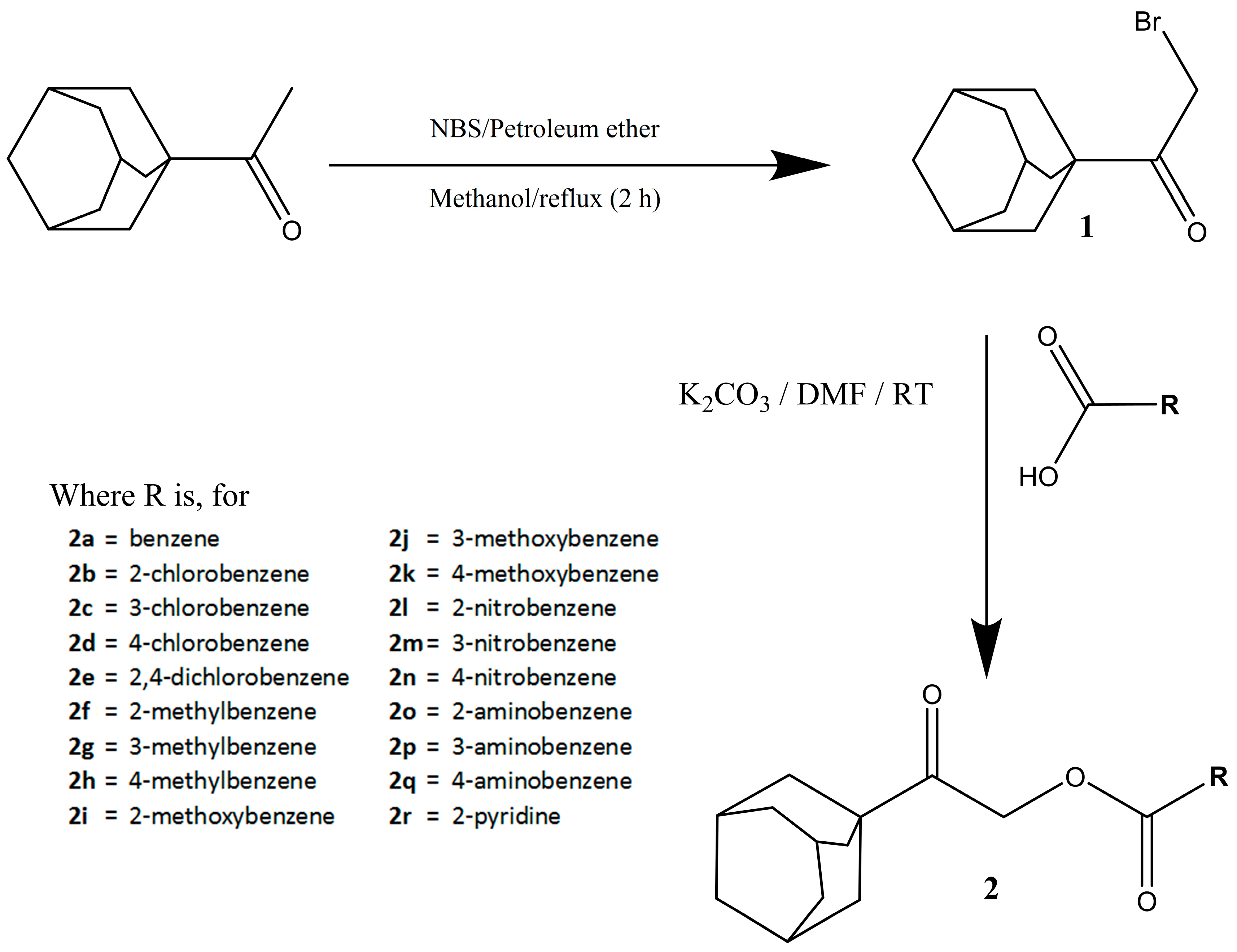
Synthesis and Crystallographic Insight into the Structural Aspects of Some Novel Adamantane-Based Ester Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. General Description of the Crystal Structure Conformations
| Compound | Refined Site Occupancy Ratio |
|---|---|
| 2a | 0.390 (10):0.610 (10) (A) |
| 0.695 (4):0.305(4) (B) | |
| 2b | 0.431 (10):0.569 (10) (A) |
| 2f | 0.716 (6):0.284 (6) (A) |
| 0.793 (4):0.207 (4) (B) | |
| 2j | 0.753 (3):0.247 (3) |
| 2l | 0.873 (4):0.127 (4) (B) |
| 2o | 0.897 (4):0.103 (4) |
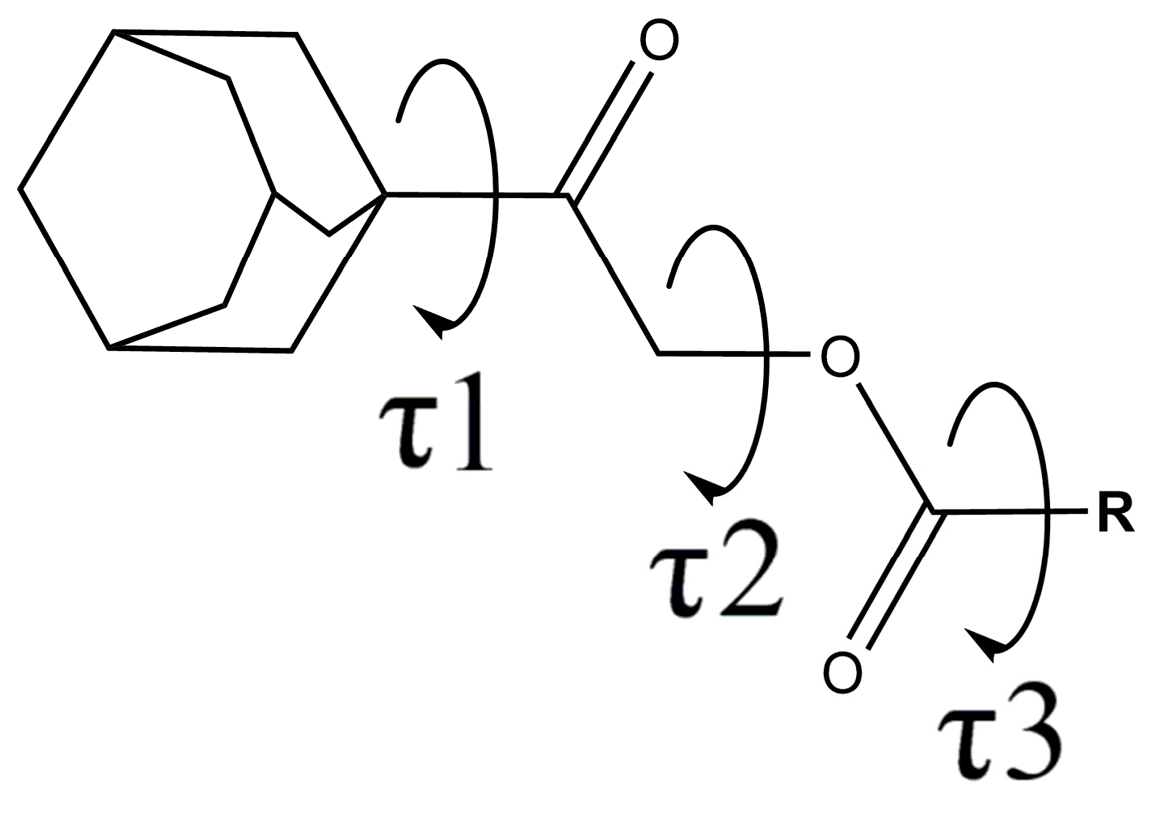
| Compound | Substituent | Torsion Angles C11—C12—O1—C13, τ2 | Torsion Angles O1—C13—C14—C15, τ3 |
|---|---|---|---|
| 2a | Benzene | −81.31, 75.84 | −9.95, 2.84 |
| 2b | 2-Chlorobenzene | 78.96, 75.23 | 11.55, −3.78 |
| 2c | 3-Chlorobenzene | 73.10 | 3.22 |
| 2d | 4-Chlorobenzene | 73.75 | −179.57 |
| 2e | 2,4-Dichlorobenzene | −69.7, −85.50 | 138.13, 135.26 |
| 2f | 2-Methylbenzene | −76.09, 77.77 | −9.58, 6.64 |
| 2g | 3-Methylbenzene | −72.91 | 1.25 |
| 2h | 4-Methylbenzene | 75.42 | −170.83 |
| 2i | 2-Methoxybenzene | −86.12, −79.34 | 39.08, 156.87 |
| 2j | 3-Methoxybenzene | −75.05 | −173.58 |
| 2k | 4-Methoxybenzene | −76.60 | 168.36 |
| 2l | 2-Nitrobenzene | −70.96, −70.23 | 126.4, 130.00 |
| 2n | 4-Nitrobenzene | 73.57 | −1.86 |
| 2o | 2-Aminobenzene | −76.5 | 171.92 |
| 2p | 3-Aminobenzene | 77.13 | −17.86 |
| 2r | 2-Pyridine | −75.07 | −17.79, 22.32 |
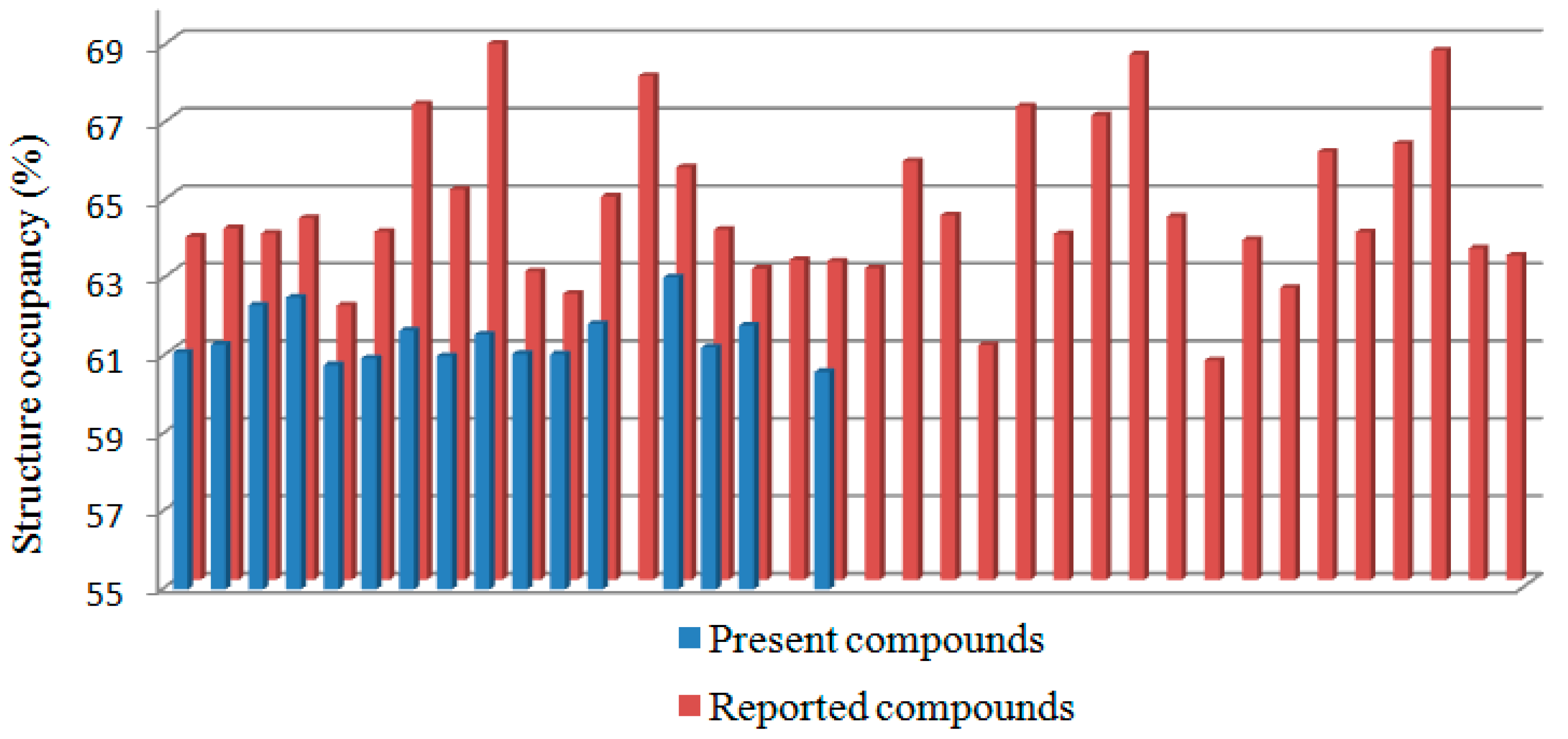
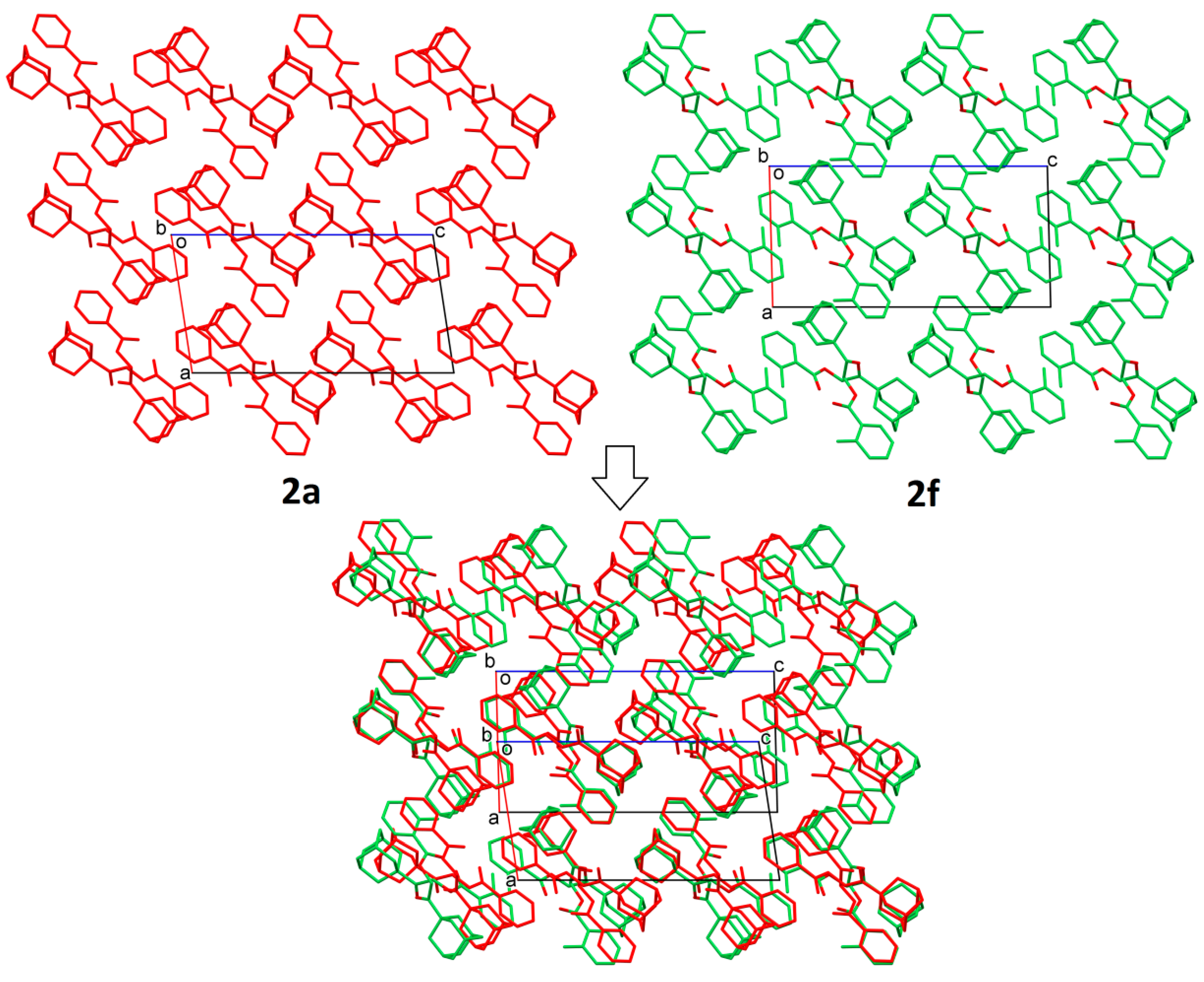
2.2. Structural Occupancy and Crystal Packing Similarity
| Compound | Packing Coefficient (%) | Compound | Packing Coefficient (%) | Compound | Packing Coefficient (%) |
|---|---|---|---|---|---|
| 2a | 61.11 | CIXVUC [20] | 63.94 | GITHUN [21] | 64.40 |
