Multivariate Quantification of the Solid State Phase Composition of Co-Amorphous Naproxen-Indomethacin
Abstract
:1. Introduction
2. Results and Discussion
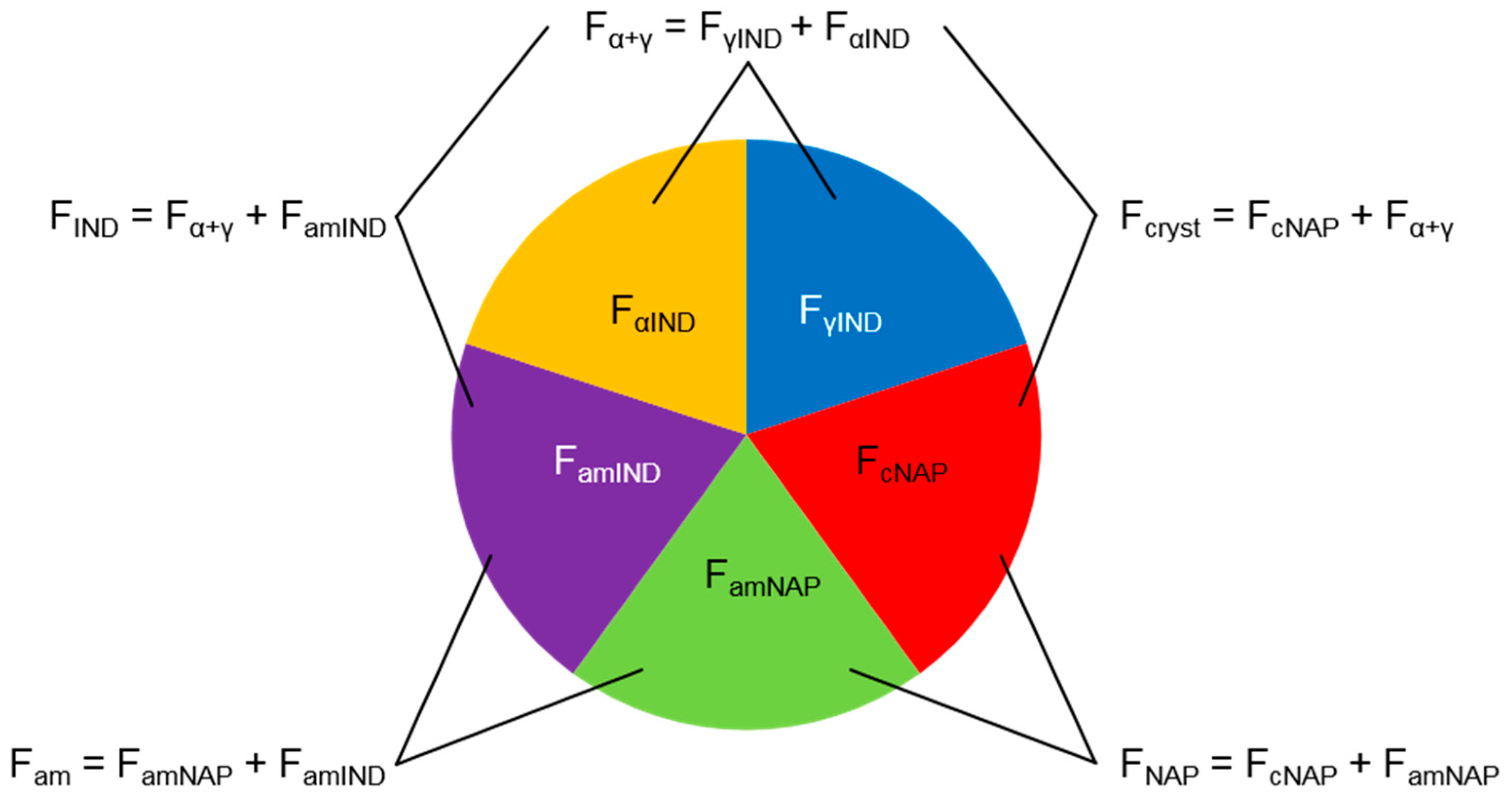
2.1. Determination of the Phase Composition of Co-Amorphous Naproxen-Indomethacin
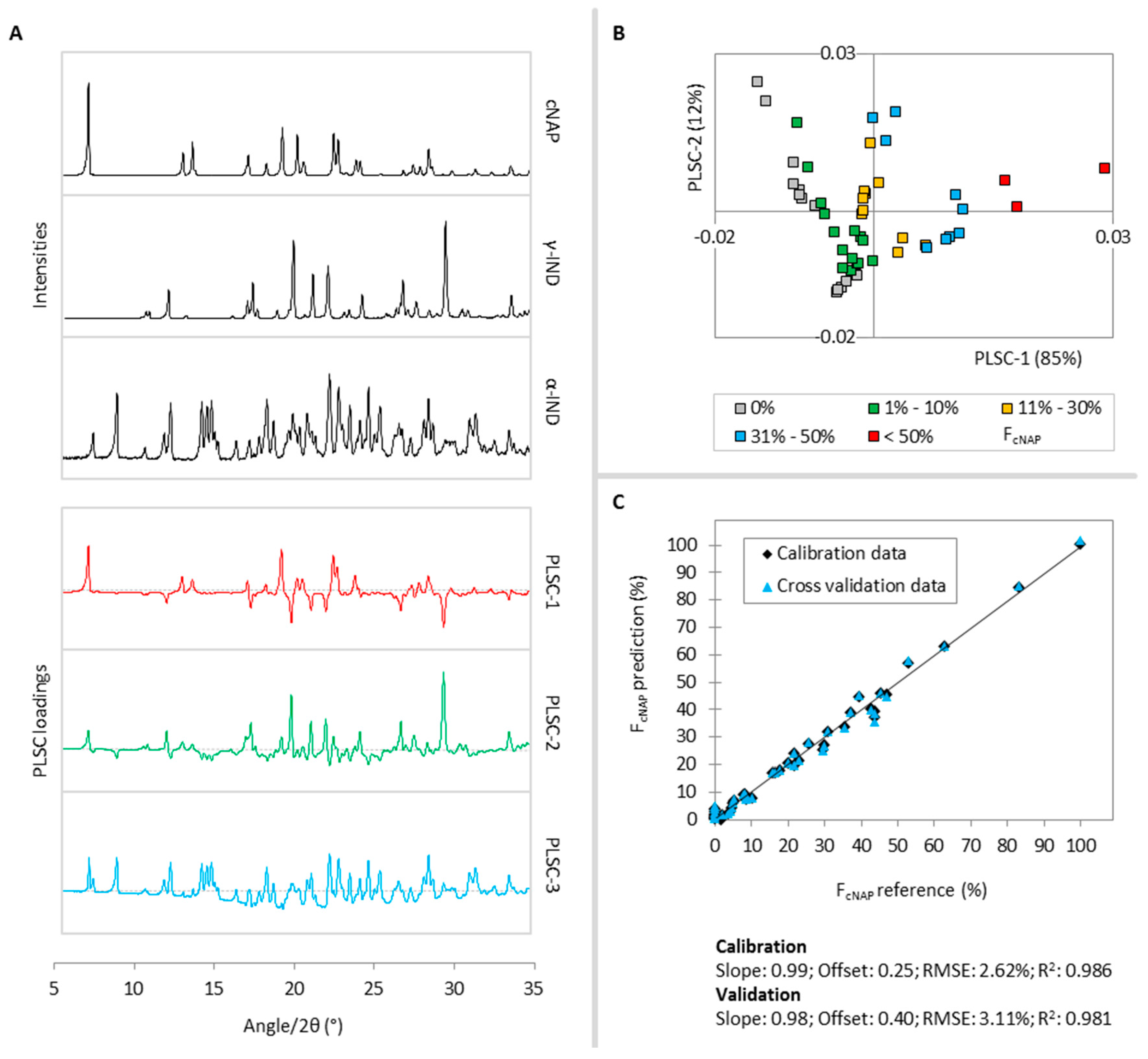
2.2. Molar Crystalline Naproxen Fraction FcNAP
2.3. Molar γ-Indomethacin Fraction FγIND

2.4. Molar α-Indomethacin Fraction FαIND

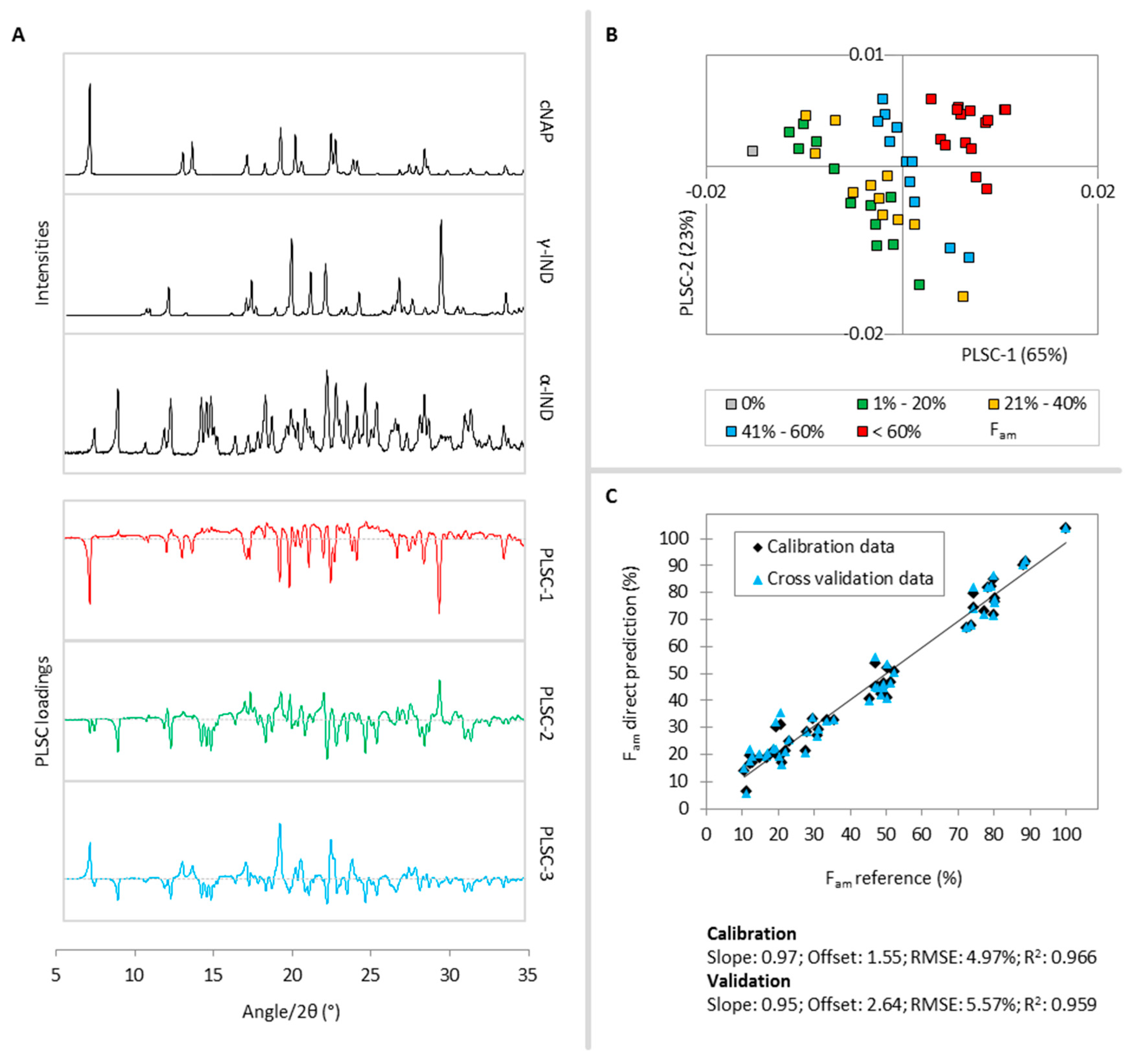
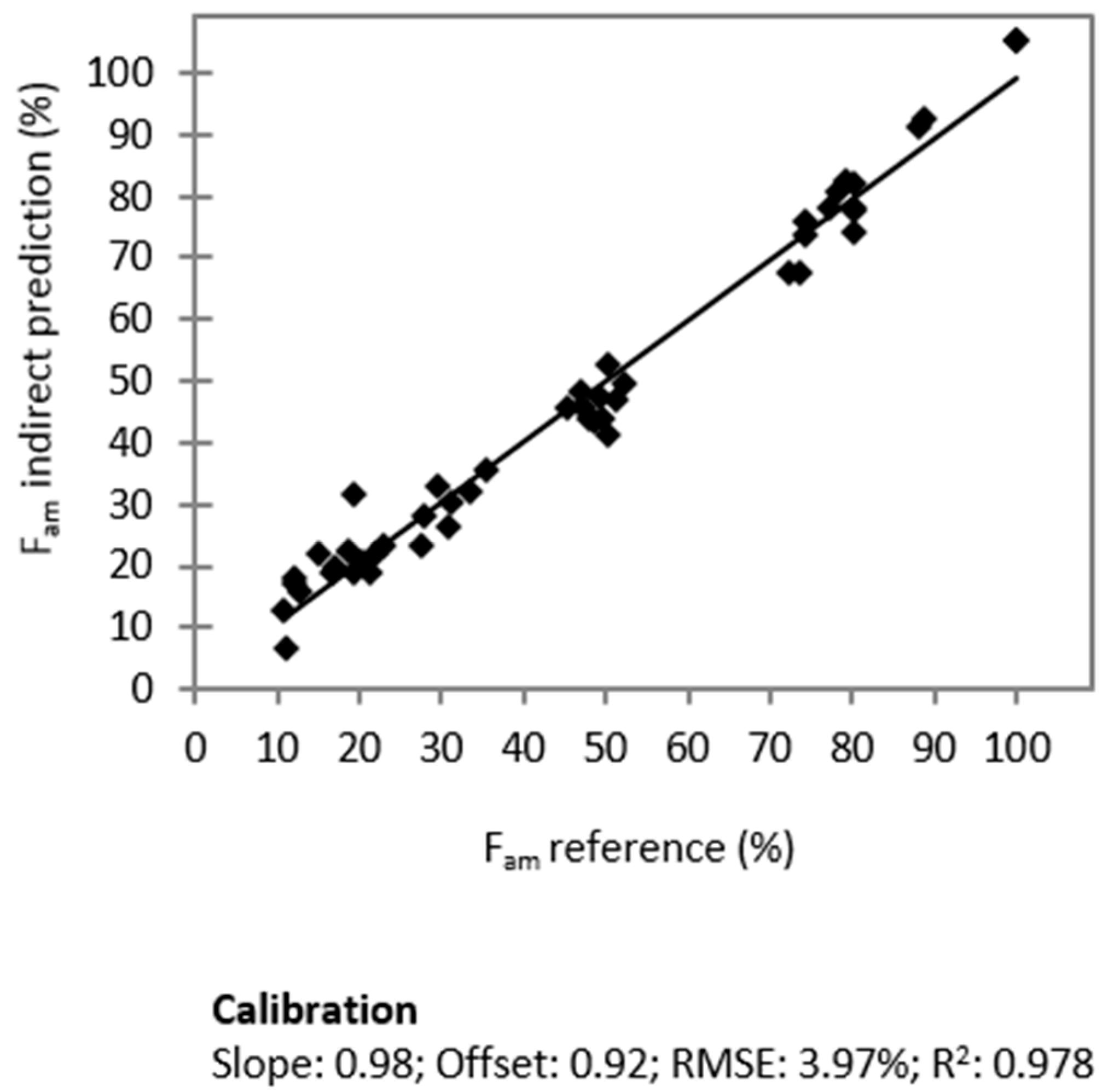
2.5. Total Molar Amorphous Fraction Fam
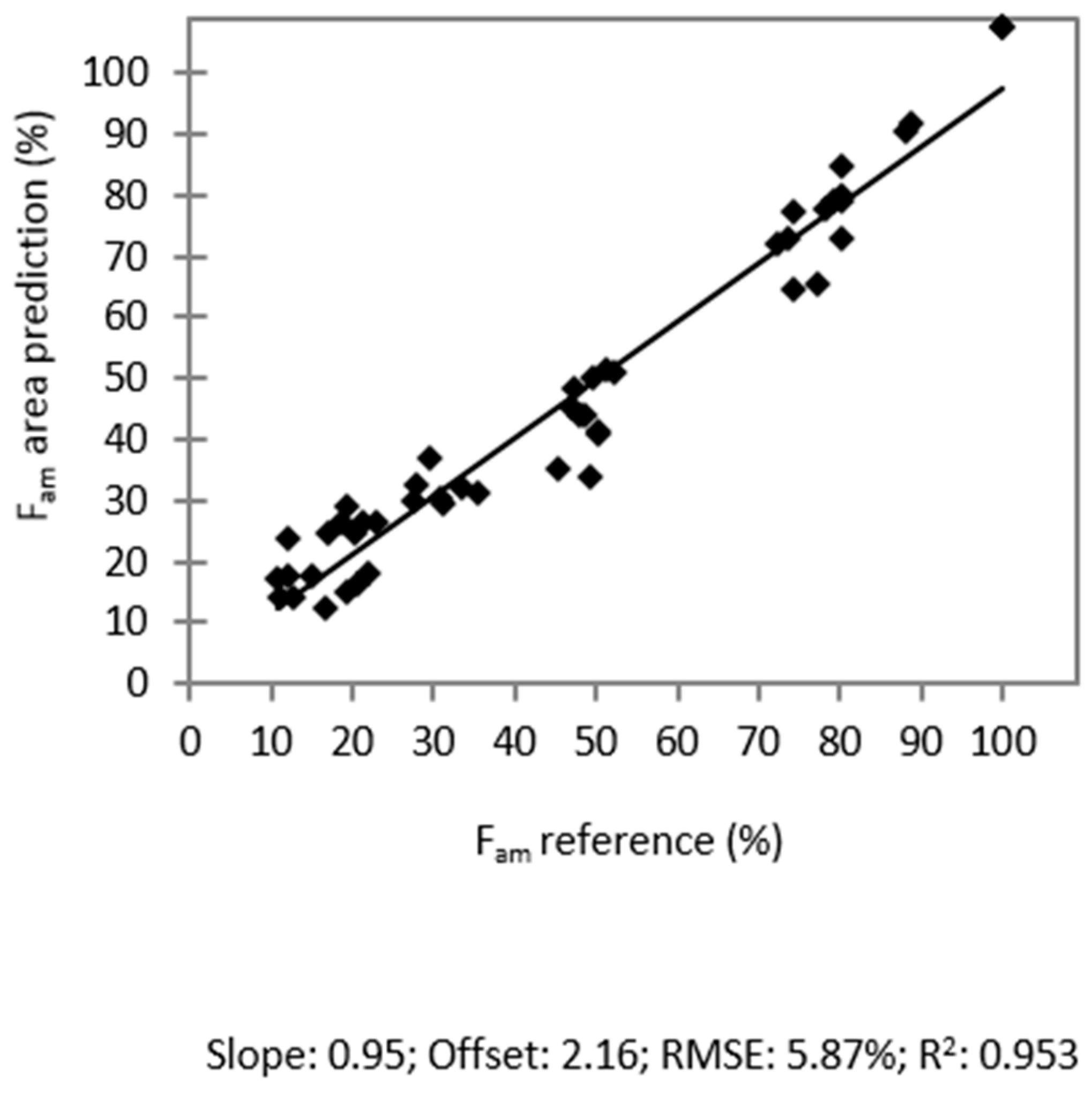
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Partial Least Squares Regression
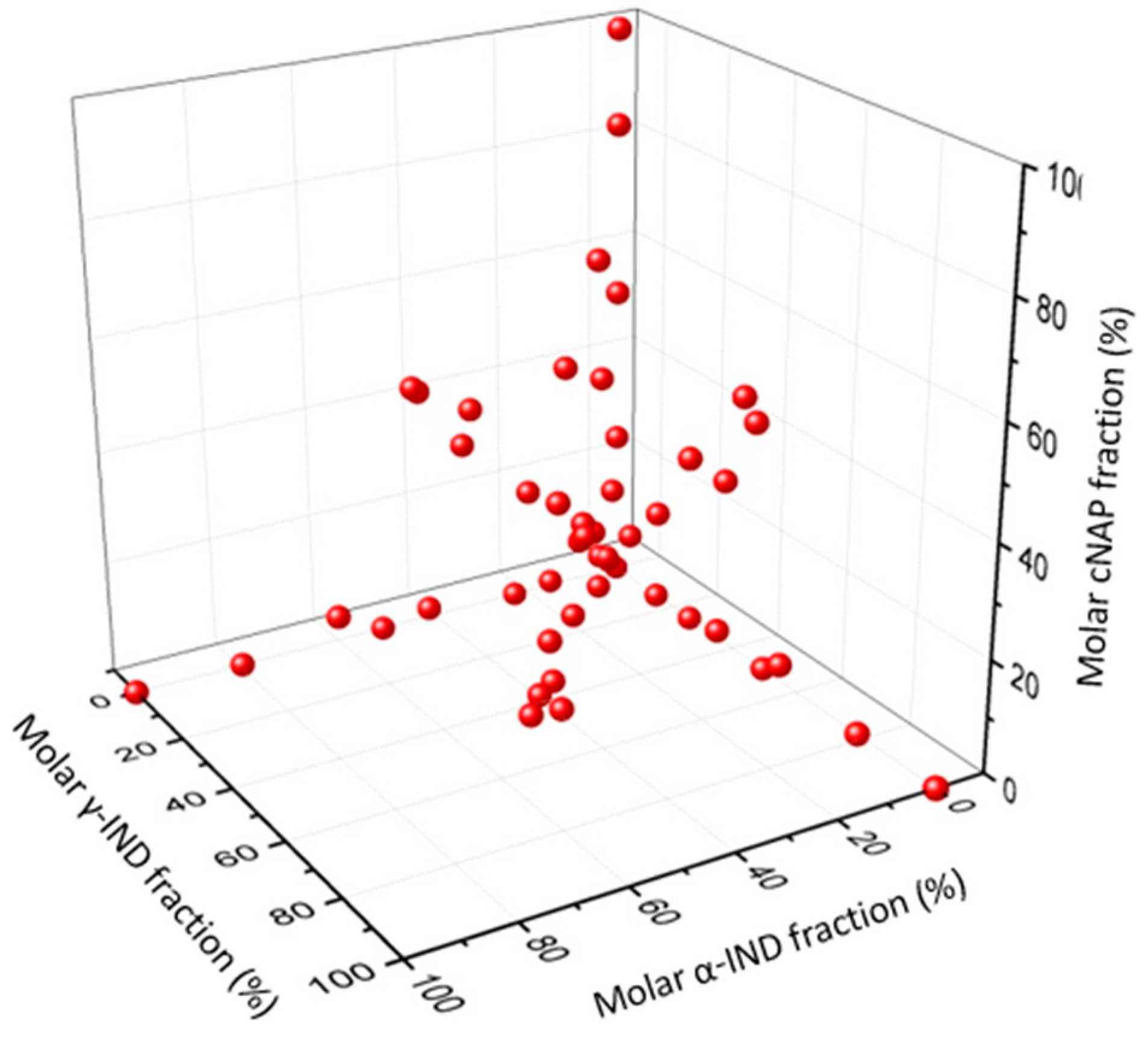
3.2.2. Preparation of the PLS Calibration Set
3.2.3. X-ray Powder Diffractometry
3.2.4. Cross Validation of the PLS Models
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| Sample | FcNAP (%) | FγIND (%) | FαIND (%) | Fam (%) |
|---|---|---|---|---|
| 1 | 3.6 | 3.6 | 3.9 | 88.9 |
| 2 | 4.2 | 3.1 | 4.5 | 88.1 |
| 3 | 8.1 | 10.7 | 8.8 | 72.4 |
| 4 | 7.9 | 9.1 | 9.4 | 73.6 |
| 5 | 17.6 | 18.6 | 16.6 | 47.2 |
| 6 | 15.8 | 15.7 | 17.1 | 51.4 |
| 7 | 22.9 | 24.9 | 22.7 | 29.5 |
| 8 | 21.6 | 27 | 23.6 | 27.8 |
| 9 | 29.5 | 28.3 | 29.9 | 12.2 |
| 10 | 0 | 37.7 | 39.4 | 22.9 |
| 11 | 0 | 34.4 | 34.7 | 30.8 |
| 12 | 0 | 43.4 | 44.5 | 12.1 |
| 13 | 0 | 44.2 | 38.8 | 17 |
| 14 | 30 | 36.5 | 0 | 33.5 |
| 15 | 43.7 | 45.6 | 0 | 10.6 |
| 16 | 47 | 41.8 | 0 | 11.1 |
