A Systematic Review of the Anxiolytic-Like Effects of Essential Oils in Animal Models
Abstract
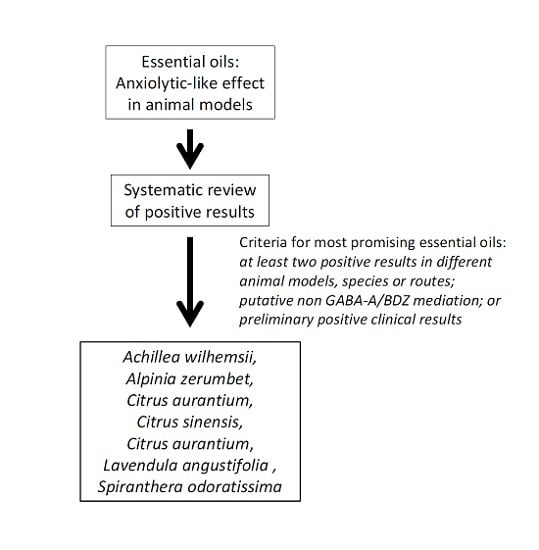
:1. Introduction
2. Results and Discussion
2.1. Abies sachalinensis
2.2. Acantholippia deserticola
2.3. Achillea umbelata
2.4. Achillea wilhemsii
| Essential Oils | Administration | Specie | Anxiety Model | Observed Effect | Motor Activity | Mechanism of Action | Observation | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Abies sachalinensis | Inhalation (acute) i.p. (acute) | Mouse | Elevated plus-maze | Anxiolytic-like No effect | Not evaluated | 10-min session DR+ | [12] | ||
| Acantholippia deserticola | i.p. | Rat | Elevated plus-maze | Anxiolytic-like? | Decrease | Female only Toxicity? DR+ | [13] | ||
| Achillea umbelata | Oral (acute) | Mice | Light/Dark | Anxiolytic-like? | Decrease | Toxicity? DR+ | [14] | ||
| Achillea wilhemsii | i.p. (acute) | Rat | Elevated plus-maze | Anxiolytic-like | Decrease? | Not mediated by BDZ Not mediated by opioid receptors | DR+ | [15] | |
| Alpinia zerumbet | i.p. (acute) | Mouse | Elevated plus-maze | No effect | Decrease | DR+ | [16] | ||
| Inhalation (acute) | Mouse | Elevated plus-maze | Anxiolytic-like | No change | Decrease 5-HTP and fluoxetine induced jumping (5-HT action) | Motor activity evaluated trough rearing DR+ | [17] | ||
| Inhalation (3 days) Inhalation (1 day) | Mouse | Elevated plus-maze Light/Dark | Anxiolytic-like No effect | No change No change | DR− | [18] | |||
| Inhalation (5–150 min) | Mouse | Elevated plus-maze | Anxiolytic-like | No change | Anxiolytic-like effect is dependent of duration of inhalation (30–120 min) DR+ | [12] | |||
| Angelica sinensis | Oral (acute) | Mouse | Elevated plus-maze Light/Dark Stress-induced hyperthermia | Anxiolytic-like Anxiolytic-like Anxiolytic-like | No change | Inverted U curve DR+ | [19] | ||
| Oral (acute) | Rat | Social Interaction | Putative Anxiolytic-like | Increase | Inverted U curve DR+ | [19] | |||
| Casimiroa pringlei | Oral (acute) | Rat | Elevated plus-maze Holeboard | No effect (compared to control) | No change | Positive effect when compared to caffeine only DR+ | [20] | ||
| Celastarus paniculatus | Oral (repeated) | Rat | Elevated plus-maze Vogel | Anxiolytic-like Anxiolytic-like | No change | [21] | |||
| Chamaecyparis obtusa | Inhaled (acute) | Mouse | Elevated plus-maze | Anxiolytic-like | Not evaluated | 10-min session DR+ | [22] | ||
| Inhaled (repeated) | Rat | Elevated plus-maze | Reversed anxiogenic-like effect of mother separation | Not evaluated | Associated with IL-6 and Ccl2 cytokines reductions | DR− | [23] | ||
| Citrus aurantium L. | Oral | Mouse | Elevated plus-maze Open Field | Anxiolytic-like | No change | DR+ | [24] | ||
| Oral | Mouse | Marble-burying Light/Dark | Anxiolytic-like | No change | DR+ | [25] | |||
| Inhalation | rats | Social interaction Open Field Elevated plus-maze | Anxiolytic-like | No change | DR+ | [26] | |||
| Oral | Mouse | Light/Dark | Anxiolytic-like | No change | 5-HT1A-receptors | DR+ | [27] | ||
| i.p. (acute) | Mouse | Elevated plus-maze | Anxiolytic-like | Not evaluated | DR+ | [28] | |||
| i.p. (acute) | Mouse | Elevated plus-maze | Anxiolytic-like | Not evaluated | GABA partial agonist? | Reduced diazepam anxiolytic-effect DR+ | [29] | ||
| Citrus aurantium subsp. bergamia | Rat | Elevated plus-maze | Anxiolytic-like? | Increase? | inverted U-shaped curve DR+ decrease increase corticosterone induced by behavioral test | [30] | |||
| Citrus junos | Inhalation | Mouse | Elevated plus-maze Light/Dark | Anxiolytic-like Anxiolytic-like | No change | DR+ | [31] | ||
| Coriandrum sativum var. microcarpum | Inhalation (repeated) | Rats | Elevated plus-maze | Decrease anxiogenic-like effect of icv beta-amyloid (1–42) | Not evaluated | Not tested in naive rats DR− | [32] | ||
| Lemon | Inhalation (continuous for 1 week) | Rat (male/female) | Elevated plus-maze | Anxiogenic-like | No change | DR− | [33] | ||
| Inhalation | Mouse | Elevated plus-maze | Anxiolytic-like? | Decrease | 5-HTergic (5-HT1A) GABA-A/BZP and DAminergic | DR− | [34] | ||
| Citrus limon | Oral (acute) | Mouse | Elevated plus-maze | Anxiolytic-like? | Decrease | inverted U-shaped curve DR+ | [35] | ||
| Citrus latifolia | Oral | Mouse | Marble-burying Light/Dark | Anxiolytic-like Anxiolytic-like | No change | inverted U-shaped curve DR+ | [36] | ||
