Fluorescence and Docking Studies of the Interaction between Human Serum Albumin and Pheophytin
Abstract
:1. Introduction
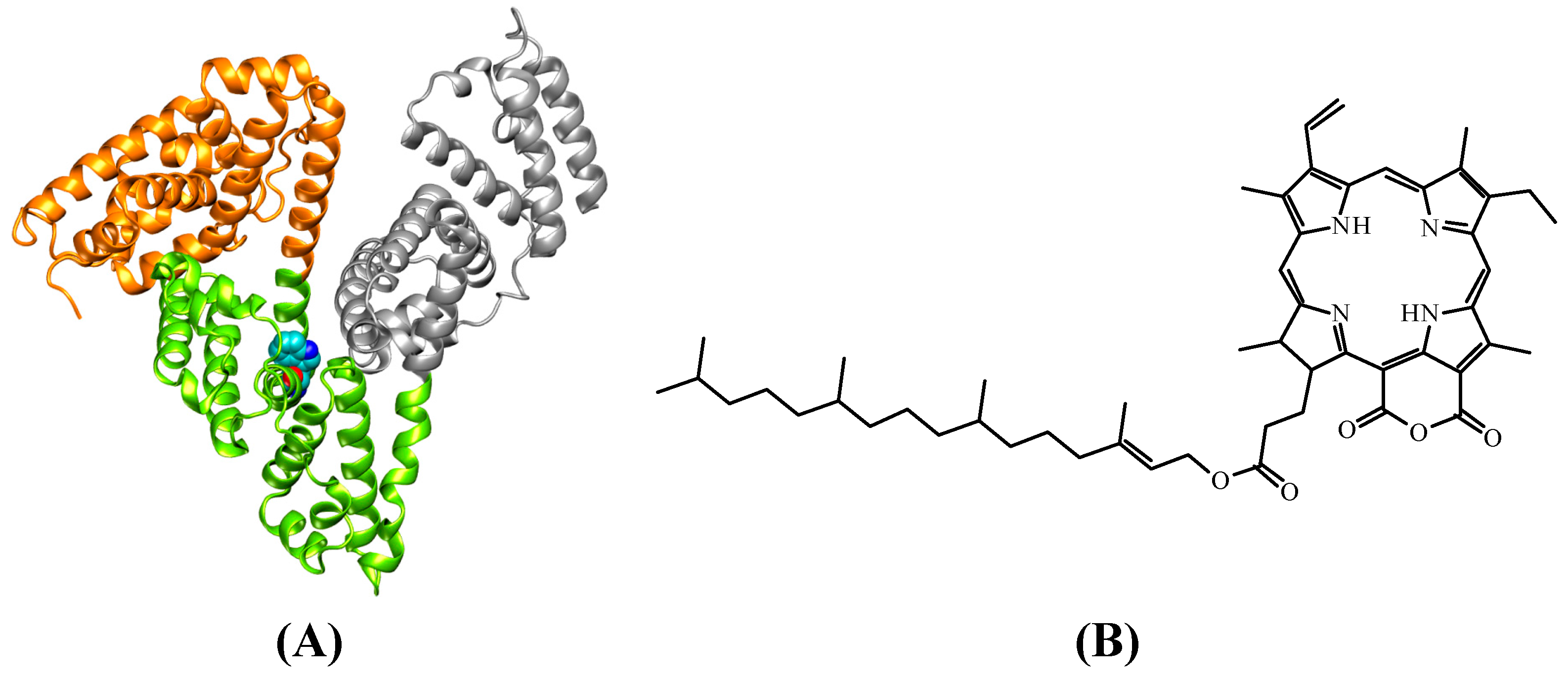
2. Results and Discussion
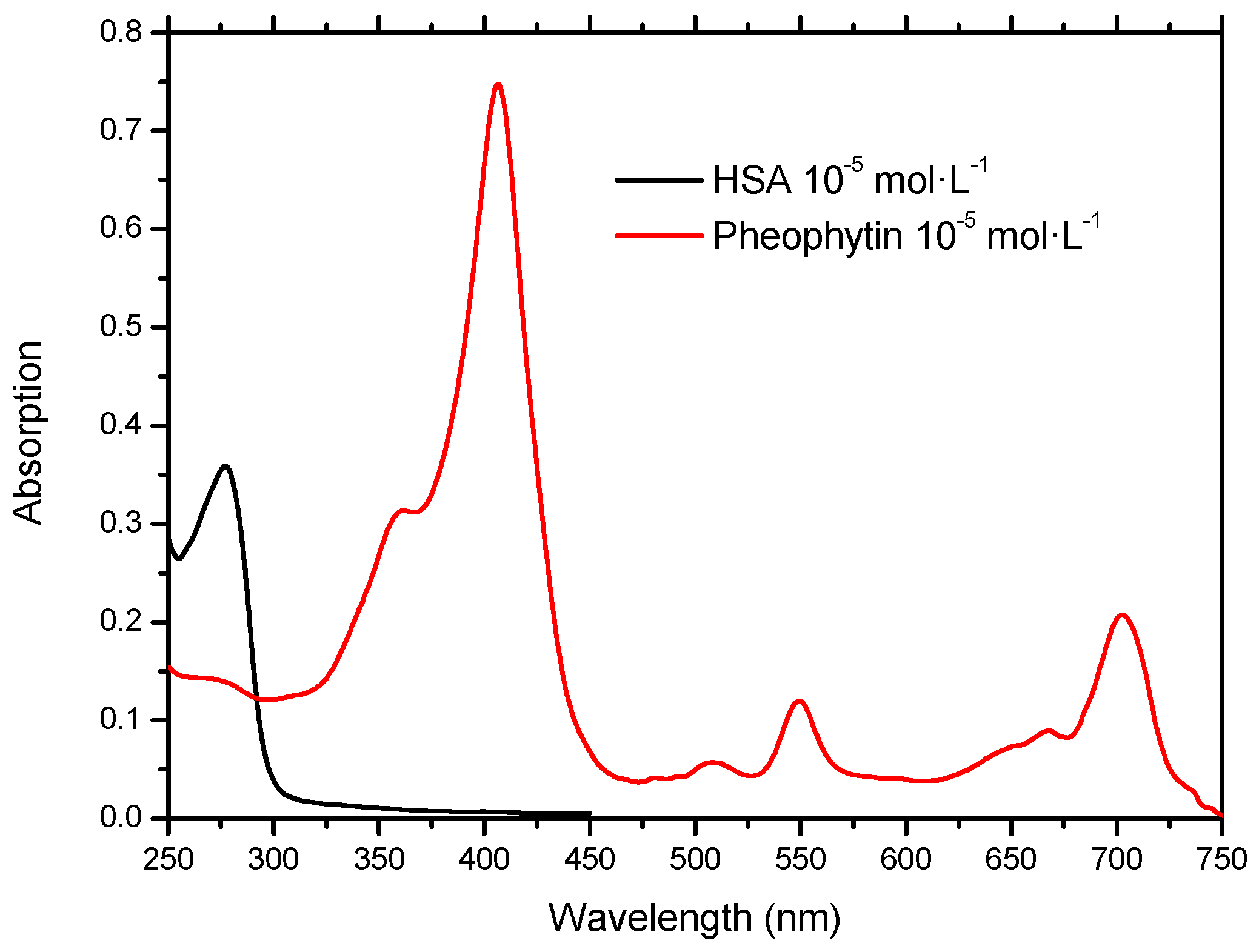
2.1. Molecular Absorption Spectroscopy in the UV-Vis
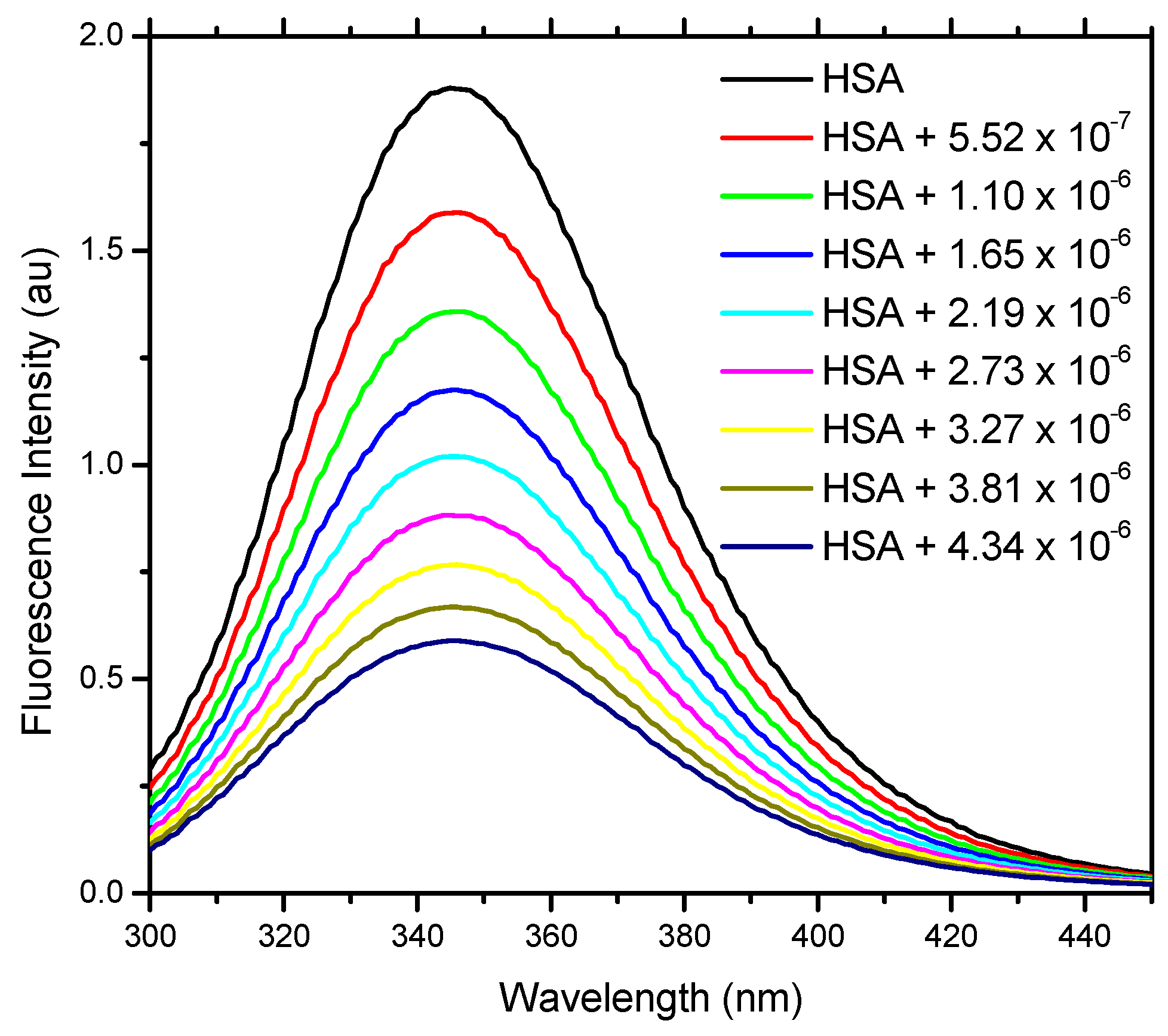
2.2. Fluorescence Spectroscopy
2.2.1. Binding Constant of Stern-Volmer Modified (Ka)
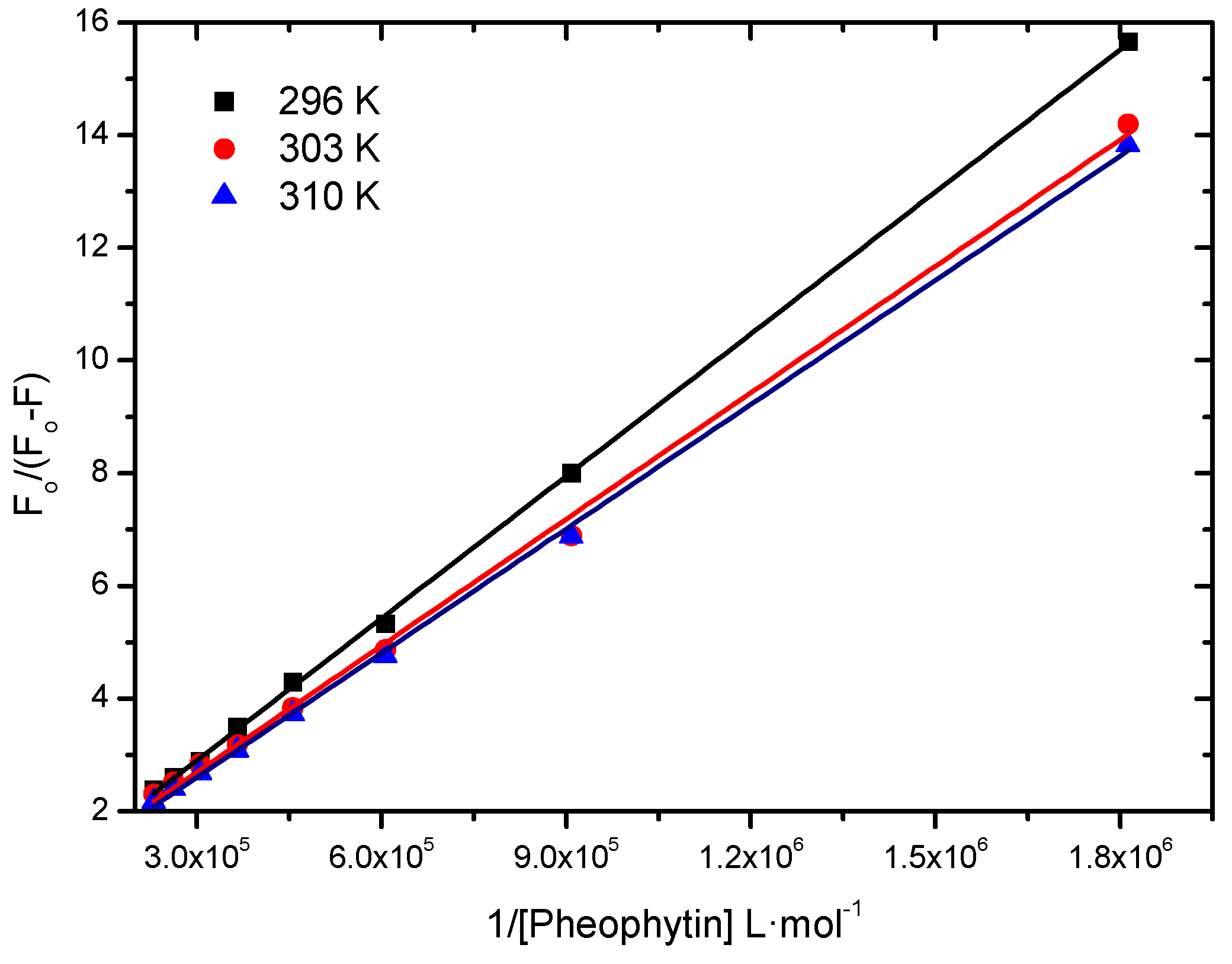