| 17 | 37.3 | 0 | 31.6 | 31.1 |
| 18 | 31 | 0 | 33.4 | 35.6 |
| 19 | 42.7 | 0 | 42.4 | 14.9 |
| 20 | 43.6 | 0 | 43.5 | 12.8 |
| 21 | 0 | 0 | 50.7 | 49.3 |
| 22 | 0 | 0 | 79.3 | 20.7 |
| 23 | 0 | 49.6 | 0 | 50.4 |
| 24 | 0 | 78.1 | 0 | 21.9 |
| 25 | 53 | 0 | 0 | 47 |
| 26 | 83.3 | 0 | 0 | 16.7 |
| 27 | 62.8 | 7.9 | 9.1 | 20.3 |
| 28 | 8.6 | 8.3 | 63.8 | 19.3 |
| 29 | 8.9 | 64.9 | 7.4 | 18.8 |
| 30 | 8.5 | 35.9 | 36.2 | 19.4 |
| 31 | 35.5 | 36 | 7.3 | 21.2 |
| 32 | 45.3 | 9.8 | 17.2 | 27.6 |
| 33 | 4.6 | 5.9 | 44.1 | 45.4 |
| 34 | 39.6 | 4.7 | 6.3 | 49.5 |
| 35 | 5.3 | 42 | 4.9 | 47.8 |
| 36 | 5 | 22.8 | 23.5 | 48.7 |
| 37 | 20 | 22.5 | 5.2 | 52.3 |
| 38 | 21.7 | 5.3 | 22.6 | 50.4 |
| 39 | 1.8 | 2.1 | 16 | 80.1 |
| 40 | 16.3 | 1.7 | 2 | 80.1 |
| 41 | 1.6 | 17.2 | 1.9 | 79.3 |
| 42 | 1.7 | 8.6 | 9.3 | 80.3 |
| 43 | 10.2 | 9 | 2.6 | 78.2 |
| 44 | 8.1 | 2 | 9.5 | 80.3 |
| 45 | 0 | 0 | 22.6 | 77.4 |
| 46 | 0 | 25.7 | 0 | 74.3 |
| 47 | 25.7 | 0 | 0 | 74.3 |
| 48 | 0 | 0 | 100 | 0 |
| 49 | 0 | 100 | 0 | 0 |
| 50 | 100 | 0 | 0 | 0 |
| 51 | 0 | 0 | 0 | 100 |
| 52 | 0 | 0 | 0 | 100 |
References
- Engers, D.; Teng, J.; Jimenez-Novoa, J.; Gent, P.; Hossack, S.; Campbell, C.; Thomson, J.; Ivanisevic, I.; Templeton, A.; Byrn, S. A solid-state approach to enable early development compounds: Selection and animal bioavailability studies of an itraconazole amorphous solid dispersion. J. Pharm. Sci. 2010, 99, 3901–3922. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aaltonen, J.; Rades, T. Commentary: Towards physicorelevant dissolution testing: The importance of solid-state analysis in dissolution. Dissolut. Technol. 2009, 16, 47–54. [Google Scholar] [CrossRef]
