Utility of 3-Acetyl-6-bromo-2H-chromen-2-one for the Synthesis of New Heterocycles as Potential Antiproliferative Agents
Abstract
:1. Introduction
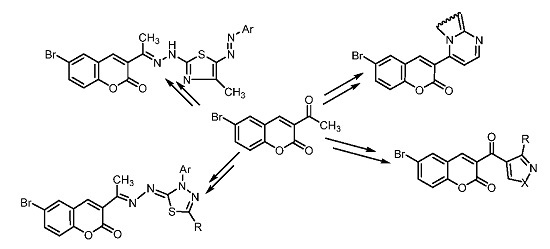
2. Results and Discussion
2.1. Chemistry
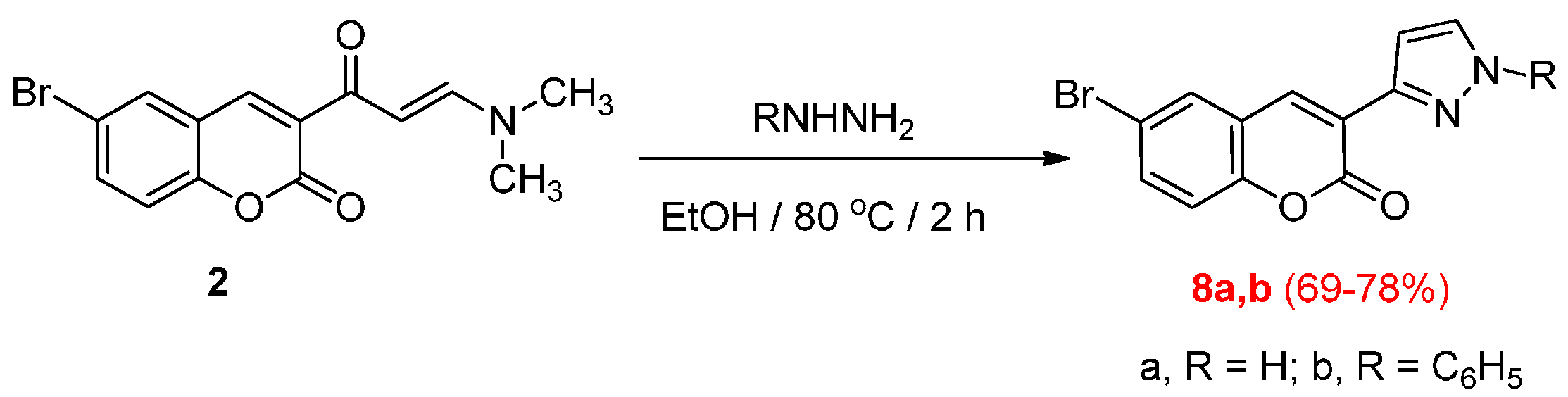