| 2b | 61.32 | CIXWAJ [20] | 64.33 | MANGIR [22] | 61.06 |
| 2c | 62.33 | CIXWEN [20] | 62.08 | OBOYIP [23] | 67.22 |
| 2d | 62.53 | CIXWIR [20] | 63.98 | OCAKUA [24] | 63.92 |
| 2e | 60.79 | CIYCAQ [20] | 67.27 | OCAQUG [25] | 66.98 |
| 2f | 60.97 | CIYCEU [20] | 65.07 | OCEFEJ [26] | 68.55 |
| 2g | 61.68 | CIYCIY [20] | 68.83 | PECZAA [27] | 64.37 |
| 2h | 61.02 | CIYCOE [20] | 62.96 | PODQIK [28] | 60.66 |
| 2i | 61.58 | CIYFUN [20] | 62.38 | PODRAD [29] | 63.77 |
| 2j | 61.08 | CIYGAU [20] | 64.89 | USIWID [30] | 62.53 |
| 2k | 61.07 | EVAFOX [31] | 68.00 | USIWOJ [32] | 66.04 |
| 2l | 61.85 | EVAJAN [33] | 65.64 | VOBYUI [34] | 63.97 |
| 2n | 63.05 | EVAJIV [35] | 64.03 | YAFWEJ [36] | 66.25 |
| 2o | 61.24 | EVAZEH [37] | 63.03 | YAFZAI [38] | 68.65 |
| 2p | 61.81 | EVEGIW [39] | 63.25 | YAHGUL [40] | 63.55 |
| 2r | 60.61 | EVEGOC [41] | 63.22 | YAHYOX [42] | 63.37 |
| AZULUD [43] | 63.85 | EVEVEH [44] | 63.04 | ||
| CIQNEW [45] | 64.07 | GARCEJ [46] | 65.80 |
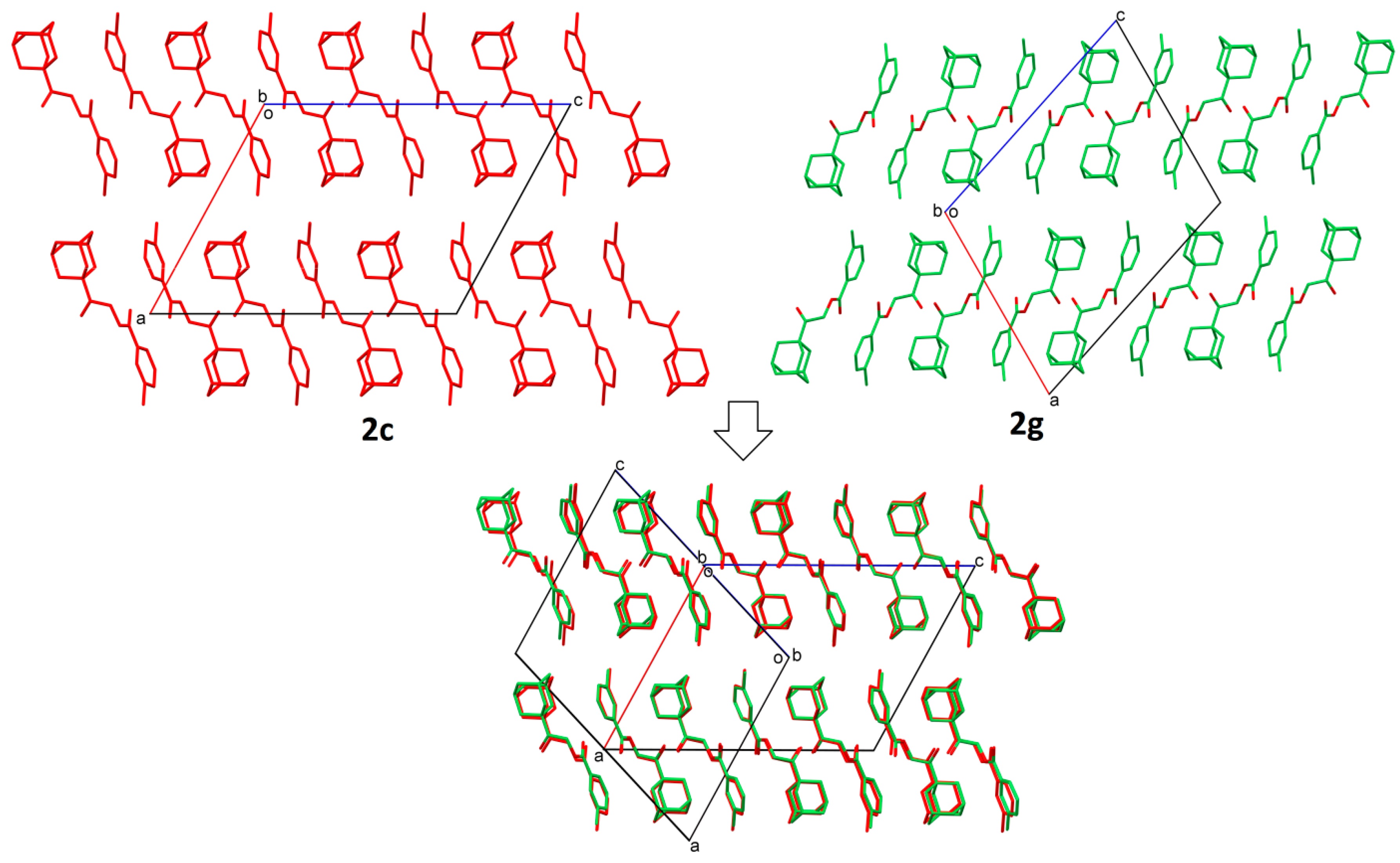
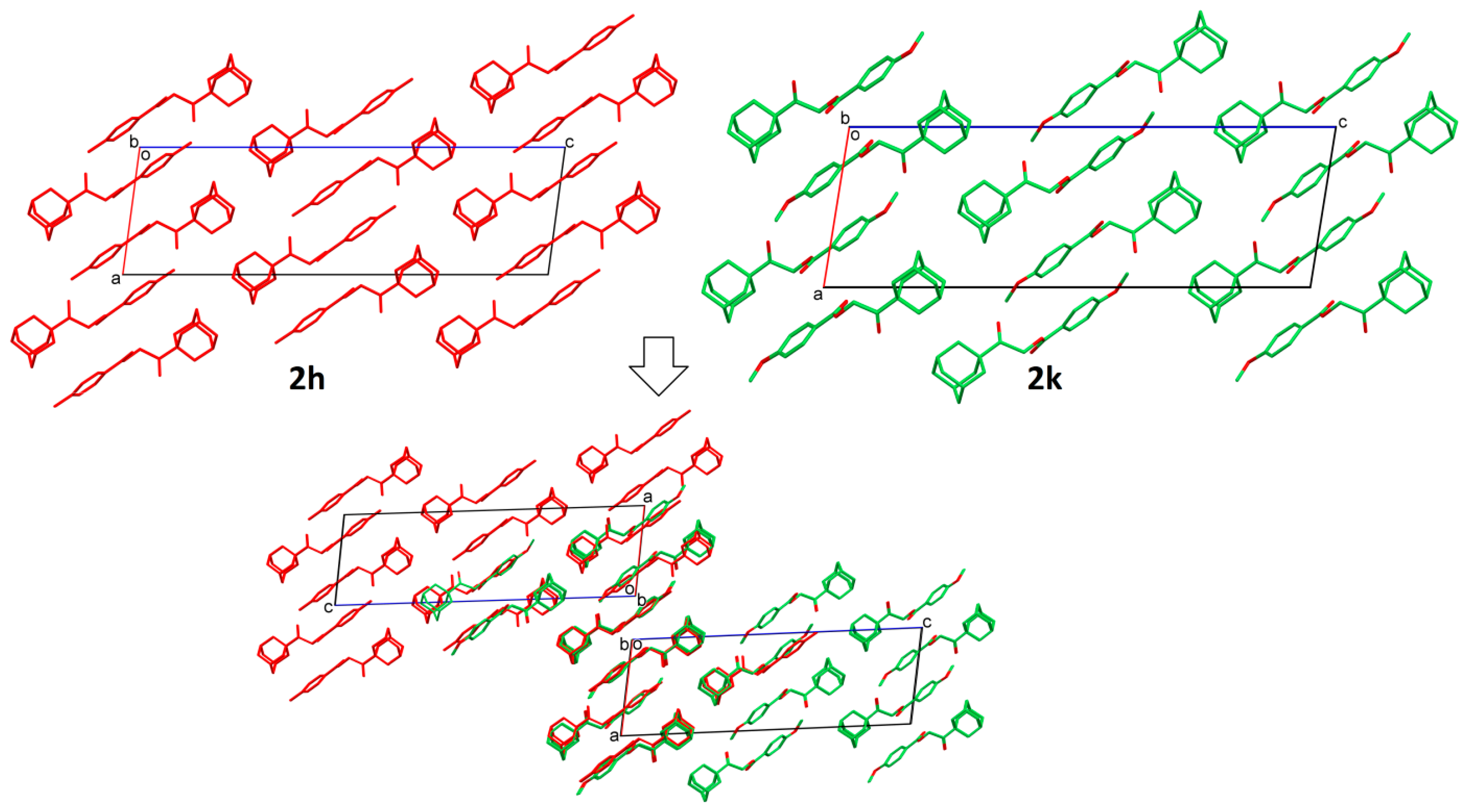
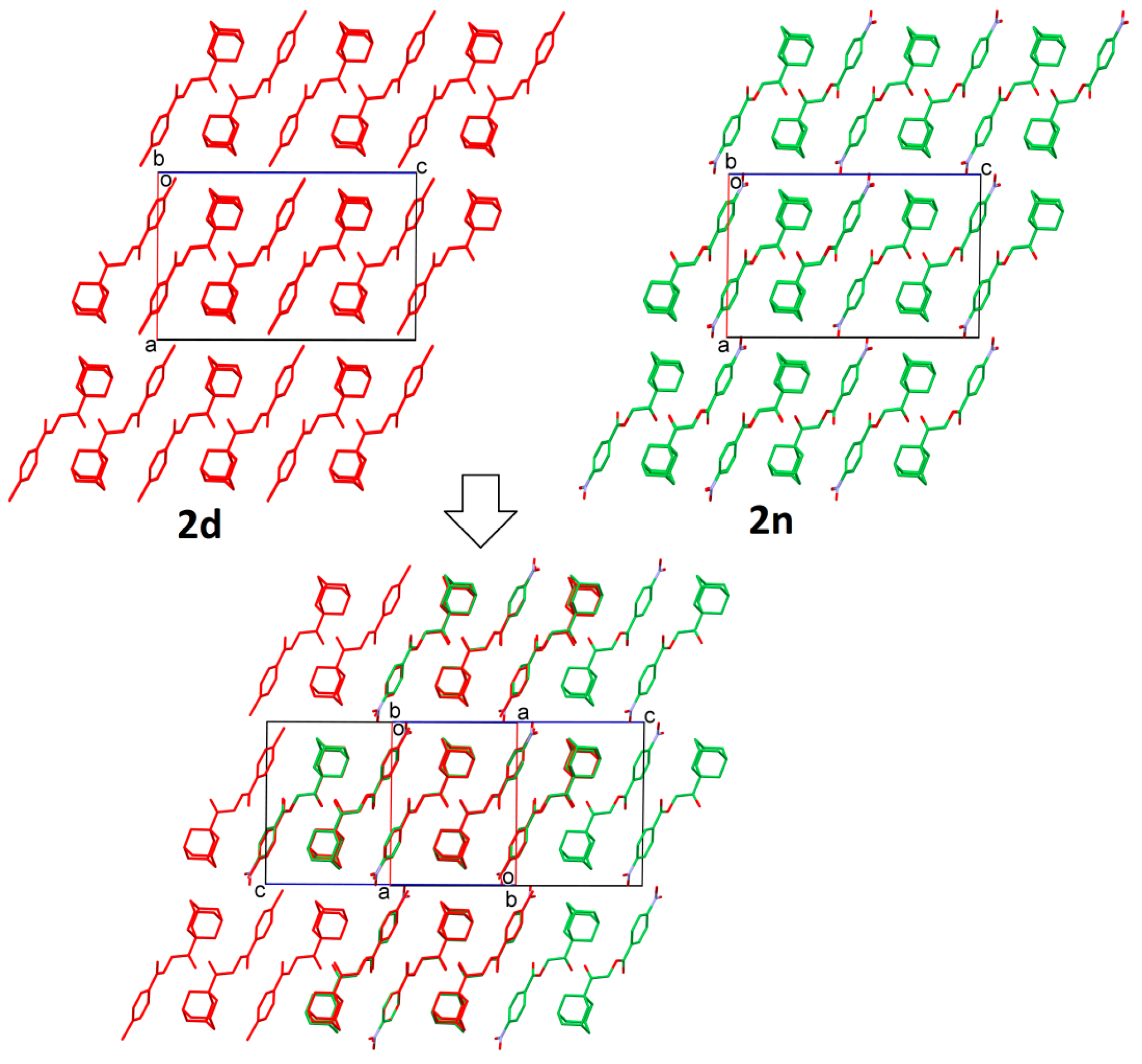
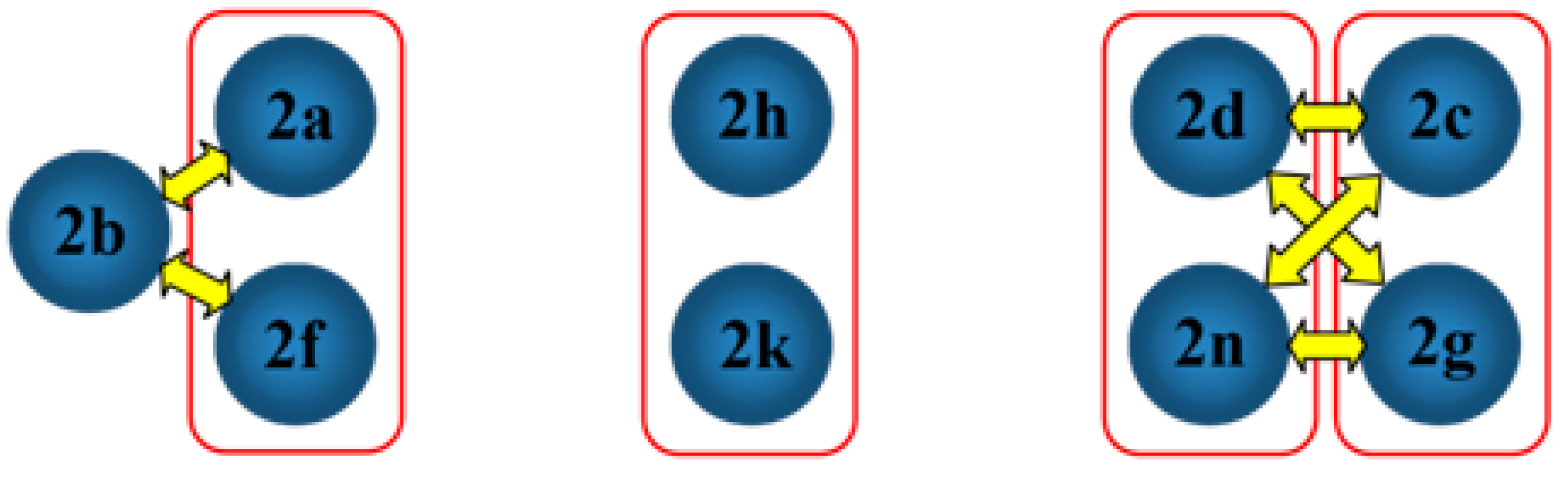
2.3. Antioxidant and Anti-Inflammatory Properties
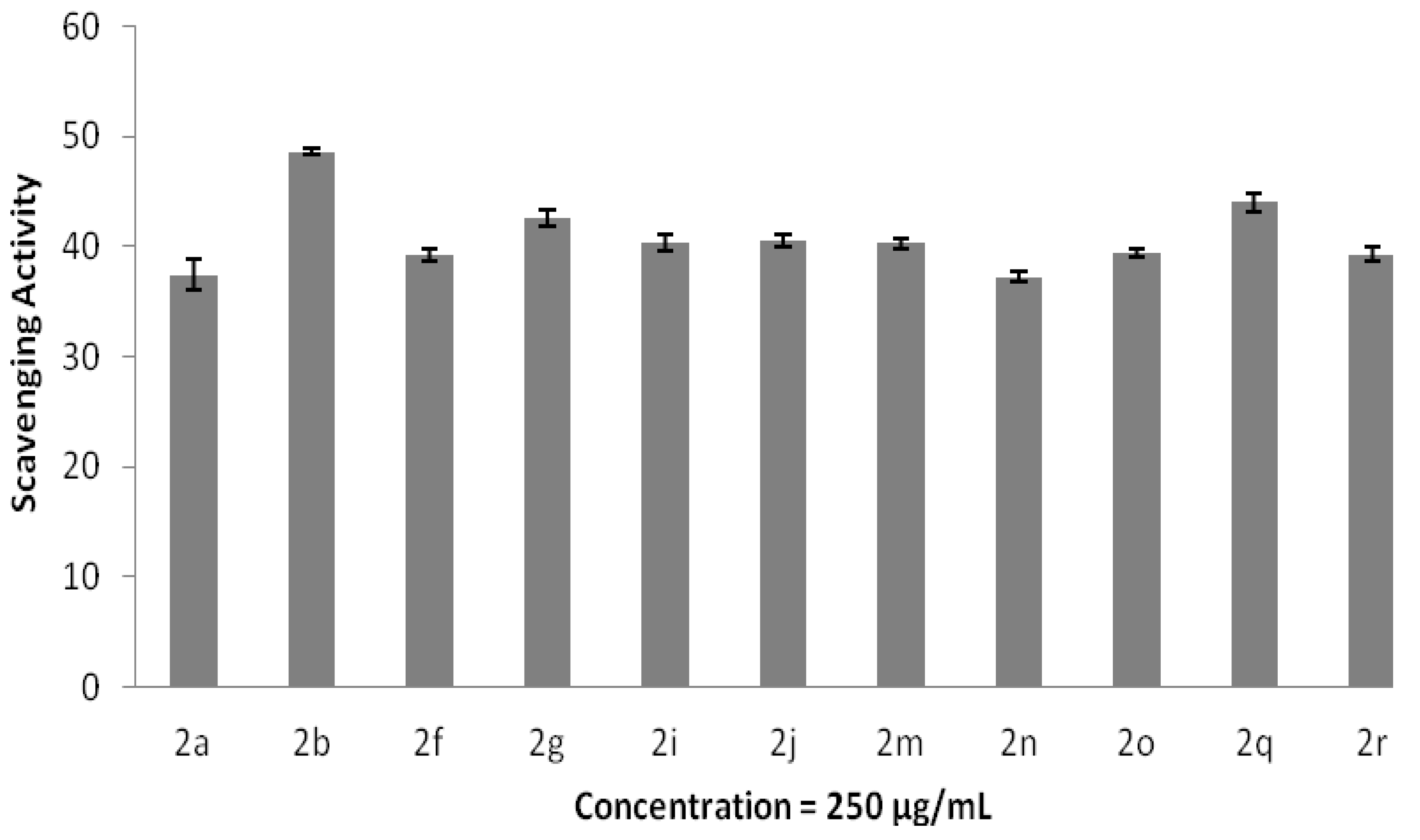
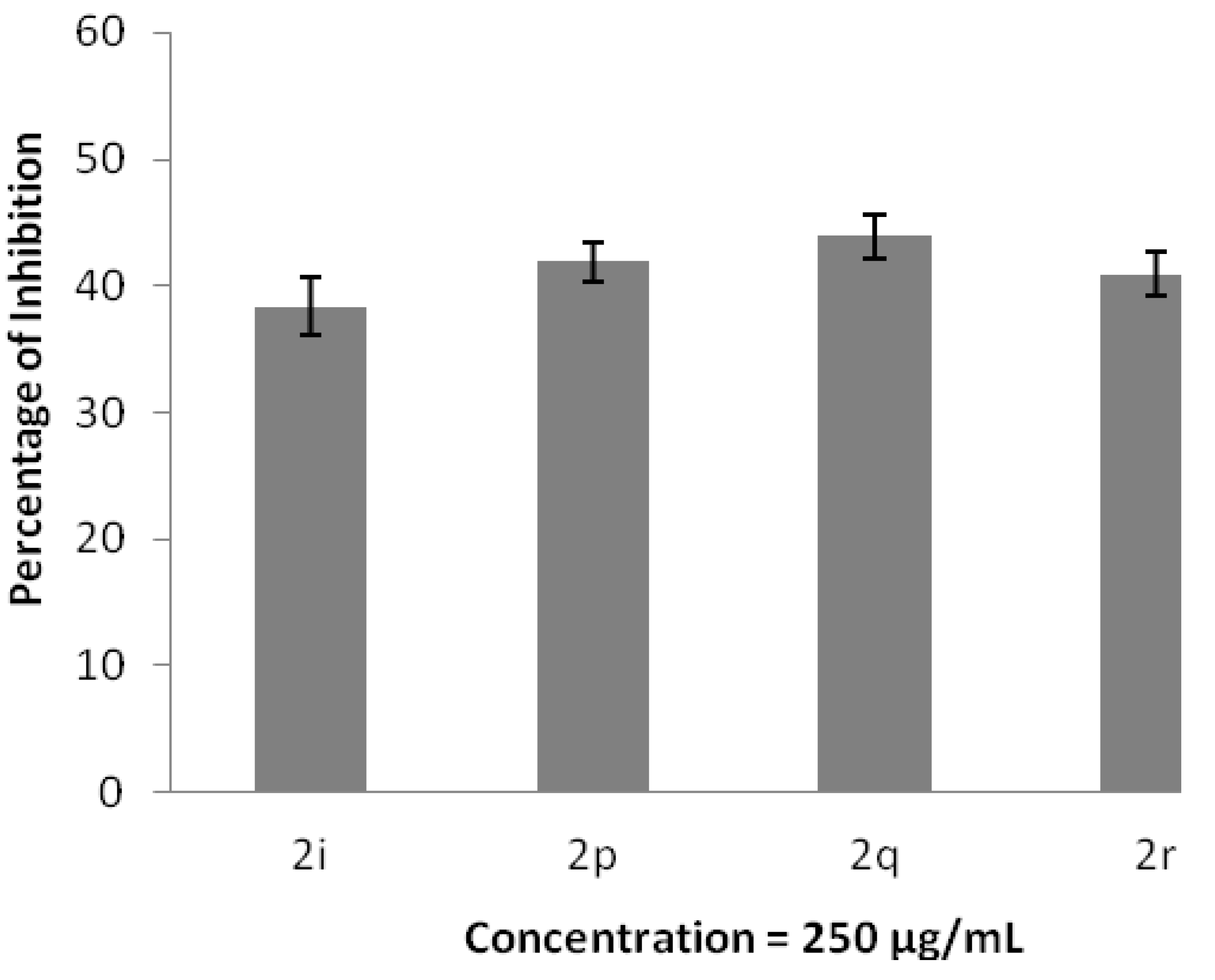
3. Experimental Section
3.1. Synthesis
3.2. Spectroscopic Details
3.3. Bioactivity Methods
3.3.1. Hydrogen Peroxide Radical Scavenging Assay
3.3.2. DPPH Radical Scavenging Assay
3.3.3. Protein Denaturation Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contribution
Conflicts of Interest
References