- Hancock, B.C.; Parks, M. What is the true solubility advantage for amorphous pharmaceuticals? Pharm. Res. 2000, 17, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Yu, L. Amorphous pharmaceutical solids: Preparation, characterization and stabilization. Adv. Drug Deliv. Rev. 2001, 48, 27–42. [Google Scholar] [CrossRef]
- Hancock, B.C.; Zografi, G. Characteristics and significance of the amorphous state in pharmaceutical systems. J. Pharm. Sci. 1997, 86, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, A.M.; Gupta, P.; Bansal, A.K. Amorphous drug delivery systems: Molecular aspects, design, and performance. Crit. Rev. Ther. Drug Carr. Syst. 2004, 21, 133–193. [Google Scholar] [CrossRef]
- Janssens, S.; van den Mooter, G. Review: Physical chemistry of solid dispersions. J. Pharm. Pharmacol. 2009, 12, 1571–1586. [Google Scholar] [CrossRef]
- Zheng, W.; Jain, A.; Papoutsakis, D.; Dannenfelser, R.-M.; Panicucci, R.; Garad, S. Selection of oral bioavailability enhancing formulations during drug discovery. Drug Dev. Ind. Pharm. 2012, 38, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Van den Mooter, G.; Wuyts, M.; Blaton, N.; Busson, R.; Grobet, P.; Augustijns, P.; Kinget, R. Physical stabilisation of amorphous ketoconazole in solid dispersions with polyvinylpyrrolidone K25. Eur. J. Pharm. Sci. 2001, 12, 261–269. [Google Scholar] [PubMed]