| Citrus reticulata | Oral | Mouse | Marble-burying Light/Dark | Anxiolytic-like No effect | No change | DR+ | [36] | ||
| Citrus sinensis | Inhalation | Rat | Light/Dark Elevated plus-maze | Anxiolytic-like Anxiolytic-like | No change | Melaleuca alternifolia essential oil used as neutral odor control DR+ | [37] | ||
| Copaifera reticulata | Oral (acute) | Rat | Elevated plus-maze | Anxiolytic-like | No change | DR+ | [38] | ||
| Cymbopogon citratus | Oral | Mouse | Open Field Elevated plus-maze Light/Dark | Anxiolytic-like | No change | DR+ | [39] | ||
| Oral | Mouse | Marble-burying Light/Dark | Anxiolytic-like | No change | GABA-A /BDZ | DR+ | [40] | ||
| Dennettia tripetala | i.p. (acute) | Mice (male/Female) | Elevated plus-maze | Anxiolytic-like * (see observation) | Not evaluated | Inconsistency in data showed in figure and text Gender not considered DR+ | [41] | ||
| Ducrosia anethfolia | Oral (acute) | Mouse | Elevated plus-maze | Anxiolytic-like | No change | DR+ | [42] | ||
| Foeniculum vulgare | Oral | Mouse | Elevated plus-maze Staircase test Open-field | Anxiolytic-like Anxiolytic-like Anxiolytic-like | No change | inverted U-shaped curve DR+ | [43] | ||
| Lavandula officinalis | i.p. | Mouse | Geller conflict Vogel conflict | Anxiolytic-like Anxiolytic-like | No change | DR+ | [44] | ||
| Lavandula angustifolia | Inhalation | Mouse | Elevated plus-maze | No effect | DR− | [34] | |||
| Inhalation (24 h) Inhalation (7 days) | Gerbil (male/female) | Elevated plus-maze | Anxiolytic-like Anxiolytic-like | Anxiolytic in male and female Inclusion of ethological measures One-tailed test DR+ | [45] | ||||
| Lavandula angustifolia | Inhalation | rat | Open Field | Anxiolytic-like | Not evaluated (total locomotion) | Increase immobility (sedation) Inclusion one group also exposed during the open-field DR+ | [46] | ||
| Inhalation | rat | Open Field | Anxiolytic-like | No change | Reduction in c-fos increases with open field exposition DR− | [47] | |||
| Inhalation | Sheep | Reaction to stress (isolation) | Mixed results | change | Nervous sheep: anxiogenic; calm sheep: anxiolytic-like effect DR− | [48] | |||
| Inhalation | Mouse | Elevated plus-maze | Anxiolytic-like | No change | Anxiolytic-like effect correlated with linalool/linalyl acetate content DR− | [49] | |||
| Inhalation | Mouse | Elevated plus-maze | Anxiolytic-like | Similar effect in stressed and non-stressed mice DR− | [50] | ||||
| Inhalation | Mouse | Elevated plus-maze Marble-burying | Anxiolytic-like Anxiolytic-like | No change | Serotonergic system (5-HT1A) Not mediated by GABA-A/BDZ | Neutral odor control DR+ | [51] | ||
| Inhalation | Mouse | Marble-burying | Anxiolytic-like | No change | Similar effects in anosmic and normal mice DR+ | [52] | |||
| Lavandula angustifolia | Inhalation | Mouse | Elevated plus-maze | Anxiolytic-like | Increase hippocampal 5-HT turn-over | Similar effects in anosmic and normal mice DR− | [53] | ||
| Lavandula angustifolia (silexan) | i.p. (repeated) | rat | Elevated plus-maze Open Field Elevated zero-maze Social interaction test Novelty-induced suppressed feeding latency test | Anxiolytic-like Anxiolytic-like Anxiolytic-like Anxiolytic-like Anxiolytic-like | Mixed results | Some results could be influenced by motor activity changes DR+ | [54] | ||
| Oral (repeated) | Mouse | Elevated plus-maze | Anxiolytic-like | No change | Non selective inhibition of voltage operated calcium channels | DR+ | [55] | ||
| Lippia alba | i.p. | Mouse | Elevated-plus-maze | Anxiolytic-like | No change | DR+ | [56] | ||
| i.p. (acute) | Rat | Elevated T-maze | Anxiolytic-like | No change | DR+ | [57] | |||
| Litsea cubeba | Oral (7 days) | Mouse | Elevated plus-maze | Anxiolytic-like? | Decrease | DR+ | [58] | ||
| Ocimum gratissimum L. | Inhalation (acute) | Mouse | Light/Dark | Anxiolytic-like? | Decrease | inverted U-shaped curve DR+ | [59] | ||
| Ocimun sanctum L. | Inhalation (repeated) | Rat | elevated plus-maze | Decrease anxiogenic-like effect of icv beta-amyloid (1–42) | No change | Not tested in naive rats DR+ | [60] | ||
| Ocimum basilicum L. | Inhalation (repeated) | Rat | elevated plus-maze | Decrease anxiogenic-like effect of icv beta-amyloid (1–42) | No change | Not tested in naive rats DR+ | [60] | ||