- Piérard, G.E.; Piérard-Franchimont, C.; Paquet, P.; Quatresooz, P. Spotlight on adapalene. Expert Opin. Drug Metab. Toxicol. 2009, 5, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Reisberg, B.; Doody, R.; Stöffler, A.; Schmitt, F.; Ferris, S.; Möbius, H.J. Memantine in moderate-to-severe alzheimer’s disease. N. Engl. J. Med. 2003, 348, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, K.S.; Sokol, M.S.; Ingram, R.L.; Subramanian, R.; Fort, R.C. Tromantadine: Inhibitor of early and late events in herpes simplex virus replication. Antimicrob. Agents Chemother. 1982, 22, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.M.; Miles, M.A.; Skinner, A.C. The anti-influenza virus drug rimantadine has trypanocidal activity. Antimicrob. Agents Chemother. 1999, 43, 985–987. [Google Scholar] [PubMed]
- De Clercq, E. Antiviral agents active against influenza a viruses. Nat. Rev. Drug Discov. 2006, 5, 1015–1025. [Google Scholar] [PubMed]
- Cady, S.D.; Luo, W.; Hu, F.; Hong, M. Structure and function of the influenza a M2 proton channel. Biochemistry 2009, 48, 7356–7364. [Google Scholar] [CrossRef] [PubMed]
- Zoidis, G.; Kolocouris, N.; Kelly, J.M.; Prathalingam, S.R.; Naesens, L.; de Clercq, E. Design and synthesis of bioactive adamantanaminoalcohols and adamantanamines. Eur. J. Med. Chem. 2010, 45, 5022–5030. [Google Scholar] [CrossRef] [PubMed]
- Von Geldern, T.W.; Trevillyan, J.M. The next big thing in diabetes: Clinical progress on DPP-IV inhibitors. Drug Dev. Res. 2006, 67, 627–642. [Google Scholar] [CrossRef]
- Havale, S.H.; Pal, M. Medicinal chemistry approaches to the inhibition of dipeptidyl peptidase-4 for the treatment of type 2 diabetes. Bioorg. Med. Chem. 2009, 17, 1783–1802. [Google Scholar] [CrossRef] [PubMed]
- Zettl, H.; Schubert-Zsilavecz, M.; Steinhilber, D. Medicinal chemistry of incretin mimetics and DPP-4 inhibitors. ChemMedChem 2010, 5, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Obando, D.; Liao, V.; Lifa, T.; Codd, R. The many faces of the adamantyl group in drug design. Eur. J. Med. Chem. 2011, 46, 1949–1963. [Google Scholar] [CrossRef] [PubMed]