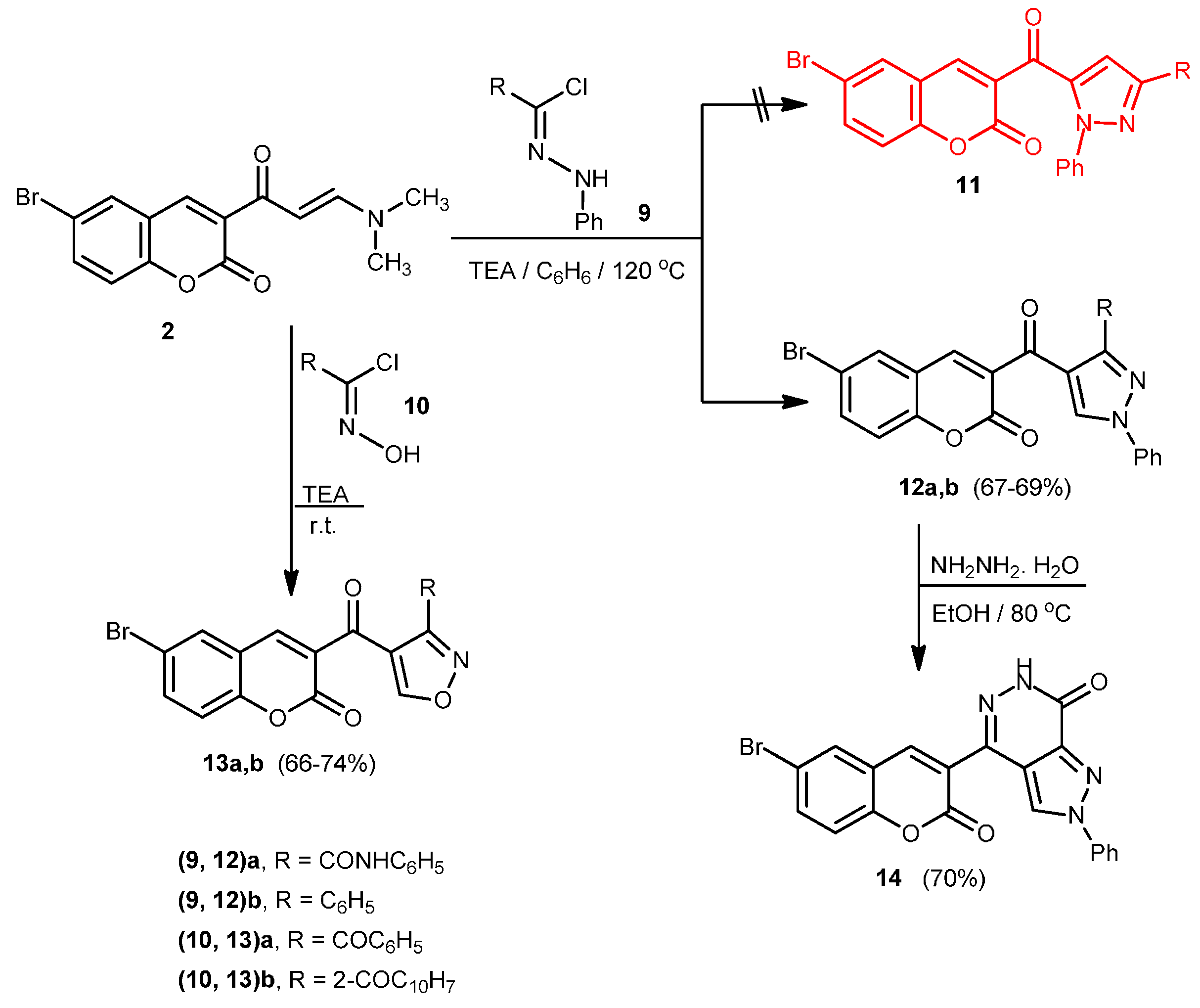
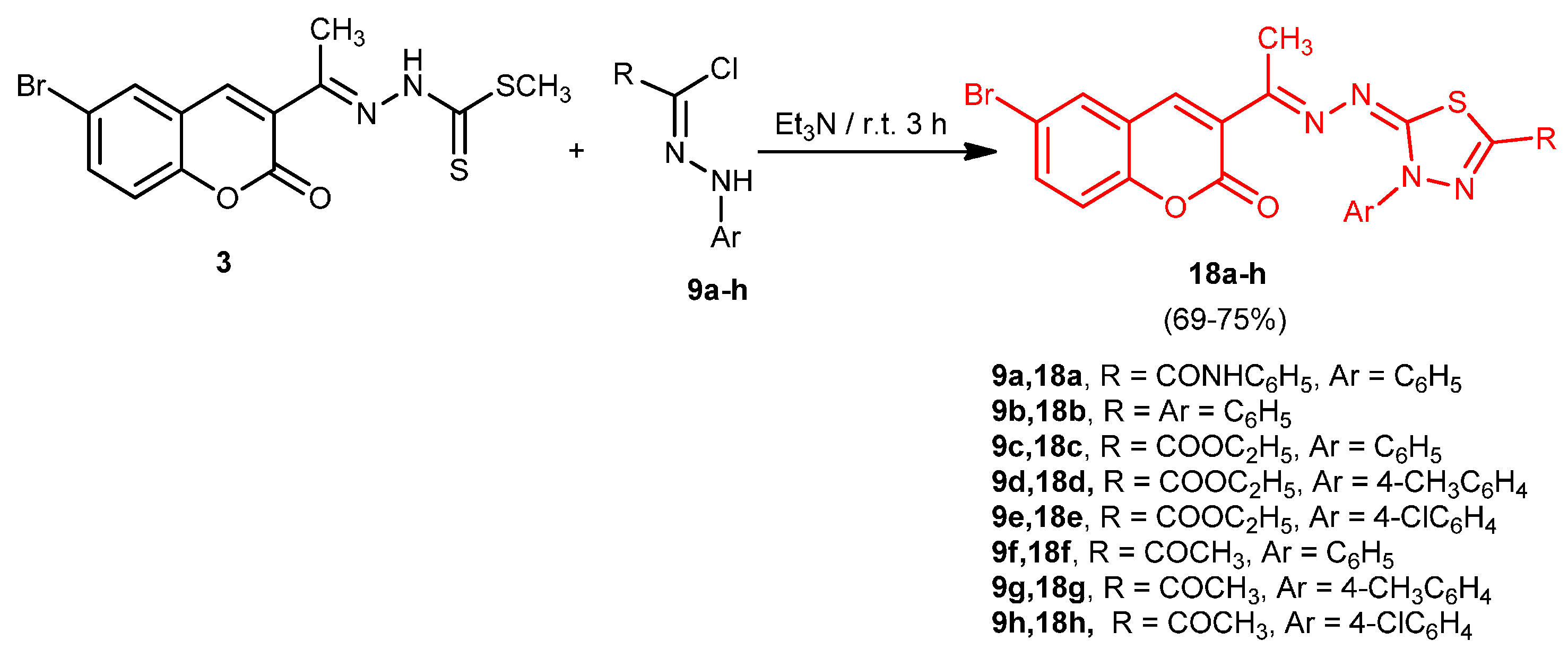
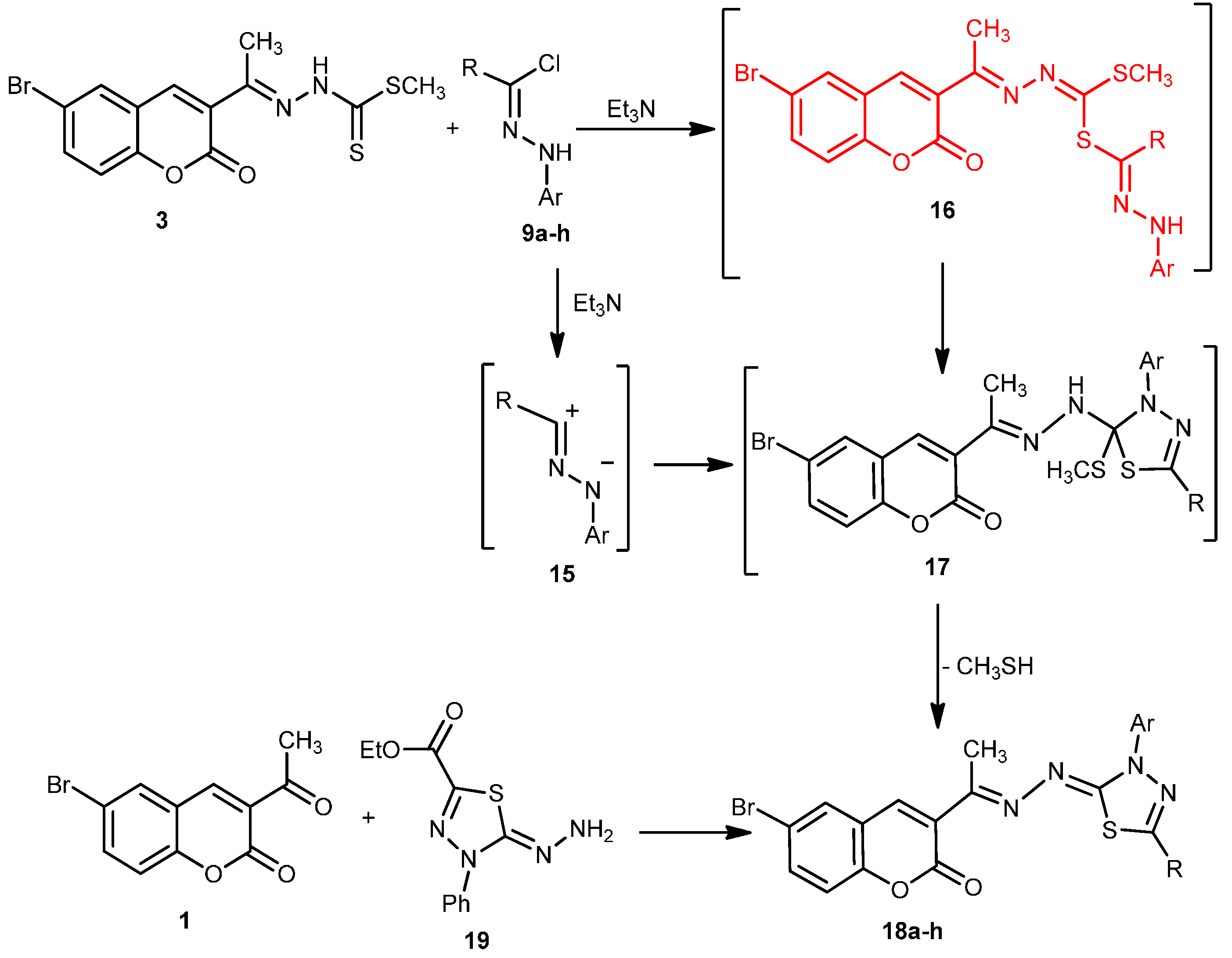
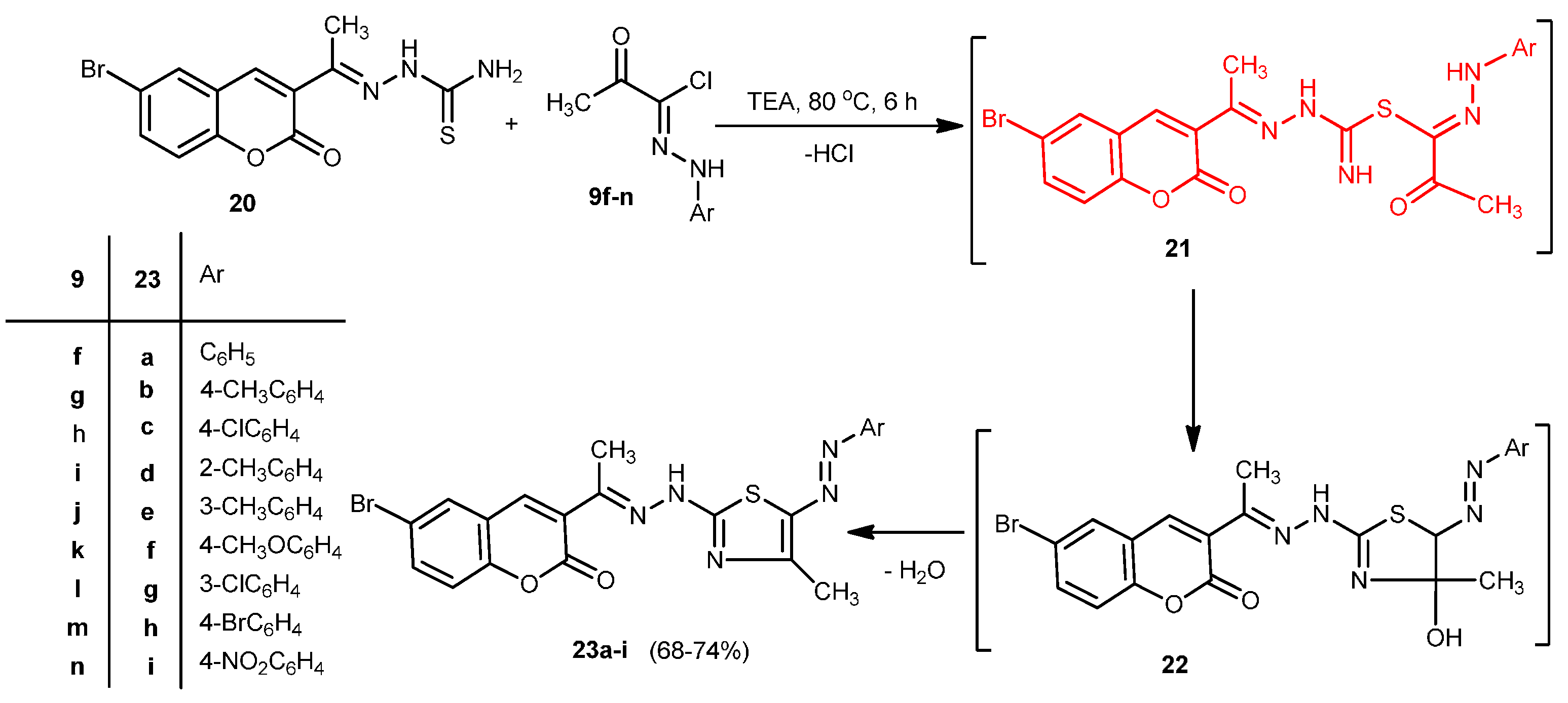
2.2. Antitumor Activity: Cytotoxicity against a Human Liver Carcinoma Cell Line (HEPG2-1)
| Compound No. | IC50 (µM) | Compound No. | IC50 (µM) |
|---|---|---|---|
| Doxorubicin | 1.40 ± 0.26 | 12b | 17.1 ± 2.28 |
| 7a | 14.2 ± 1.43 | 13a | 15.3 ± 1.69 |
| 7b | 10.0 ± 0.97 | 18c | 17.4 ± 1.03 |
| 7c | 2.70 ± 0.28 | 18f | 13.0 ± 1.20 |
| 7d | 14.6 ± 0.59 | 18a | 4.90 ± 0.69 |
| 7e | 12.8 ± 0.85 | 23c | 9.10 ± 1.29 |
| 8a | 9.80 ± 1.36 | 23d | 15.5 ± 1.49 |
| 12a | 8.20 ± 1.54 | 23g | 3.50 ± 0.23 |
- -
- Among the fused pyrimidine derivatives, pyrazolo[1,5-a]pyrimidine 7c is the most active one (IC50 = 2.70 ± 0.28 µM).