| Piper guineense | Inhalation (acute) | Mouse | Light/Dark | Anxiolytic-like? | Mixed results | DR+ | [61] | ||
| Propolis | Oral (14 days) | Mouse | Elevated plus-maze | Anxiolytic-like | No change | Effect in stressed mice DR+ | [62] | ||
| Rat | Elevated plus-maze Open-field | Anxiolytic-like? | Increase | Female (estrous phase not specified) DR+ | [63] | ||||
| Rose centifolia | i.p. | Mouse | Geller conflict Vogel | Anxiolytic-like Anxiolytic-like | No change | DR+ | [64] | ||
| Rose | Inhaled | Rat | Elevated plus-maze | Anxiolytic-like | Not evaluated | DR+ | [65] | ||
| Inhalation (acute) | Mouse | Elevated plus-maze | No effect | No change | Ethanol as control DR− | [34] | |||
| Rose damascena | Prolonged Inhalation (24 h) Repeated Inhalation (14 days) | Gerbil | Elevated plus-maze Light/Dark | No effect Anxiolytic-like? | Mixed | DR− | [66] | ||
| Santalum album L. | Inhalation (acute) | Mouse | Elevated plus-maze | Anxiolytic-like in stressed mice | Not evaluated | No effect in non-stressed mice DR− | [67] | ||
| Spiranthera odoratissima A. St. Hil. | Oral (acute) | Mouse | Elevated plus-maze Light/Dark Open-field | Anxiolytic-like Anxiolytic-like Anxiolytic-like | No change | 5-HTergic (5-HT1A) Not mediated by GABA-A/BDZ | DR+ | [68] | |
| Stachys tibetica | Oral (3 days) Oral (7 days) | Rat | Elevated plus-maze Social Interaction Light/Dark Holeboard Elevated plus-maze | Anxiolytic-like Anxiolytic-like Anxiolytic-like Anxiolytic-like Anxiolytic-like | No change | DR+ | [69] | ||
| Thujopsis dolabrata | Elevated plus-maze | Anxiolytic-like | Decrease | 10 min session DR+ | [70] |
| Compound | Experimental Protocol | Anxiolytic-Like Effect and/or Mechanism | Animal Tested | Reference |
|---|---|---|---|---|
1,4-Cineole | Elevated plus-maze test Holeboard test | Increased exploration of the open arms Increased head dipping | Mice | [71] |
α-Asarone | Elevated plus-maze test Holeboard test Open field test | Decreases in open-arm exploration Increased time spent for head dips Increase in the total number of line crossings | Rats | [72] |
α-Pinene  | Elevated plus-maze test | Increased open arm exploration in the elevated plus maze | Mice | [73] |
β-Caryophyllene | Elevated plus-maze test Light/dark test Marble-burying test Open-field test | Increased open arm exploration Increased time spent in light side, and number of transitions Decreased number of marbles buried Increased time spent in the center | Mice | [68,74] |
Citral | Elevated plus-maze test | No effect was observed | Mice | [75] |
Myrcene | ||||
Carvacrol | Elevated plus-maze test | Increased % time spent and % entries in the open arms | Mice | [76] |
Carvacryl acetate | Elevated plus-maze test Light-dark box test Marble-burying test | Increased mouse motor activity Anxiolytic-like effect | Mice | [77] |
(R)-(-)-Carvone | Elevated-T maze test | Reduced avoidance latency without any effect in escape time | Rats | [57] |
Citronellol | Geller conflict test | Increased punished behavior at dose that did not change unpunished behavior | Mice | [64] |
2-Phenethyl alcohol | Vogel conflict tests | Increased punished licking | ||
(E)-Methyl isoeugenol | Elevated plus-maze test Light/dark test Open field test | Increased time spent and % entries in the open arms Increased number of transitions and time spent in light side Increased crossing of the center | Mice | [78] |
Nerol | Elevated plus-maze test Open field test Light/dark test Rota rod test | Increased number of entries and time of permanence in the open arms Decrease in motor activity Increased time of permanence in the room clear No modification in time spent or number of falls in the revolving bar | Mice | [79] |
Isopropyl N-methylanthranilate | Light/dark test Open field test | Increased time spent in the light side without effect on the number of crossing | Mice | [80] |
Methyl N-methylanthranilate | ||||
Isopulegol | Elevated plus-maze test Holeboard test Open field test | Increased number of entries and time spent in the open arms Increase number of head dips Did not change the number of crossing | Mice | [81] |
(R)-(+)-Limonene | Elevated plus-maze test Light/dark test | Increase in time spent and in the number of entries in the open arms Increased time spent in the light side of the light/dark apparatus | Mice | [31,82] |