- Al-Omar, M.A.; Al-Abdullah, E.S.; Shehata, I.A.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial, and anti-inflammatory activities of novel 5-(1-adamantyl)-4-arylideneamino-3-mercapto-1,2,4-triazoles and related derivatives. Molecules 2010, 15, 2526–2550. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, G.; Russier, J.; Dumortier, H.; Bianco, A. Enhancement of anti-inflammatory drug activity by multivalent adamantane-based dendrons. Biomaterials 2012, 33, 5610–5617. [Google Scholar] [CrossRef] [PubMed]
- Antoniadou-Vyza, E.; Avramidis, N.; Kourounakis, A.; Hadjipetrou, L. Anti-inflammatory properties of new adamantane derivatives. Design, synthesis, and biological evaluation. Arch. Pharm. 1998, 331, 72–78. [Google Scholar] [CrossRef]
- Priyanka, B.; Anitha, K.; Shirisha, S.; Dipankar, B.; Rajesh, K. Evaluation of anti-oxidant activity of ethanolic root extract of albizla lebbeck l. Int. Res. J. Pharm. Appl. Sci. 2013, 3, 93–101. [Google Scholar]
- Joyce, D.A. Oxygen radicals in disease. Advers. Drug React. Bull. 1987, 127, 476–479. [Google Scholar] [CrossRef]
- Farber, J.L. Mechanisms of cell injury by activated oxygen species. Environ. Health Perspect. 1994, 102, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Repasky, E.A. Opposing roles for heat and heat shock proteins in macrophage functions during inflammation: A function of cell activation state? Front. Immunol. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Saso, L.; Valentini, G.; Casini, M.; Grippa, E.; Gatto, M.; Leone, M.; Silvestrini, B. Inhibition of heat-induced denaturation of albumin by nonsteroidal antiinflammatory drugs (NSAIDs): Pharmacological implications. Arch. Pharm. Res. 2001, 24, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Kumar Chandraju Sadolalu, C.; Chia Tze, S.; Ooi Chin, W.; Quah Ching, K.; Chandraju, S.; Fun, H.K. Conformational studies of 2-(4-bromophenyl)-2-oxoethyl benzoates. Z. Kristallogr. Cryst. Mater. 