.; Beraldo, H. Equilibrium and kinetic studies of iron(II) and iron(III) complexes of some α(N)-heterocyclic thiosemicarbazones. Reduction of the iron(III) complexes of 2-formylpyridine thiosemicarbazone and 2-acetylpyridine thiosemicarbazone by cellular thiol-like reducing agents. J. Inorg. Biochem. 1997, 65, 267–275. [Google Scholar]
- Rebolledo, A.P.; de Lima, G.M.; Gambi, L.N.; Speziali, N.L.; Maia, D.F.; Pinheiro, C.B.; Ardisson, J.D.; Cortés, M.E.; Beraldo, H. Tin(IV) complexes of 2-benzoylpyridine N(4)-phenyl-thiosemicarbazone: Spectral characterization, structural studies and antifungal activity. Appl. Organomet. Chem. 2003, 17, 945–951. [Google Scholar] [CrossRef]
- El-Sawaf, A.K.; West, D.X.; El-Saied, F.A.; El-Bahnasawy, R.M. Iron(III), Cobalt(II), Nickel(II), Copper(II) and Zinc(II) complexes of 4-formylantipyrine thiosemicarbazone. Syn. React. Inorg. Met. 1997, 27, 1127–1147. [Google Scholar] [CrossRef]
- Alonso, R.; Bermejo, E.; Carballo, R.; Castiñeiras, A.; Pérez, T. The supramolecular chemistry of thiosemicarbazones derived from pyrrole: A structural view. J. Mol. Struct. 2002, 606, 155–173. [Google Scholar] [CrossRef]
- Brousse, B.N.; Moglioni, A.G.; Alho, M.M.; Álvarez-Larena, Á.; Moltrasio, G.Y.; DAccorso, N.B. Behavior of thiosemicarbazones derived from some terpenones under acetylating conditions. Arkivoc 2002, 10, 14–23. [Google Scholar]
- Kaupp, G.; Metwally, M.A.; Amer, F.A.; Abdel-latif, E. Quantitative Gas-solid diazotization of 3-aminopyrazolo[3,4-b]pyridine derivatives and azo dye syntheses by means of solid-solid reactions. Eur. J. Org. Chem. 2003, 8, 1545–1551. [Google Scholar] [CrossRef]
- Abdel-Latif, E.; Kaupp, G.; Metwally, M.A. Brand new quantitative solid-state synthesis of N-pyrazolylazomethines. J. Chem. Res. 2005, 3, 187–189. [Google Scholar] [CrossRef]
- Abdel-Latif, E.; Metwally, M.A. Waste-Free Solid-state organic syntheses: Solvent-free alkylation, heterocyclization, and azo-coupling reactions. Monatsh. Chem. Chem. Mon. 2007, 138, 771–776. [Google Scholar] [CrossRef]
- Bondock, S.; El-Azap, H.; Kandeel, E.-E.M.; Metwally, M.A. Eco-friendly solvent-free synthesis of thiazolylpyrazolederivatives. Monatsh. Chem. Chem. Mon. 2008, 139, 1329–1335. [Google Scholar] [CrossRef]
- Mustafa, S.M.; Nair, V.A.; Chittoor, J.P.; Krishnapillai, S. Synthesis of 1,2,4-triazoles and thiazoles from thiosemicarbazide and its derivatives. Mini Rev. Org. Chem. 2004, 1, 375–385. [Google Scholar] [CrossRef]
- Kaupp, G. Solid-state molecular syntheses: complete reactions without auxiliaries based on the new solid-state mechanism. Cryst. Eng. Comm. 2003, 5, 117–133. [Google Scholar] [CrossRef]
- Ferguson, A.D.; Taylor, T.P. Standard methods for the determination of the colour fastness of textiles and leather. J. Soc. Dyers Colour. 1980, 96, 18–23. [Google Scholar] [CrossRef]
- Al-Etaibi, A.M.; El-Apasery, M.A.; Ibrahim, M.R.; Al-Awadi, N.A. A facile synthesis of new monoazo disperse dyes derived from 4-hydroxyphenylazopyrazole-5-amines: Evaluation of microwave assisted dyeing behavior. Molecules 2012, 17, 13891–13909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chipalkatti, H.R.; Desai, N.F.; Giles, C.H.; Macaulay, N. The influence of the substrate upon the light fading of azo dyes. J. Soc. Dyers Colour. 1954, 70, 487–501. [Google Scholar] [CrossRef]
- Műller, C. Recent developments in the chemistry of disperse dyes and their intermediate. Am. Dyest. Rep. 1970, 59, 37–44. [Google Scholar]
- Morita, M. Synthesis and antibacterial activity of antipyrine derivatives. IV thiazoles. Yakugaku Zasshi 1962, 82, 57–64. [Google Scholar] [PubMed]
- Sample Availability: Samples of the compounds (7–9 series) are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaffer, H.E.; Khalifa, M.E. Eco-Friendly Synthesis of Some Thiosemicarbazones and Their Applications as Intermediates for 5-Arylazothiazole Disperse Dyes. Molecules 2015, 20, 21982-21991. https://doi.org/10.3390/molecules201219820
Gaffer HE, Khalifa ME. Eco-Friendly Synthesis of Some Thiosemicarbazones and Their Applications as Intermediates for 5-Arylazothiazole Disperse Dyes. Molecules. 2015; 20(12):21982-21991. https://doi.org/10.3390/molecules201219820
Chicago/Turabian StyleGaffer, Hatem E., and Mohamed E. Khalifa. 2015. "Eco-Friendly Synthesis of Some Thiosemicarbazones and Their Applications as Intermediates for 5-Arylazothiazole Disperse Dyes" Molecules 20, no. 12: 21982-21991. https://doi.org/10.3390/molecules201219820