| T (K) | Ka (L∙mol−1) | r2 |
|---|---|---|
| 296 | 4.48 ± 0.06 × 104 | 0.9997 |
| 303 | 5.43 ± 0.13 × 104 | 0.9978 |
| 310 | 5.95 ± 0.08 × 104 | 0.9992 |
2.2.2. Thermodynamics Parameters (ΔG°, ΔH°, ΔS°)

| T (K) | ΔH° (kJ∙mol−1) | ΔS° (kJ∙mol−1∙K−1) | ΔG° (kJ∙mol−1) | r2 |
|---|---|---|---|---|
| 296 | −26.4 | |||
| 303 | 15.5 ± 1.2 | 0.145 | −27.4 | 0.9313 |
| 310 | −28.4 |
2.2.3. Fluorescence Quenching; Static vs. Dynamic
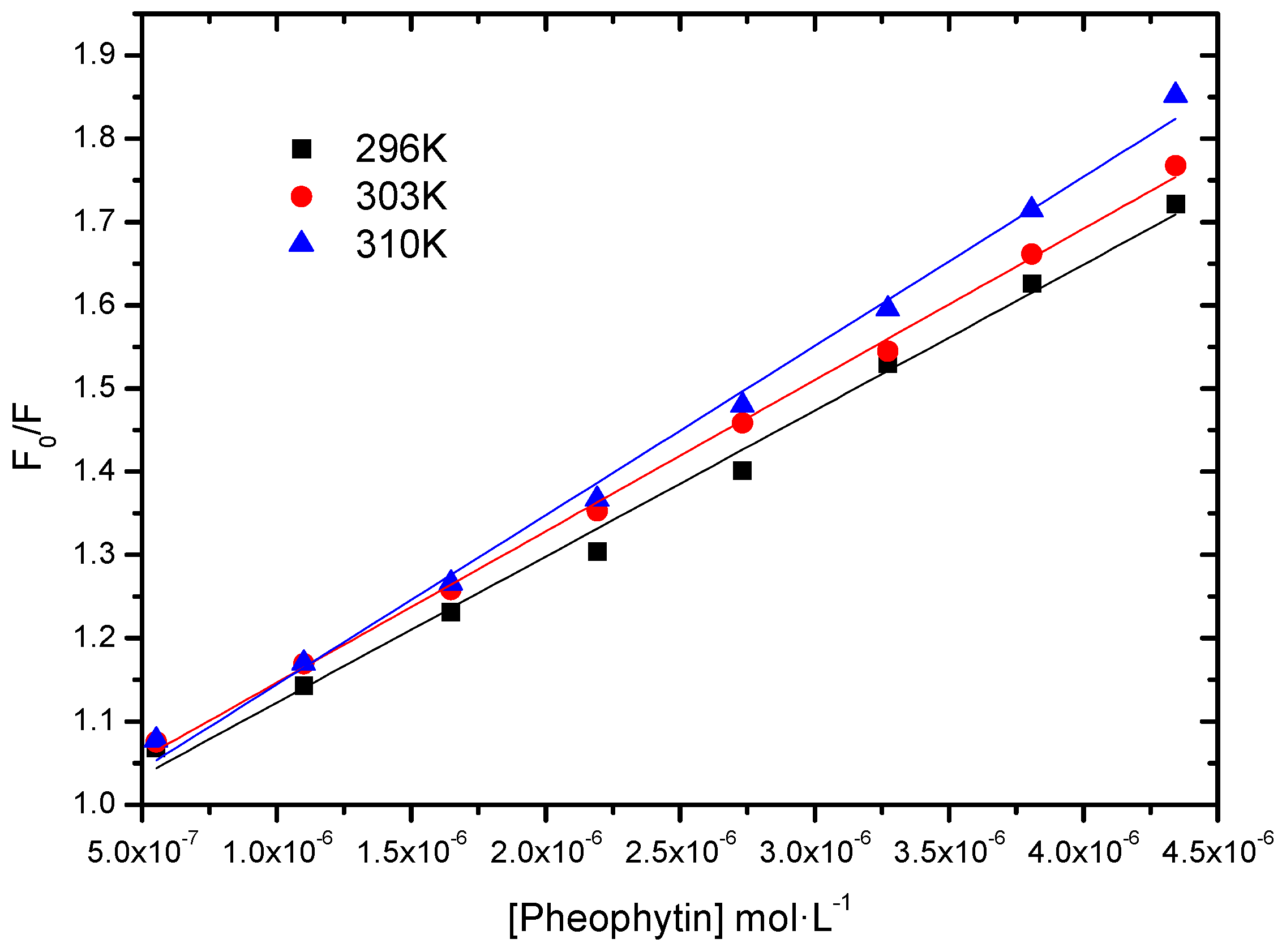