2014, 229. [Google Scholar] [CrossRef]
- Jin, Y.; Guo, J.N.; Lin, K.; Tang, G.; Zhao, Y.F. Benzoylmethyl 4-chlorobenzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2008, 64. [Google Scholar] [CrossRef] [PubMed]
- Komarov, I.V.; Gorichko, M.V.; Shishkin, O.V.; Kornilov, M.Y. Short communications—Unusual by-product in the bromination of 3,3-dibromocamphor. Russ. J. Org. Chem. 1999, 35, 1388–1389. [Google Scholar]
- Fun, H.K.; Shahani, T.; Garudachari, B.; Isloor, A.M.; Satyanarayan, M.N. 2-(4-bromophenyl)-2-oxoethyl 4-methylbenzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67. [Google Scholar] [CrossRef]
- Fun, H.K.; Quah, C.K.; Vijesh, A.M.; Isloor, A.M.; Arulmoli, T. 2-(4-chlorophenyl)-2-oxoethyl 3,4-dimethoxybenzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Chia, T.S.; Shenvi, S.; Isloor, A.M.; Garudachari, B. 2-(2,4-dichlorophenyl)-2-oxoethyl 4-methoxybenzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Loh, W.S.; Garudachari, B.; Isloor, A.M.; Satyanarayan, M.N. 2-(4-fluorophenyl)-2-oxoethyl 4-methoxybenzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ibrar, A.; Korzanski, A.; Kubicki, M. 2-(4-methylphenyl)-2-oxoethyl 3-bromobenzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68. [Google Scholar] [CrossRef] [PubMed]
- Chidan Kumar, C.S.; Yohannan Panicker, C.; Fun, H.-K.; Sheena Mary, Y.; Harikumar, B.; Chandraju, S.; Quah, C.K.; Ooi, C.W. Molecular structure, FT-IR, first order hyperpolarizability, NBO analysis, HOMO and LUMO analysis of 2-(4-chlorophenyl)-2-oxoethyl 3-methylbenzoate by HF and density functional methods. Spectrochim. Acta A 2014, 128, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Chidan Kumar, C.S.; Panicker, C.Y.; Fun, H.