(+)-Limonene epoxide | Marble burying test | Reduction in number of buried marbles | Mice | [83] |
Linalool | Light/dark test Elevated plus-maze test | Increased time spent in the light side and increased social interaction Increased number of visits to the open arms | Mice | [84] |
Linalool oxide | Light/dark test | Increased number of crossings and time spent in the light side | Mice | [85] |
Myrtenol | Elevated plus-maze test Light/dark test | Increase in open arm exploration Elevated time spent in the light side of light/dark apparatus | Rats | [86] |
Phytol | Elevated plus-maze test | Increased social interaction and decreased number of marbles buried | Mice | [87] |
Pulegone | Elevated plus-maze test Open field test Rota rod test Grasping test Conditioning place preference (CPP) test | Increased mouse motor activity Increased ambulatory activity of mice in a dose-dependent and bell-shaped manner Decreased performance on the Rota rod apparatus Decreased grasping strength Decreased percentage of time spent in the least preferred compartment | Mice | [88] |
Thymol | Brief mechanical restraint Open-field test | Reduced struggle latency and increased struggle bouts, which would be indicative of fear reduction No effect on motor activity | Quail | [89] |
Vanillin | Elevated plus-maze test Bright and dark arena | Increase in the percentile ratio of open arm to total arm entries and reduction in the time spent in the closed arms Increased number of bright chamber entries, time spent, and rears in bright arena | Rats | [90] |
2.5. Alpinia zerumbet
2.6. Angelica sinensis
2.7. Chamaecyparis obtusa
2.8. Casimiroa pringlei
2.9. Celastarus paniculatus
2.10. Citrus Genus
2.10.1. Citrus sp.
2.10.2. Citrus aurantium (Sour/Bitter Orange)
2.10.3. Citrus aurantium subsp. bergamia (Bergamot)
2.10.4. Citrus latifolia and C. reticulata
2.10.5. Citrus junos
2.10.6. Citrus limon
2.10.7. Citrus sinensis (Sweet Orange)
2.11. Copaifera reticulata Ducke
2.12. Coriandrum sativum Var. microcarpum
2.13. Cymbopogon citratus
2.14. Dennettia tripetala
2.15. Ducrosia anethifolia
2.16. Foeniculum vulgare
2.17. Lavendula angustifolia
2.18. Lippia alba
2.19. Litsea cubeba
2.20. Ocimum basilicum L. and Ocimun sanctum L. Essential Oil
2.21. Ocimum gratissimum L.
2.22. Piper guineense
2.23. Propolis
2.24. Rose
2.25. Santalum album L.
2.26. Spiranthera odoratissima A. St. Hil.
2.27. Stachys tibetica
2.28. Thujopsis dolabrata
2.29. Constituents from Essential Oils with Anxiolytic-Like Activity
2.29.1. 1,4-Cineole
2.29.2. α-Asarone
2.29.3. α-Pinene
2.29.4. β-Caryophyllene
2.29.5. Citral and Myrcene
2.29.6. Carvacrol
2.29.7. Carvacryl Acetate
2.29.8. (R)-(−)-Carvone
2.29.9. Citronellol and 2-Phenethyl Alcohol
2.29.10. (E)-Methyl Isoeugenol
2.29.11. Nerol
2.29.12. Isopropyl N-Methylanthranilate and Methyl N-Methylanthranilate
2.29.13. Isopulegol
2.29.14. (R)-(+)-Limonene and (+)-Limonene Epoxide
2.29.15. Linalool and Linalool Oxide
2.29.16. Myrtenol
2.29.17. Phytol
2.29.18. Pulegone
2.29.19. Thymol
2.29.20. Vanillin
2.30. General Discussion
3. Methodology
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nash, J.R.; Nutt, D.J. Pharmacotherapy of anxiety. Handb. Exp. Pharmacol. 2005, 169, 469–501. [Google Scholar]
- Eisenberg, D.M.; Davis, R.B.; Ettner, S.L.; Appel, S.; Wilkey, S.; van Rompay, M.; Kessler, R.C. Trends in alternative medicine use in the United States, 1990–1997: Results of a follow-up national survey. JAMA 1998, 280, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Andreatini, R.; Faustino, T.T.; Almeida, R.B. Medicinal plants for the treatment of generalized anxiety disorder: A review of controlled clinical studies. Rev. Bras. Psiquiatr. 2010, 32, 429–436. [Google Scholar]
- Kasper, S.; Gastpar, M.; Müller, W.E.; Volz, H.P.; Möller, H.J.; Dienel, A.; Schläfke, S. Efficacy and safety of silexan, a new, orally administered lavender oil preparation, in subthreshold anxiety disorder—Evidence from clinical trials. Wien Med. Wochenschr. 2010, 160, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Griebel, G.; Holmes, A. 50 years of hurdles and hope in anxiolytic drug discovery. Nat. Rev. Drug Discov. 2013, 12, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Tsang, H.W.; Ho, T.Y. A systematic review on the anxiolytic effects of aromatherapy on rodents under experimentally induced anxiety models. Rev. Neurosci. 2010, 21, 141–152. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, D.P. Anxiolytic essential oils. Nat. Prod. Chem. Res. 2012, 1, 102–102. [Google Scholar]