- Hancock, B.C.; Shamblin, S.L.; Zografi, G. Molecular mobility of amorphous pharmaceutical solids below their glass transition temperatures. Pharm. Res. 1995, 12, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Serajuddin, A.T.M. Salt formation to improve drug solubility. Adv. Drug Deliv. Rev. 2007, 59, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Srinarong, P.; de Waard, H.; Frijlink, H.W.; Hinrichs, W.L. Improved dissolution behavior of lipophilic drugs by solid dispersions: The production process as starting point for formulation considerations. Expert Opin. Drug Deliv. 2011, 8, 1121–1140. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.R.; Ursekar, B.; Kapadia, C.J. Design, optimization, preparation and evaluation of dispersion granules of valsartan and formulation into tablets. Curr. Drug Deliv. 2009, 1, 28–37. [Google Scholar] [CrossRef]
- Laitinen, R.; Löbmann, K.; Strachan, C.J.; Grohganz, H.; Rades, T. Emerging trends in the stabilization of amorphous drugs. Int. J. Pharm. 2013, 6, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Chieng, N.; Aaltonen, J.; Saville, D.; Rades, T. Physical characterization and stability of amorphous indomethacin and ranitidine hydrochloride binary systems prepared by mechanical activation. Eur. J. Pharm. 2009, 71, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Allesø, M.; Chieng, N.; Rehder, S.; Rantanen, J.; Rades, T.; Aaltonen, J. Enhanced dissolution rate and synchronized release of drugs in binary systems through formulation: Amorphous naproxen-cimetidine mixtures prepared by mechanical activation. J. Control. Release 2009, 136, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Descamps, M.; Willart, J.; Dudognon, E.; Caron, V. Transformation of pharmaceutical compounds upon milling and comilling: The role of Tg. J. Pharm. Sci. 2007, 96, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Hoppu, P.; Jouppila, K.; Rantanen, J.; Schantz, S.; Juppo, A.M. Characterisation of blends of paracetamol and citric acid. J. Pharm. Pharmacol. 2007, 59, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Yoshihashi, Y.; Yonemochi, E.; Fujii, K.; Uekusa, H.; Terada, K. Cocrystallization and amorphization induced by drug-excipient interaction improves the physical properties of acyclovir. Int. J. Pharm. 2012, 422, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Löbmann, K.; Grohganz, H.; Laitinen, R.; Strachan, C.; Rades, T. Amino acids as co-amorphous stabilizers for poorly water soluble drugs—Part 1: Preparation, stability and dissolution enhancement. Eur. J. Pharm. 2013, 85, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Löbmann, K.; Laitinen, R.; Strachan, C.; Rades, T.; Grohganz, H. Amino acids as co-amorphous stabilizers for poorly water-soluble drugs—Part 2: Molecular interactions. Eur. J. Pharm. 2013, 85, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.; Löbmann, K.; Rades, T.; Grohganz, H. Improving Co-Amorphous Drug Formulations by the Addition of the Highly Water Soluble Amino Acid, Proline. Pharmaceutics 2014, 6, 416–435. [Google Scholar] [CrossRef] [PubMed]