-K.; Mary, Y.S.; Harikumar, B.; Chandraju, S.; Quah, C.K.; Ooi, C.W. FT-IR, molecular structure, first order hyperpolarizability, HOMO and LUMO analysis, MEP and NBO analysis of 2-(4-chlorophenyl)-2-oxoethyl 3-nitrobenzoate. Spectrochim. Acta A 2014, 126, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Arshad, S.; Garudachari, B.; Isloor, A.M.; Satyanarayan, M.N. 2-oxo-2-phenylethyl benzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Arshad, S.; Garudachari, B.; Isloor, A.M.; Satyanarayan, M.N. 2-(4-bromophenyl)-2-oxoethyl 4-bromobenzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Loh, W.S.; Garudachari, B.; Isloor, A.M.; Satyanarayan, M.N. 2-(4-bromophenyl)-2-oxoethyl 4-methoxybenzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67. [Google Scholar] [CrossRef]
- Fun, H.K.; Loh, W.S.; Garudachari, B.; Isloor, A.M.; Satyanarayan, M.N. 2-(4-chlorophenyl)-2-oxoethyl 3-(trifluoromethyl)benzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67. [Google Scholar] [CrossRef] [PubMed]
- Chidan Kumar, C.S.; Fun, H.K.; Tursun, M.; Ooi, C.W.; Chandraju, S.; Quah, C.K.; Parlak, C. Synthesis, molecular structure, FT-IR and XRD investigations of 2-(4-chlorophenyl)-2-oxoethyl 2-chlorobenzoate: A comparative DFT study. Spectrochim. Acta A 2014, 124, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Arshad, S.; Garudachari, B.; Isloor, A.M.; Satyanarayan, M.N. 2-(4-chlorophenyl)-2-oxoethyl 2,4-difluorobenzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Arshad, S.; Garudachari, B.; Isloor, A.M.; Shivananda, K.N. 2-(4-fluorophenyl)-2-oxoethyl 