- Pimenta, F.C.F.; Correia, N.D.A.; Albuquerque, K.L.G.D.; de Sousa, D.P.; da Rosa, M.R.D.; Pimenta, M.B.F.; Diniz, M.F.F.M.; de Almeida, R.N. Naturally occurring anxiolytic substances from aromatic plants of genus citrus. J. Med. Plants Res. 2012, 6, 342–347. [Google Scholar] [CrossRef]
- De Sousa, D.P. Analgesic-like activity of essential oils constituents. Molecules 2011, 16, 2233–2252. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, R.N.; Agra, M.F.; Maior, F.N.S.; de Sousa, D.P. Essential oils and their constituents, anticonvulsant activity. Molecules 2011, 16, 2726–2742. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, H.M.; Wilkinson, J.M. Bio logical activities of lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Satou, T.; Matsuura, M.; Takahashi, M.; Umezu, T.; Hayashi, S.; Sadamoto, K.; Koike, K. Anxiolytic-like effect of essential oil extracted from Abies sachalinensis. Flavour Frag. J. 2011, 26, 416–420. [Google Scholar] [CrossRef]
- Benites, J.; Bustos, L.; Rios, D.; Bravo, F.; López, J.; Gajardo, S.; Rojo, L.; Buc-Calderon, P. Antidepressant and anxiolytic-like effects of essential oil from Acantholippia deserticola Phil in female rats. Bol. Latinoam. Caribe Plant. Med. Aromat. 2013, 12, 413–419. [Google Scholar]
- Radulović, N.S.; Dekić, M.S.; Ranđelović, P.J.; Stojanović, N.M.; Stojanović-Radić, Z.Z.; Zarubica, A.R. Toxic essential oils: anxiolytic, antinociceptive and antimicrobial properties of the yarrow Achillea umbellata Sibth. et Sm. (Asteraceae) volatiles. Food Chem. Toxicol. 2012, 50, 2016–2026. [Google Scholar] [CrossRef] [PubMed]
- Majnooni, M.B.; Mohammadi-Farani, A.; Gholivand, M.B.; Nikbakht, M.R.; Bahrami, G.R. Chemical composition and anxiolytic evaluation of Achillea wilhelmsii C. Koch essential oil in rat. Res Pharm. Sci. 2013, 8, 269–275. [Google Scholar] [PubMed]
- De Araújo, F.Y.; Silva, M.I.; Moura, B.A.; de oliveira, G.V.; Leal, L.K.; Vasconcelos, S.M.; Viana, G.S.; de Moraes, M.O.; de Sousa, F.C.; Macêdo, D.S. Central nervous system effects of the essential oil of the leaves of Alpinia zerumbet in mice. J. Pharm. Pharmacol. 2009, 61, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Matsuura, M.; Satou, T.; Hayashi, S.; Koike, K. Effects of the essential oil from leaves of Alpinia zerumbet on behavioral alterations in mice. Nat. Prod. Commun. 2009, 4, 129–132. [Google Scholar] [PubMed]
- Satou, T.; Murakami, S.; Matsuura, M.; Hayashi, S.; Koike, K. Anxiolytic effect and tissue distribution of inhaled Alpinia zerumbet essential oil in mice. Nat. Prod. Commun. 2010, 5, 143–146. [Google Scholar]
- Chen, S.W.; Min, L.; Li, W.J.; Kong, W.X.; Li, J.F.; Zhang, Y.J. The effects of angelica essential oil in three murine tests of anxiety. Pharmacol. Biochem. Behav. 2004, 79, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Landaverde, N.A.; Juárez-Flores, B.I.; Jiménez-Capdeville, M.E.; Ortiz-Pérez, M.D. Anxiolytic and sedative effects of essential oil from Casimiroa pringlei on Wistar rats. J. Med. Plants Res. 2009, 3, 791–798. [Google Scholar]
- Rajkumar, R.; Kumar, E.P.; Sudha, S.; Suresh, B. Evaluation of anxiolytic potential of Celastrus oil in rat models of behavior. Fitoterapia 2007, 78, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, H.; Hata, E.; Satou, T.; Yoshikawa, M.; Hayashi, S.; Masuo, Y.; Koike, K. Effect on emotional behavior and stress by inhalation of the essential oil from Chamaecyparis obtusa. Nat. Prod. Commun. 2013, 8, 515–518. [Google Scholar] [PubMed]
- Park, H.J.; Kim, S.K.; Kang, W.S.; Woo, J.M.; Kim, J.W. Effects of essential oil from Chamaecyparis obtusa on cytokine genes in the hippocampus of maternal separation rats. Can. J. Physiol. Pharmacol. 2014, 92, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Freitas, M.I.; Costa, M. Anxiolytic and sedative effects of extracts and essential oil from Citrus aurantium L. Biol. Pharm. Bull. 2002, 25, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Pultrini, A.M.; Galindo, L.A.; Costa, M. Effects of the essential oil from Citrus aurantium L. in experimental anxiety models in mice. Life Sci. 2006, 78, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.P.; Fassin, J.J.; Baziloni, E.M.F.; Almeida, R.N.; Mattei, R.; Leite, J.R. Behavioral effects of essential oil of Citrus aurantium L. inhalation in rats. Rev. Bras. Farmacogn. 2008, 18, 661–666. [Google Scholar] [CrossRef]