| T (K) | KSV (L∙mol−1) | kq (L∙mol−1∙s−1) | r2 |
|---|---|---|---|
| 296 | 1.75 ± 0.07 × 105 | 1.75 × 1013 | 0.9927 |
| 303 | 1.82 ± 0.03 × 105 | 1.82 × 1013 | 0.9980 |
| 310 | 2.03 ± 0.06 × 105 | 2.03 × 1013 | 0.9946 |
2.3. Molecular Modeling Calculation

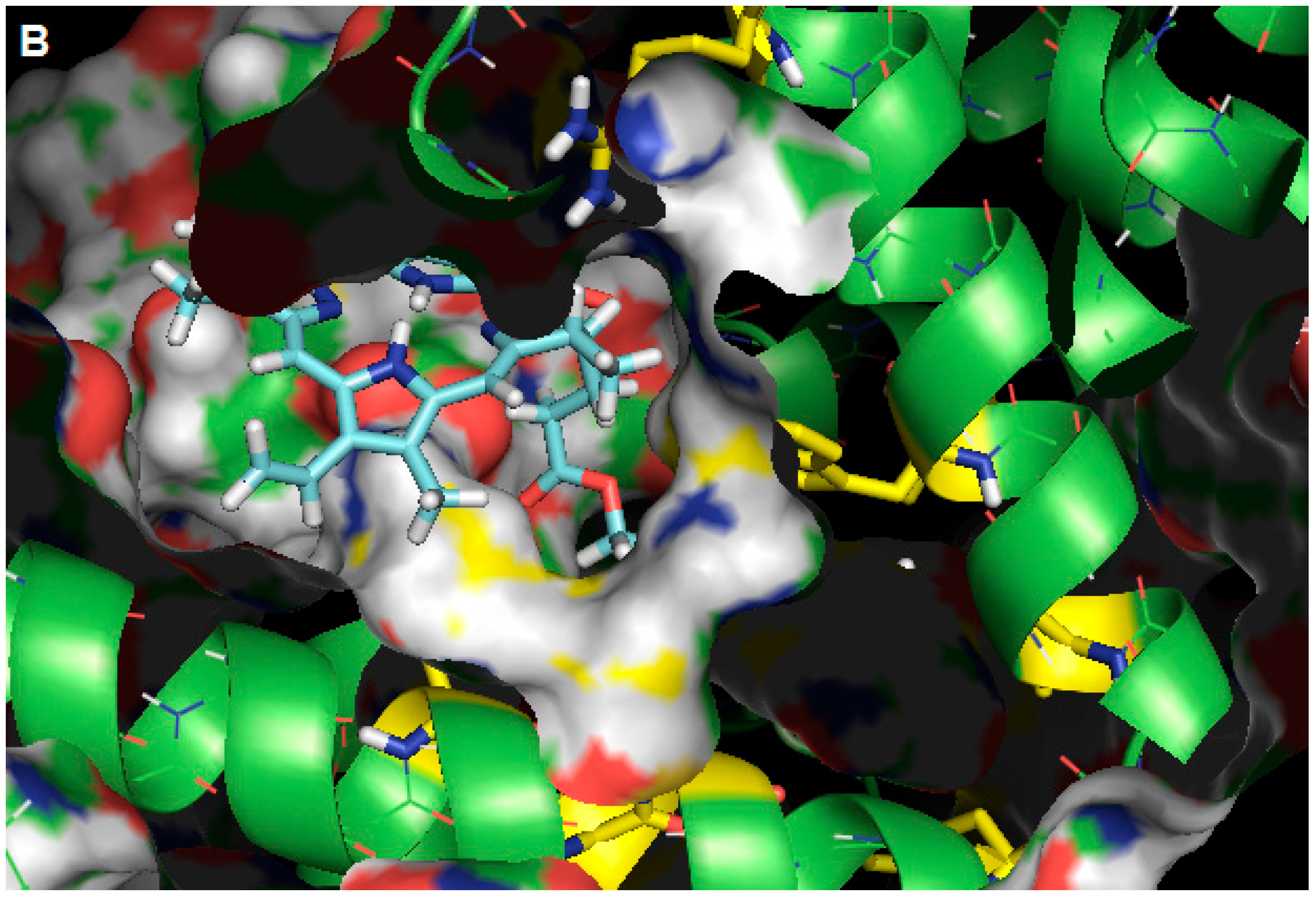
3. Experimental Section
3.1. Spectroscopic Experiments
3.1.1. Instruments and Materials
3.1.2. Methodology for UV-Vis Spectroscopy