3-(trifluoromethyl)benzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Asik, S.I.J.; Garudachari, B.; Isloor, A.M.; Satyanarayan, M.N. 2-(4-chlorophenyl)-2-oxoethyl 2-methoxybenzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Loh, W.S.; Garudachari, B.; Isloor, A.M.; Satyanarayana, M.N. 2-(4-bromophenyl)-2-oxoethyl 4-hydroxybenzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Yeap, C.S.; Garudachari, B.; Isloor, A.M.; Satyanarayan, M.N. 2-(4-bromophenyl)-2-oxoethyl 4-chlorobenzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Loh, W.S.; Garudachari, B.; Isloor, A.M.; Satyanarayana, M.N. 2-(4-chlorophenyl)-2-oxoethyl 4-methylbenzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Quah, C.K.; Garudachari, B.; Isloor, A.M.; Satyanarayan, M.N. 2-(4-bromophenyl)-2-oxoethyl 2-methoxybenzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67. [Google Scholar] [CrossRef]
- Fun, H.K.; Ooi, C.W.; Garudachari, B.; Isloor, A.M.; Satyanarayan, M.N. 2-(4-bromophenyl)-2-oxoethyl 2-methylbenzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67. [Google Scholar] [CrossRef]
- Fun, H.K.; Shahani, T.; Garudachari, B.; Isloor, A.M.; Shivananda, K.N. 2-(4-chlorophenyl)-2-oxoethyl 4-hydroxybenzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Shahani, T.; Garudachari, B.; Isloor, A.M.; Satyganarayan, M.N. 2-(4-chlorophenyl)-2-oxoethyl benzoate. Acta Crystallogr. Sect. E: Struct. Rep. Online 2011, 67. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Wang, X.L.; Gao, Y.X.; Tang, G.; Zhao, Y.F. Benzoylmethyl 4-methoxybenzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63. [Google Scholar] [CrossRef]
- Isloor, A.M.; Garudachari, B.; Satyanarayan, M.N.; Gerber, T.; Hosten, E.; Betz, R. 2-(4-fluorophenyl)-2-oxoethyl 2-methoxybenzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68. [Google Scholar] [CrossRef] [PubMed]