- -
- Pyrazole derivative 12a (substituted with CONHPh group at position 3) has in vitro inhibitory activity more than pyrazole derivative 12b (substituted with Ph group at position 3)
- -
- For 1,3,4-thiadiazole derivatives 18a, 18c and 18f, compound 18a (IC50 = 4.90 ± 0.69 µM) (with a chlorine atom as electron-withdrawing group on the aryl moiety) has promising antitumor activity while the other 1,3,4-thiadiazole derivatives 18c and 18f have moderate activities (IC50 = 17.4 ± 1.03 and 13.0 ± 1.20 µM, respectively).
- -
- Among thiazole derivatives 23c, 23d and 23g, compound 23g (IC50 = 3.50 ± 0.23 µM) (with a chlorine atom as electron-withdrawing group on the aryl moiety) has promising antitumor activity, while the other thiazole derivatives 23c and 23d have moderate activities (IC50 = 9.10 ± 1.29 and 15.5 ± 1.49 µM, respectively).
3. Experimental Protocols
3.1. General Information
3.2. Chemistry
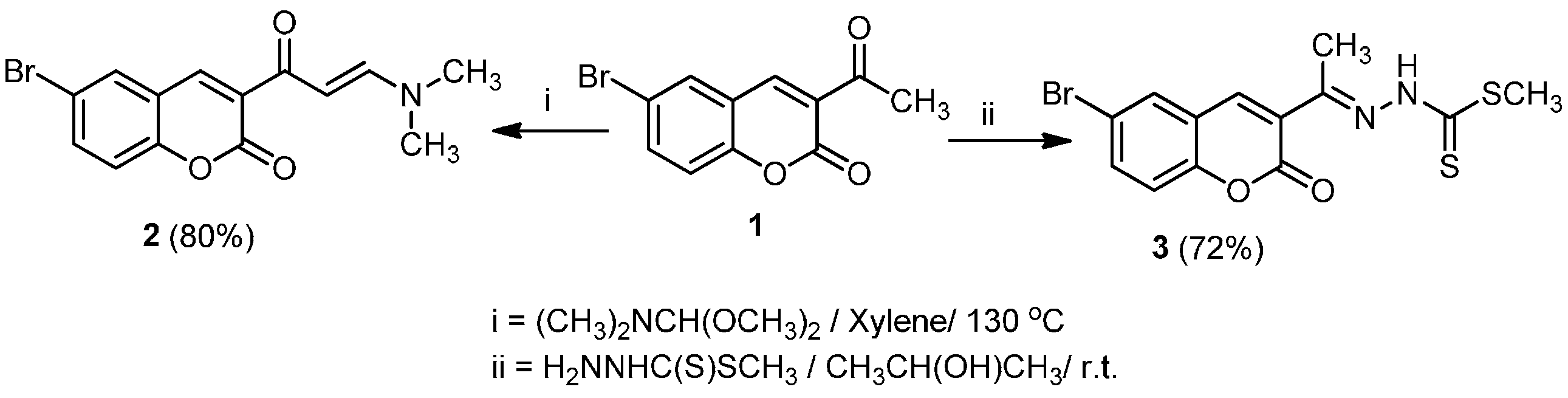
3.2.1. Synthesis of 6-Bromo-3-(3-(dimethylamino)acryloyl)-2H-chromen-2-one (2)
3.2.2. Synthesis of Methyl 2-(1-(6-Bromo-2-oxo-2H-chromen-3-yl)ethylidene)hydrazine carbodithioate (3)
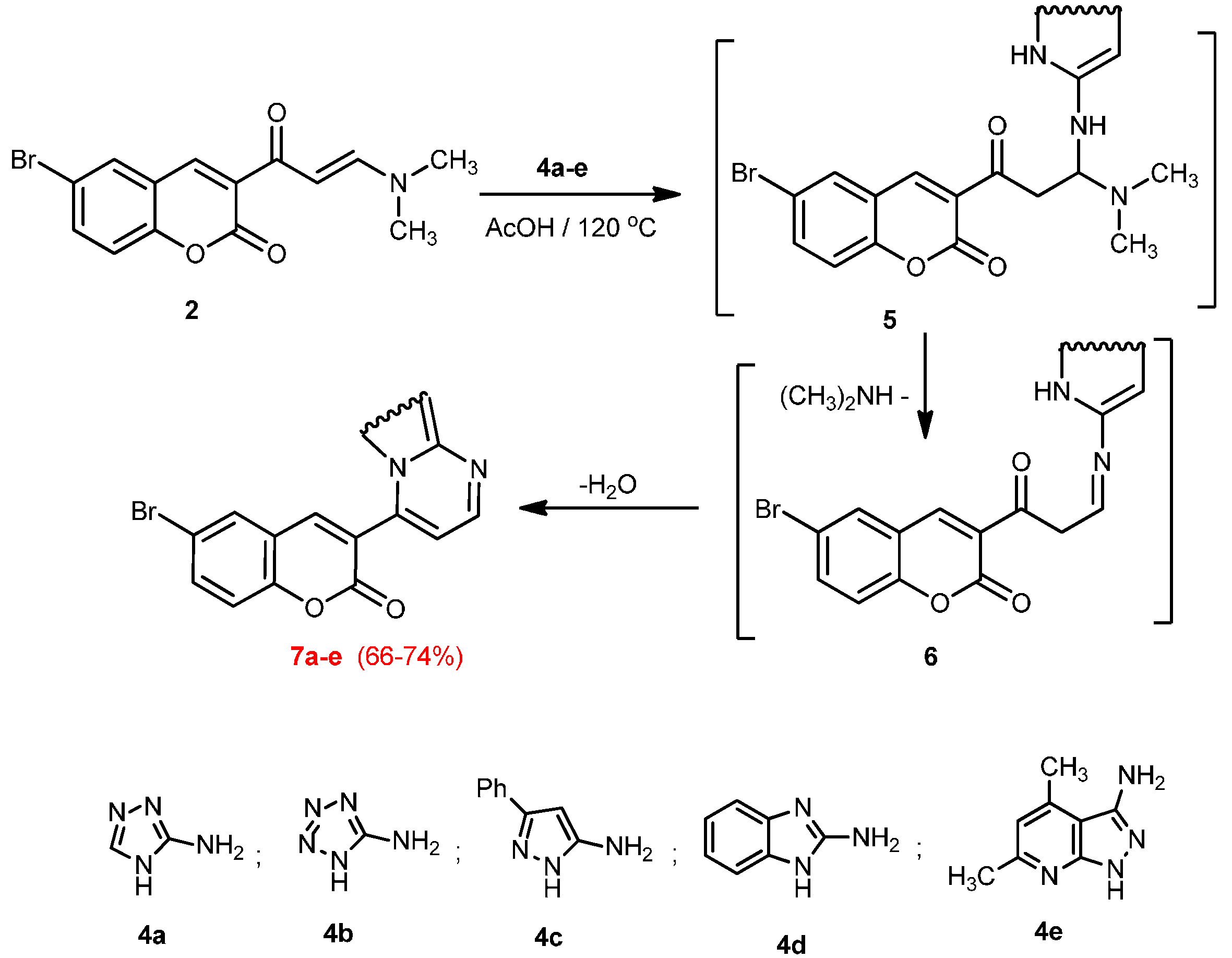
3.2.3. Reactions of Enaminone 2 with Heterocyclic Amines 4a–e
3.2.4. Reactions of Enaminone 2 with Hydrazines
3.2.5. Reactions of Enaminone 2 with Hydrazonoyl Chlorides 9a, 9b and Hydroximoyl Chlorides 10a, 10b
3.2.6. Reaction of pyrazole 12a with hydrazine hydrate
3.2.7. General Procedure for Synthesis of 1,3,4-Thiadiazole Derivatives 18a–h
3.2.8. Alternative Synthesis of 18c
3.2.9. General Procedure for the Synthesis of 1,3-Thiazole Derivatives 23a–i
3.3. Evaluation of the Antitumor Activity Using Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Bhattacharyya, S.S.; Mandal, S.K.; Biswas, R.; Paul, S.; Pathak, S.; Boujedaini, N.; Belon, P.; Khuda-Bukhsh, A.R. A synthetic coumarin (4-methyl-7-hydroxy coumarin) has anti-cancer potentials against DMBA-induced skin cancer in mice. Eur. J. Pharmacol. 2009, 614, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.K.; Sharma, P.K.; Dudhe, R.; Chaudhary, A.; Verma, P.K. Synthesis, analgesic and ulcerogenic activity of novel pyrimidine derivative of coumarin moiety. Anal. Univ. Bucuresti-Chim. 2010, 19, 9–21. [Google Scholar]
- Pavurala, S.; Vedula, R.R. An efficient, multicomponent synthesis of pyrazolyl-triazolothiadiazinyl chromen-2-ones. J. Heterocycl. Chem. 2015, 52, 306–309. [Google Scholar] [CrossRef]