3.1.3. Methodology for Fluorescence Spectroscopy
3.2. Computational Experiments
Methodology for Docking Study
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rodrigues, M.I.A.; Furlan, A. Estudo Morfológico da Superfície das Sementes de Portulacaceae através da Microscopia Eletrônica de Varredura. In Proceedings of the 54° Congresso Nacional de Botânica 3ª Reunião Amazônica de Botânica, Amazônia-UNAMA, Manaus, Brazil, 13–18 July 2003; p. 65.
- Ekpo, A.; Eseyin, O.; Kalu, N.; Jackson, O.; Edoho, E.J. Studies on the Biochemical Effects of Talinum triangulare in Rat. J. Pharmacol. Toxicol. 2007, 3, 300–303. [Google Scholar]
- Kohda, H.; Yamoaka, Y.; Morinaga, S.; Ishak, M.; Darise, M. Saponins from Talinum triangulare. Chem. Pharm. Bull. 1992, 40, 2557–2558. [Google Scholar] [CrossRef]
- Amorim, A.P.O.; Amorim, T.A.; Oliveira, M.C.C.; Echevarria, A. Antioxidant, Iron Chelating and Tyrosinase Inhibitory Activities of Extracts from Talinum triangulare Leach Stem. Antioxidants 2013, 3, 90–99. [Google Scholar] [CrossRef]
- Amorim, A.P.; Oliveira, M.C.C.; Esteves, A.S.; Carvalho, M.G.; Gattas, C.R. Avaliação da atividade antitumoral de Talinum triangulare Leach. In Proceedings of the 7° Simpósio Iberoamericano de Plantas Medicinais e o 2° Simpósio Iberoamericano de Investigação em Câncer, Ilhéus, BA, Brazil, 27–30 October 2014; p. 79.