- Costa, C.A.; Cury, T.C.; Cassettari, B.O.; Takahira, R.K.; Flório, J.C.; Costa, M. Citrus aurantium L. essential oil exhibits anxiolytic-like activity mediated by 5-HT(1A)-receptors and reduces cholesterol after repeated oral treatment. BMC Complement. Altern. Med. 2013, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Saketi, S.; Bananej, M.; Jahromy, M.H. Effect of Citrus aurantium L. essential oil and its interaction with fluoxetine on anxiety in male mice. J. Behav. Brain Sci. 2014, 4, 285–290. [Google Scholar] [CrossRef]
- Khosravi, M.; Khakpour, S.; Adibi, L.; Jahromy, M.H. A study of the effect of Citrus aurantium L. essential oil on anxiety and its interaction with GABAergic pathways in male mice. J. Behav. Brain Sci. 2014, 4, 470–476. [Google Scholar] [CrossRef]
- Saiyudthong, S.; Marsden, C.A. Acute effects of bergamot oil on anxiety-related behavior and corticosterone level in rats. Phytother. Res. 2011, 25, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Satou, T.; Miyahara, N.; Murakami, S.; Hayashi, S.; Koike, K. Differences in the effects of essential oil from Citrus junos and (+)-limonene on emotional behavior in mice. J. Essent. Oil Res. 2012, 24, 493–500. [Google Scholar] [CrossRef]
- Cioanca, O.; Hritcu, L.; Mihasan, M.; Trifan, A.; Hancianu, M. Inhalation of coriander volatile oil increased anxiolytic-antidepressant-like behaviors and decreased oxidative status in beta-amyloid (1–42) rat model of Alzheimer’s disease. Physiol. Behav. 2014, 28, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, I.; Lariviere, W.R.; Fiorenzani, P.; Sacerdote, P.; Aloisi, A.M. Effects of long-term exposure of lemon essential oil odor on behavioral, hormonal and neuronal parameters in male and female rats. Brain Res. 2004, 1001, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Komiya, M.; Takeuchi, T.; Harada, E. Lemon oil vapor causes an anti-stress effect via modulating the 5-HT and DA activities in mice. Behav. Brain Res. 2006, 172, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.M.L.; Gonçalves, S.C.; de Almeida, A.A.; da Costa, J.P.; Marques, T.H.; Feitosa, C.M.; Saldanha, G.B.; de Freitas, R.M. Sedative, anxiolytic and antidepressant activities of Citrus limon (Burn) essential oil in mice. Pharmazie 2011, 66, 623–627. [Google Scholar]
- Gargano, A.C.; Almeida, C.A.R.; Costa, M. Essencial oils from Citrus latifolia and Citrus reticulata reduce anxiety and prolong ether sleeping time in mice. Tree For. Sci. Biotechnol. 2008, 2, 121–124. [Google Scholar]
- Faturi, C.B.; Leite, J.R.; Alves, P.B.; Canton, A.C.; Teixeira-Silva, F. Anxiolytic-like effect of sweet orange aroma in Wistar rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Curio, M.; Jacone, H.; Perrut, J.; Pinto, A.C.; Filho, V.F.; Silva, R.C. Acute effect of Copaifera reticulata Ducke copaiba oil in rats tested in the elevated plus-maze: An ethological analysis. J. Pharm. Pharmacol. 2009, 61, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.M.; Costa, C.A.; Freire, A.O.; Santos, J.G.J.; Costa, M. Neurobehavioral effect of essential oil of Cymbopogon citratus in mice. Phytomedicine 2009, 16, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.A.; Kohn, D.O.; de Lima, V.M.; Gargano, A.C.; Flório, J.C.; Costa, M. The GABAergic system contributes to the anxiolytic-like effect of essential oil from Cymbopogon citratus (lemongrass). J. Ethnopharmacol. 2011, 137, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Oyemitan, I.A.; Elusiyan, C.A.; Akanmu, M.A.; Olugbade, T.A. Hypnotic, anticonvulsant and anxiolytic effects of 1-nitro-2-phenylethane isolated from the essential oil of Dennettia tripetala in mice. Phytomedicine 2013, 20, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemi, V.; Rabbani, M.; Ghanadi, A.; Davari, E. Evaluation of antianxiety and sedative effects of essential oil of Ducrosia anethifolia in mice. Clinics 2010, 65, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Mesfin, M.; Asres, K.; Shibeshi, W. Evaluation of anxiolytic activity of the essential oil of the aerial part of Foeniculum vulgare Miller in mice. BMC Complement. Altern. Med. 2014, 14, 310. [Google Scholar] [CrossRef] [PubMed]