- Abdelhafez, O.M.; Amin, K.M.; Batran, R.Z.; Maher, T.J.; Nada, S.A.; Sethumadhavan, S. Synthesis, anticoagulant and PIVKA-II induced by new 4-hydroxycoumarin derivatives. Bioorg. Med. Chem. 2010, 18, 3371–3378. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.Z.; Watanabe, T.; Kawamoto, M.; Nishiyama, K.; Yamashita, H.; Ishii, M.; Iwamura, M.; Furuta, T. Coumarin-4-ylmethoxycarbonyls as photo-triggers for alcohols and phenols. Org. Lett. 2003, 5, 4867–4870. [Google Scholar] [CrossRef] [PubMed]
- Sunthitikawinsakul, A.; Kongkathip, N.; Kongkathip, B.; Phonnakhu, S.; Daly, J.W.; Spande, T.F.; Nimit, Y.; Rochanaruangrai, S. Coumarins and carbazoles from Clausena excavate exhibited antimycobacterial and antifungal activities. Planta Med. 2003, 69, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Weigt, S.; Huebler, N.; Strecker, R.; Braunbeck, T.; Broschard, T.H. Developmental effects of coumarin and the anticoagulant coumarin derivative warfarin on zebrafish (Danio rerio) embryos. Reprod. Toxicol. 2012, 33, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.A.; Cooperwood, J.S.; Khan, M.O.; Rahman, T. In-vitro antiproliferative activity of benzopyranone derivatives in comparison with standard chemotherapeutic drugs. Arch. Pharm. 2011, 344, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.A.; Badisa, V.L.D.; Latinwo, L.M.; Waryoba, C.; Ugochukwu, N. In vitro cytotoxicity of benzopyranone derivatives with basic side chain against human lung cell lines. Anticancer Res. 2010, 30, 4613–4617. [Google Scholar] [PubMed]
- Musa, M.A.; Khan, M.O.F.; Cooperwood, J.S. Synthesis and antiproliferative activity of coumarin-estrogen conjugates against breast cancer cell lines. Lett. Drug Des. Discov. 2009, 6, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Kokotos, G.; Theodorou, V.; Tzougraki, C.; Deforce, D.L.; van den Eeckhout, E.G. Synthesis and in vitro cytotoxicity of aminocoumarin platinum (II) complexes. Bioorg. Med. Chem. Lett. 1997, 7, 2165–2168. [Google Scholar] [CrossRef]
- Cui, J.; Li, S. Inhibitors and prodrugs targeting CYP1: A novel approach in cancer prevention and therapy. Curr. Med. Chem. 2014, 21, 519–552. [Google Scholar] [CrossRef] [PubMed]
- Steffen, U.S.; Weber, B.; Siegers, C. Antitumor-activities of coumarin, 7-hydroxy-coumarin and its glucuronide in several human tumor cell lines. Res. Commun. Mol. Pathol. Pharmacol. 1998, 99, 193–206. [Google Scholar]