- Ofusori, D.A.; Adelakun, A.E.; Ayoka, A.O.; Oluwayinka, O.P.; Omotoso, E.O.; Odukoya, S.A.; Adeyemi, D.O. Water Leaf (Talinum triangulare) enhances Cerebral Functions in Swiss Albino Mice. J. Neurol. Sci. 2008, 25, 239–246. [Google Scholar]
- Majorek, K.A.; Porebski, P.J.; Dayal, A.; Zimmerman, M.D.; Jablonska, K.; Stewart, A.J.; Chruszcz, M.; Minor, W. Structural and Immunologic Characterization of Bovine, Horse, and Rabbit Serum Albumins. J. Mol. Immunol. 2012, 52, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Chuang, V.T.G.; Maruyama, T.; Otagiri, M. Human Serum Albumin—New Insights on its Structural Dynamics, Functional Impacts and Pharmaceutical Applications. J. Pharm. Sci. 2012, 101, 3033–3046. [Google Scholar] [CrossRef] [PubMed]
- Peters, T. Serum Albumin, 1st ed.; Anfinsen, C.B., Ed.; Academic Press: New York, NY, USA, 1985; Volume 1, pp. 170–246. [Google Scholar]
- Paul, B.K.; Samanta, A.; Guchhait, N. Exploring Hydrophobic Subdomain IIA of the Protein Bovine Serum Albumin in the Native, Intermediate, Unfolded, and Refolded States by a Small Fluorescence Molecular Reporter. J. Phys. Chem. B 2010, 114, 6183–6196. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.C.; He, X.M.; Munson, S.H.; Twigg, P.D.; Gernert, K.M.; Broom, M.B.; Miller, T.Y. Three-Dimensional Structure of Human Serum Albumin. Science 1989, 244, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Sugio, S.; Kashima, S.; Mochizuki, S.; Noda, M.; Kobayashi, K. Crystal Structure of Human Serum Albumin at 2.5 Å resolution. Protein Eng. Design Sel. 1999, 12, 439–446. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 1st ed.; Springer: New York, NY, USA, 2006; pp. 923–928. [Google Scholar]
- Ben Dror, S.; Bronshtein, I.; Garini, Y.; O’Neal, W.G.; Jacobi, P.A.; Ehrenberg, B. The Localization and Photosensitization of Modified Chlorin Photosensitizers in Artificial Membranes. J. Photochem. Photobiol. 2009, 8, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, R. The Use of Spectrophotometry UV-Vis for the Study of Porphyrins. In Macro to Nano Spectroscopy, 1st ed.; Uddin, J., Ed.; InTech Europe: Rijeka, Croatia, 2012; Volume 1, pp. 87–108. [Google Scholar]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A Review of NIR Dyes in Cancer Targeting and Imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tian, J.N.; Zhang, J.; Hu, Z.; Chen, X. Interaction of Magnolol with Bovine Serum Albumin: A Fluorescence—Quenching Study. Anal. Bioanal. Chem. 2003, 376, 864–867. [Google Scholar] [PubMed]
- Tian, J.; Liu, X.; Zhao, Y.; Zhao, S. Studies on the Interaction between Tetraphenylporphyrin Compounds and Bovine Serum Albumin. J. Lumin. 2007, 22, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Eftink, M.R. Fluorescence Quenching Reactions: Probing Biological Macromolecular Structures. In Biophysical Biochemical Aspects of Fluorescence Spectroscopy, 1st ed.; Dgurvy, T.G., Ed.; Plenum Press: New York, NY, USA, 1991; Volume 1, pp. 1–41. [Google Scholar]
- Eftink, M.R.; Ghiron, C.A. Fluorescence Quenching Studies with Protein. Analytical Bioanal. Chem. 1981, 114, 199–227. [Google Scholar] [CrossRef]
- Li, Q.; Seeger, S. Deep UV Sensing of the Interaction of Porphyrin with Bovine Serum Albumin Protein. Sens. Actuators B Chem. 2009, 139, 118–134. [Google Scholar] [CrossRef] [Green Version]
- Borissevitch, I.E.; Tominaga, T.T.; Schmitt, C.C. Photophysical Studies on the Interaction of Two Water-Soluble Porphyrins with Bovine Serum Albumin. Effects upon the Porphyrin Triplet State Characteristics. J. Photochem. Photobiol. A 1998, 114, 201–207. [Google Scholar] [CrossRef]
- Borissevitch, I.E.; Tominaga, T.T.; Imasato, H.; Tabak, M. Fluorescence and Optical Absorption Study of Interaction of Two Water Soluble Porphyrins with Bovine Serum Albumin. The role of Albumin and Porphyrin Aggregation. J. Lumin. 1996, 69, 65–76. [Google Scholar] [CrossRef]