- Umezu, T.; Nagano, K.; Ito, H.; Kosakai, K.; Sakaniwa, M.; Morita, M. Anticonflict effects of lavender oil and identification of its active constituents. Pharmacol. Biochem. Behav. 2006, 85, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Bradley, B.F.; Starkey, N.J.; Brown, S.L.; Lea, R.W. Anxiolytic effects of Lavandula angustifolia odor on the Mongolian gerbil elevated plus maze. J. Ethnopharmacol. 2007, 111, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.; Annett, J.M.; Doherty, B.; Leslie, J.C. Anxiolytic effects of lavender oil inhalation on open-field behavior in rats. Phytomedicine 2007, 14, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.; Norwood, K.; Leslie, J.C. Chlordiazepoxide and lavender oil alter unconditioned anxiety-induced c-fos expression in the rat brain. Behav. Brain Res. 2011, 224, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hawken, P.A.; Fiol, C.; Blache, D. Genetic differences in temperament determine whether lavender oil alleviates or exacerbates anxiety in sheep. Physiol. Behav. 2012, 105, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Satou, T.; Ohashi, M.; Hayashi, S.; Sadamoto, K.; Koike, K. Interspecies comparison of chemical composition and anxiolytic-like effects of lavender oils upon inhalation. Nat. Prod Commun. 2011, 6, 1769–1774. [Google Scholar] [PubMed]
- Takahashi, M.; Yoshino, A.; Yamanaka, A.; Asanuma, C.; Satou, T.; Hayashi, S.; Masuo, Y.; Sadamoto, K.; Koike, K. Effects of inhaled lavender essential oil on stress-loaded animals: Changes in anxiety-related behavior and expression levels of selected mRNAs and proteins. Nat. Prod. Commun. 2012, 7, 1539–1544. [Google Scholar] [PubMed]
- Chioca, L.R.; Ferro, M.M.; Baretta, I.P.; Oliveira, S.M.; Silva, C.R.; Ferreira, J.; Losso, E.M.; Andreatini, R. Anxiolytic-like effect of lavender essential oil inhalation in mice: participation of serotonergic but not GABAA/benzodiazepine neurotransmission. J. Ethnopharmacol. 2013, 147, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Chioca, L.R.; Antunes, V.D.; Ferro, M.M.; Losso, E.M.; Andreatini, R. Anosmia does not impair the anxiolytic-like effect of lavender essential oil inhalation in mice. Life Sci. 2013, 92, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Yamanaka, A.; Asanuma, C.; Asano, H.; Satou, T.; Koike, K. Anxiolytic-like effect of inhalation of essential oil from Lavandula officinalis: Investigation of changes in 5-HT turnover and involvement of olfactory stimulation. Nat. Prod. Commun. 2014, 9, 1023–1026. [Google Scholar] [PubMed]
- Kumar, V. Characterization of anxiolytic and neuropharmacological activities of Silexan. Wien Med. Wochenschr. 2013, 163, 89–94. [Google Scholar] [PubMed]
- Schuwald, A.M.; Nöldner, M.; Wilmes, T.; Klugbauer, N.; Leuner, K.; Müller, W.E. Lavender oil-potent anxiolytic properties via modulating voltage dependent calcium channels. PLoS ONE 2013, 8, 1–9. [Google Scholar]
- Vale, T.G.; Matos, F.J.; de Lima, T.C.; Viana, G.S. Behavioral effects of essential oils from Lippia alba (Mill.) N.E. Brown chemotypes. J. Ethnopharmacol. 1999, 67, 127–133. [Google Scholar] [CrossRef]
- Hatano, V.Y.; Torricelli, A.S.; Giassi, A.C.; Coslope, L.A.; Viana, M.B. Anxiolytic effects of repeated treatment with an essential oil from Lippia alba and (R)-(−)-carvone in the elevated T-maze. Braz. J. Med. Biol. Res. 2012, 45, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Tseng, Y.H.; Chu, F.H.; Wen, T.Y.; Cheng, W.W.; Chen, Y.T.; Tsao, N.W.; Wang, S.Y. Neuropharmacological activities of fruit essential oil from Litsea cubeba Persoon. J. Wood Sci. 2012, 58, 538–543. [Google Scholar] [CrossRef]
- Tankam, J.M.; Ito, M. Sedative, anxiolytic and antidepressant-like effects of inhalation of the essential oil of Ocimum gratissimum L. from Cameroon in mice. J. Pharmacogn. Phytochem. 2014, 2, 1–9. [Google Scholar]
- Gradinariu, V.; Cioanca, O.; Hritcu, L.; Trifan, A.; Gille, E.; Hancianu, M. Comparative efficacy of Ocimum sanctum L. and Ocimum basilicum L. essential oils against amyloid beta (1–42)-induced anxiety and depression in laboratory rats. Phytochem. Rev. 2015, 14, 567–575. [Google Scholar] [CrossRef]