- Zagotto, G.; Gia, O.; Baccichetti, F.; Uriarte, E.; Palumbo, M. Synthesis and photobiological properties of 4-hydroxymethyl-4′-methylpsoralen derivatives. Photochem. Photobiol. 1993, 58, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Williamson, R.C.N.; Lyndon, P.T.; Tudway, A.J.C. Effects of anticoagulation and ileal resection on the development and spread of experimental intestinal carcinomas. Br. J. Cancer 1980, 42, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.J.; Ketcham, A.S.; Wexler, H. Warfarin therapy as an adjunct to the surgical treatment of malignant tumors in mice. Cancer Res. 1969, 29, 2191–2194. [Google Scholar] [PubMed]
- Yang, E.B.; Zhao, Y.N.; Zhang, K.; Mack, P. Daphnetin, one of coumarin derivatives, is a protein kinase inhibitor. Biochem. Biophys. Res. Commun. 1999, 260, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Shiv Jee, K.; Vipin Kumar, G.; Pramod Kumar, S.; Nitin, K.; Rupesh, D.; Jitendra, K.G. Thiazoles: Having diverse biological activities. Med. Chem. Res. 2012, 21, 2123–2132. [Google Scholar]
- Shawali, A.S. 1,3,4-Thiadiazoles of pharmacological interest: Recent trends in their synthesis via tandem 1,3-dipolar cycloaddition. J. Adv. Res. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Ahmed, O.M.; Abdelhamid, A.O. Synthesis and anti-tumor activities of new [1,2,4]triazolo[1,5-a]pyrimidine derivatives. Eur. J. Chem. 2014, 5, 334–338. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Ahmed, S.A.; Abdelhamid, A.O. Synthesis and cytotoxicity evaluation of some novel thiazoles, thiadiazoles, and pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidin-5(1H)-one incorporating triazole moiety. Molecules 2015, 20, 1357–1376. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Salah, T.A.; Abdelhamid, A.O. Synthesis, characterization and pharmacological evaluation of some novel thiadiazoles and thiazoles incorporating pyrazole moiety as potent anticancer agents. Monatsh. Chem. 2015, 146, 149–158. [Google Scholar] [CrossRef]
- Gomha, S.M.; Khalil, K.D. A convenient ultrasound-promoted synthesis and cytotoxic activity of some new thiazole derivatives bearing a coumarin nucleus. Molecules 2012, 17, 9335–9347. [Google Scholar] [CrossRef] [PubMed]
- Gomha, S.M.; Abdel-aziz, H.M. Synthesis and antitumor activity of 1,3,4-thiadiazole derivatives bearing coumarine ring. Heterocycles 2015, 91, 583–592. [Google Scholar] [CrossRef]
- Abbas, I.M.; Gomha, S.M.; Elneairy, M.A.A.; Elaasser, M.M.; Mabrouk, B.K.A. Fused triazolo[4,3-a]pyrimidinones: Synthesis and biological evaluation as antimicrobial and anti-cancer agents. Turk. J. Chem. 2015, 39, 510–531. [Google Scholar] [CrossRef]