- He, W.; Li, Y.; Tian, J.; Liu, H.; Hu, Z.; Chen, X. Spectroscopic Studies on Binding of Shikonin to Human Serum Albumin. J. Photochem. Photobiol. A 2005, 174, 53–61. [Google Scholar] [CrossRef]
- Ross, P.D.; Subramanian, S. Thermodynamics of Protein Association Reactions: Forces Contributing to Stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Timaseff, S.N. Thermodynamics of Protein Interactions. In Proteins of Biological Fluids, 1st ed.; Peeters, H., Ed.; Oxford Pergamon Press: Oxford, UK, 1972; Volume 1, pp. 511–519. [Google Scholar]
- Brown, J.R.; Schockcey, P. Serum Albumin: Structure and Characterization of it Binding Sites. In Lipid-Protein Interaction, 1st ed.; Jost, P., Griffith, O.H., Eds.; John Wiley & Sons: New York, NY, USA, 1989; Volume 1, pp. 25–68. [Google Scholar]
- Brune, D.; Kim, S. Predicting Protein Diffusion Coefficients. Biophysics 1993, 90, 3835–3839. [Google Scholar] [CrossRef]
- Fasano, M.; Curry, S.; Terreno, E.; Galliano, M.; Fanali, G.; Narciso, P.; Notari, S.; Ascenzi, P. The Extraordinary Ligand Binding Properties of Human Serum Albumin. IUBMB Life 2005, 57, 787–796. [Google Scholar] [CrossRef] [PubMed]
- He, X.M.; Carter, D.C. Atomic Structure and Chemistry of Human Serum Albumin. Nature 1992, 358, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.; Ho, J.X. Structure of Serum Albumin. Adv. Protein Chem. 1994, 45, 153–203. [Google Scholar] [PubMed]
- Moriyama, Y.; Ohta, D.; Hachiya, K.; Mitsui, Y.; Takeda, K.J. Fluorescence Behavior of Tryptophan Residues of Bovine and Human Serum Albumins in Ionic Surfactant Solutions: A Comparative Study of the Two and One Tryptophan(s) of Bovine and Human Albumins. J. Protein Chem. 1996, 15, 265–272. [Google Scholar] [CrossRef]
- Amorim, A.P.O.; Carvalho-Jr, A.R.; Castro, R.N.; Lopes, N.P.; Oliveira, M.C.C.; Carvalho, M.G. Chemical Compounds Isolated from Talinum triangulare (Portulacaceae). Food Chem. 2014, 160, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Wardell, M.; Wang, Z.; Ho, J.X.; Robert, J.; Ruker, F.; Ruble, J.; Carter, D.C. The Atomic Structure of Human Methemalbumin at 1.9 Å. Biochem. Biophys. Res. Commun. 2002, 291, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Dewar, M.J.S.; Zoebisch, E.G.; Healy, E.F.; Stewart, J.J.P. Development and use of Quantum Mechanical Molecular Models. 76. AM1: A new General Purpose Quantum Mechanical Molecular Model. J. Am. Chem. Soc. 1985, 107, 3902–3909. [Google Scholar] [CrossRef]
- Jones, G; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and Validation of a Genetic Algorithm for Flexible Docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Korb, O.; Stützle, T.; Exner, T.E. Empirical Scoring Functions for Advanced Protein-Ligand Docking with Plants. J. Chem. Inf. Model. 2009, 49, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaves, O.A.; Amorim, A.P.d.O.; Castro, L.H.E.; Sant’Anna, C.M.R.; De Oliveira, M.C.C.; Cesarin-Sobrinho, D.; Netto-Ferreira, J.C.; Ferreira, A.B.B. Fluorescence and Docking Studies of the Interaction between Human Serum Albumin and Pheophytin. Molecules 2015, 20, 19526-19539. https://doi.org/10.3390/molecules201019526
Chaves OA, Amorim APdO, Castro LHE, Sant’Anna CMR, De Oliveira MCC, Cesarin-Sobrinho D, Netto-Ferreira JC, Ferreira ABB. Fluorescence and Docking Studies of the Interaction between Human Serum Albumin and Pheophytin. Molecules. 2015; 20(10):19526-19539. https://doi.org/10.3390/molecules201019526
Chicago/Turabian StyleChaves, Otávio Augusto, Ana Paula de O. Amorim, Larissa H. E. Castro, Carlos Mauricio R. Sant’Anna, Márcia C. C. De Oliveira, Dari Cesarin-Sobrinho, José Carlos Netto-Ferreira, and Aurélio B. B. Ferreira. 2015. "Fluorescence and Docking Studies of the Interaction between Human Serum Albumin and Pheophytin" Molecules 20, no. 10: 19526-19539. https://doi.org/10.3390/molecules201019526