- Löbmann, K.; Strachan, C.; Grohganz, H.; Rades, T.; Korhonen, O.; Laitinen, R. Co-amorphous simvastatin and glipizide combinations show improved physical stability without evidence of intermolecular interactions. Eur. J. Pharm. 2012, 81, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Löbmann, K.; Laitinen, R.; Grohganz, H.; Strachan, C.; Rades, T.; Gordon, K.C. A theoretical and spectroscopic study of co-amorphous naproxen and indomethacin. Int. J. Pharm. 2013, 453, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Löbmann, K.; Laitinen, R.; Grohganz, H.; Gordon, K.C.; Strachan, C.; Rades, T. Coamorphous drug systems: Enhanced physical stability and dissolution rate of indomethacin and naproxen. Mol. Pharm. 2011, 8, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, G.A.; Forbes, R.A.; Reutzel-Edens, S.M. Characterization of the solid state: Quantitative issues. Adv. Drug Deliv. Rev. 2001, 48, 67–90. [Google Scholar] [CrossRef]
- Brittain, H.G.; Bogdanowich, S.J.; Bugay, D.E.; DeVincentis, J.; Lewen, G.; Newman, A.W. Physical characterization of pharmaceutical solids. Pharm. Res. 1991, 8, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Chipera, S.J.; Bish, D.L. Fitting Full X-ray Diffraction Patterns for Quantitative Analysis: A Method for Readily Quantifying Crystalline and Disordered Phases. AMPC 2013, 3, 47–53. [Google Scholar] [CrossRef]