- Vijesh, A.M.; Isloor, A.M.; Prabhu, V.; Ahmad, S.; Malladi, S. Synthesis, characterization and anti-microbial studies of some novel 2,4-disubstituted thiazoles. Eur. J. Med. Chem. 2010, 45, 5460–5464. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.O.; Zohdi, H.F.; Rateb, N.M. Reactions with hydrazonoyl halides XXI: Reinvestigation of the reactions of hydrazonoyl bromides with 1,1-dicyanothioacetanilide. J. Chem. Res. 1998, 184–185, 920–932. [Google Scholar]
- Arshad, A.; Osman, H.; Bagley, M.C.; Lam, C.K.; Mohamad, S.; Zahariluddin, A.S.M. Synthesis and antimicrobial properties of some new thiazolyl coumarin derivatives. Eur. J. M. Chem. 2011, 46, 3788–3794. [Google Scholar]
- Asiri, A.M.; Zayed, M.E.M.; Ng, S.W. Ethyl (Z)-2-chloro-2-(2-phenylhydrazin-1-ylidene)acetate. Acta Cryst. 2011, 67. [Google Scholar] [CrossRef] [PubMed]
- Eweiss, N.F.; Osman, A. Synthesis of heterocycles-2. New routes to acetylthiadiazolines and arylazothiazoles. J. Heterocycl. Chem. 1980, 17, 1713–1717. [Google Scholar] [CrossRef]
- Shawali, A.S.; Osman, A. Reaction of dimethylphenacylsulfonium bromide with N-nitrosoacetarylamides and reactions of the products with nucleophiles. Bull. Chem. Soc. Jpn. 1976, 49, 321–324. [Google Scholar] [CrossRef]
- Shawali, A.S.; Osman, A. Synthesis and reactions of phenyl carbamoyl-aryl hydrazidic chlorides. Tetrahedron 1971, 27, 2517–2528. [Google Scholar] [CrossRef]
- Wolkoff, P. A new method of preparing hydrazonyl halides. Can. J. Chem. 1975, 53, 1333–1335. [Google Scholar] [CrossRef]
- Klančnik, A.; Piskernik, S.; Jeršek, B.; Možina, S.S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Gangadevi, V.; Muthumary, J. Preliminary studies on cytotoxic effect of fungal taxol on cancer cell lines. Afr. J. Biotechnol. 2007, 6, 1382–1386. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the synthesized compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomha, S.M.; Zaki, Y.H.; Abdelhamid, A.O. Utility of 3-Acetyl-6-bromo-2H-chromen-2-one for the Synthesis of New Heterocycles as Potential Antiproliferative Agents. Molecules 2015, 20, 21826-21839. https://doi.org/10.3390/molecules201219803
Gomha SM, Zaki YH, Abdelhamid AO. Utility of 3-Acetyl-6-bromo-2H-chromen-2-one for the Synthesis of New Heterocycles as Potential Antiproliferative Agents. Molecules. 2015; 20(12):21826-21839. https://doi.org/10.3390/molecules201219803
Chicago/Turabian StyleGomha, Sobhi M., Yasser H. Zaki, and Abdou O. Abdelhamid. 2015. "Utility of 3-Acetyl-6-bromo-2H-chromen-2-one for the Synthesis of New Heterocycles as Potential Antiproliferative Agents" Molecules 20, no. 12: 21826-21839. https://doi.org/10.3390/molecules201219803