- Bruker. Apex2, Saint and Sadabs; Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Sheldrick, G.M. A short history of shelx. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Ruch, R.J.; Cheng, S.; Klaunig, J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from chinese green tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Farasat, M.; Khavari-Nejad, R.A.; Nabavi, S.M.B.; Namjooyan, F. Antioxidant activity, total phenolics and flavonoid contents of some edible green seaweeds from northern coasts of the persian gulf. Iran. J. Pharm. Res. 2014, 13, 163–170. [Google Scholar] [PubMed]
- Ashok Kumar, B.S.; Saran, G.; Harshada, R.; Manoj, B.; Archana, P.G. Evaluation of anti-arthritic activity of vitex negundo by in vitro protein denaturation method. J. Tradit. Med. 2014, 1, 1–3. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, C.S.C.; Kwong, H.C.; Mah, S.H.; Chia, T.S.; Loh, W.-S.; Quah, C.K.; Lim, G.K.; Chandraju, S.; Fun, H.-K. Synthesis and Crystallographic Insight into the Structural Aspects of Some Novel Adamantane-Based Ester Derivatives. Molecules 2015, 20, 18827-18846. https://doi.org/10.3390/molecules201018827
Kumar CSC, Kwong HC, Mah SH, Chia TS, Loh W-S, Quah CK, Lim GK, Chandraju S, Fun H-K. Synthesis and Crystallographic Insight into the Structural Aspects of Some Novel Adamantane-Based Ester Derivatives. Molecules. 2015; 20(10):18827-18846. https://doi.org/10.3390/molecules201018827
Chicago/Turabian StyleKumar, C. S. Chidan, Huey Chong Kwong, Siau Hui Mah, Tze Shyang Chia, Wan-Sin Loh, Ching Kheng Quah, Gin Keat Lim, Siddegowda Chandraju, and Hoong-Kun Fun. 2015. "Synthesis and Crystallographic Insight into the Structural Aspects of Some Novel Adamantane-Based Ester Derivatives" Molecules 20, no. 10: 18827-18846. https://doi.org/10.3390/molecules201018827