- Chung, F.H. Quantitative interpretation of X-ray diffraction patterns of mixtures. I. Matrix-flushing method for quantitative multicomponent analysis. J. Appl. Crystallogr. 1974, 7, 519–525. [Google Scholar] [CrossRef]
- Rumondor, A.C.F.; Taylor, L.S. Application of Partial Least-Squares (PLS) modeling in quantifying drug crystallinity in amorphous solid dispersions. Int. J. Pharm. 2010, 398, 155–160. [Google Scholar] [CrossRef] [PubMed]
- De La Torre, A.G.; Bruque, S.; Aranda, M.A.G. Rietveld quantitative amorphous content analysis. J. Appl. Crystallogr. 2001, 34, 196–202. [Google Scholar] [CrossRef]
- Shah, B.; Kakumanu, V.K.; Bansal, A.K. Analytical techniques for quantification of amorphous/crystalline phases in pharmaceutical solids. J. Pharm. Sci. 2006, 95, 1641–1665. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, H. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Bish, D.L.; Howard, S.A. Quantitative phase analysis using the Rietveld method. J. Appl. Crystallogr. 1988, 21, 86–91. [Google Scholar] [CrossRef]
- Caliandro, R.; di Profio, G.; Nicolotti, O. Multivariate analysis of quaternary carbamazepine-saccharin mixtures by X-ray diffraction and infrared spectroscopy. J. Pharm. Biomed. Anal. 2013, 78–79, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Atef, E.; Chauhan, H.; Prasad, D.; Kumari, D.; Pidgeon, C. Quantifying Solid-State Mixtures of Crystalline Indomethacin by Raman Spectroscopy Comparison with Thermal Analysis. ISRN Chromatogr. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Wiklund, S.; Nilsson, D.; Eriksson, L.; Sjöström, M.; Wold, S.; Faber, K. A randomization test for PLS component selection. J. Chemom. 2007, 21, 427–439. [Google Scholar] [CrossRef]
- Martens, H.A.; Dardenne, P. Validation and verification of regression in small data sets. Chemometr. Intell. Lab. Syst. 1998, 1–2, 99–121. [Google Scholar] [CrossRef]
- Bansal, P.; Hall, M.; Realff, M.J.; Lee, J.H.; Bommarius, A.S. Multivariate statistical analysis of X-ray data from cellulose: A new method to determine degree of crystallinity and predict hydrolysis rates. Bioresour. Technol. 2010, 101, 4461–4471. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beyer, A.; Grohganz, H.; Löbmann, K.; Rades, T.; Leopold, C.S. Multivariate Quantification of the Solid State Phase Composition of Co-Amorphous Naproxen-Indomethacin. Molecules 2015, 20, 19571-19587. https://doi.org/10.3390/molecules201019571
Beyer A, Grohganz H, Löbmann K, Rades T, Leopold CS. Multivariate Quantification of the Solid State Phase Composition of Co-Amorphous Naproxen-Indomethacin. Molecules. 2015; 20(10):19571-19587. https://doi.org/10.3390/molecules201019571
Chicago/Turabian StyleBeyer, Andreas, Holger Grohganz, Korbinian Löbmann, Thomas Rades, and Claudia S. Leopold. 2015. "Multivariate Quantification of the Solid State Phase Composition of Co-Amorphous Naproxen-Indomethacin" Molecules 20, no. 10: 19571-19587. https://doi.org/10.3390/molecules201019571