- Tankam, J.M.; Ito, M. Inhalation of the essential oil of Piper guineense from Cameroon shows sedative and anxiolytic-like effects in mice. Biol. Pharm. Bull. 2013, 36, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Xuan, H.Z.; Shou, Q.Y.; Zhan, Z.G.; Lu, X.; Hu, F.L. Therapeutic effects of propolis essential oil on anxiety of restraint-stressed mice. Hum. Exp. Toxicol. 2012, 31, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.S.; Oliveira, G.B.; Monteiro, M.C.; Machado, C.S.; Torres, Y.R.; Prediger, R.D.; Maia, C.S. Antidepressant- and anxiolytic-like activities of an oil extract of propolis in rats. Phytomedicine 2014, 21, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Umezu, T.; Ito, H.; Nagano, K.; Yamakoshi, M.; Oouchi, H.; Sakaniwa, M.; Morita, M. Anticonflict effects of rose oil and identification of its active constituents. Life Sci. 2002, 72, 91–102. [Google Scholar] [CrossRef]
- De Almeida, R.N.; Motta, S.C.; Faturi, C.B.; Catallani, B.; Leite, J.R. Anxiolytic-like effects of rose oil inhalation on the elevated plus-maze test in rats. Pharmacol. Biochem. Behav. 2004, 77, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Bradley, B.F.; Starkey, N.J.; Brown, S.L.; Lea, R.W. The effects of prolonged rose odor inhalation in two animal models of anxiety. Physiol. Behav. 2007, 92, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Satou, T.; Miyagawa, M.; Seimiya, H.; Yamada, H.; Hasegawa, T.; Koike, K. Prolonged anxiolytic-like activity of sandalwood (Santalum album L.) oil in stress-loaded mice. Flavour Frag. J. 2014, 29, 35–38. [Google Scholar] [CrossRef]
- Galdino, P.M.; Nascimento, M.V.; Florentino, I.F.; Lino, R.C.; Fajemiroye, J.O.; Chaibub, B.A.; de Paula, J.R.; de Lima, T.C.; Costa, E.A. The anxiolytic-like effect of an essential oil derived from Spiranthera odoratissima A. St. Hil. leaves and its major component, β-caryophyllene, in male mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 38, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Bhat, Z.A.; Kumar, V.; Khan, N.A.; Chashoo, I.A.; Zargar, M.I.; Shah, M.Y. Effects of Stachys tibetica essential oil in anxiety. Eur. J. Integr. Med. 2012, 4, 169–176. [Google Scholar] [CrossRef]
- Matsuura, T.; Yamaguchi, T.; Zaike, Y.; Yanagihara, K.; Ichinose, M. Reduction of the chronic stress response by inhalation of hiba (Thujopsis dolabrata) essential oil in rats. Biosci. Biotechnol. Biochem. 2014, 78, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.B.; Feitosa, M.L.; Silva, M.I.; Noronha, E.C.; Moura, B.A.; Venâncio, E.T.; Rios, E.R.; de Sousa, D.P.; de Vasconcelos, S.M.; Fonteles, M.M.; et al. Anxiolytic-like effect of the monoterpene 1,4-cineole in mice. Pharmacol. Biochem. Behav. 2010, 96, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Sur, B.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.H. Alpha-asarone, a major component of Acorus gramineus, attenuates corticosterone-induced anxiety-like behaviors via modulating TrkB signaling process. Korean J. Physiol. Pharmacol. 2014, 18, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Satou, T.; Kasuya, H.; Maeda, K.; Koike, K. Daily inhalation of α-pinene in mice: effects on behavior and organ accumulation. Phytother. Res. 2014, 28, 1284–1287. [Google Scholar] [CrossRef] [PubMed]
- Bahi, A.; Al Mansouri, S.; Al Memari, E.; Al Ameri, M.; Nurulain, S.M.; Ojha, S. β-Caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiol. Behav. 2014, 135, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Vale, T.G.; Furtado, E.C.; Santos, J.G.J.; Viana, G.S. Central effects of citral, myrcene and limonene, constituents of essential oil chemotypes from Lippia alba (Mill.) n.e. Brown. Phytomedicine 2002, 9, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Melo, F.H.; Venâncio, E.T.; de Sousa, D.P.; de França Fonteles, M.M.; de Vasconcelos, S.M.; Viana, G.S.; de Sousa, F.C. Anxiolytic-like effect of Carvacrol (5-isopropyl-2-methylphenol) in mice: involvement with GABAergic transmission. Fundam Clin. Pharmacol. 2010, 24, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.F.; Costa, L.M.; Silva, O.A.; de Almeida, A.A.C.; Cerqueira, G.S.; de Sousa, D.P.; de Freitas, R.M. Anxiolytic-like effects of carvacryl acetate, a derivative of carvacrol, in mice. Pharmacol. Biochem. Behav. 2013, 112, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Fajemiroyem, J.O.; Galdino, P.M.; de Paula, J.A.; Rocha, F.F.; Akanmu, M.A.; Vanderlinde, F.A.; Zjawiony, J.K.; Costa, E.A. Anxiolytic and antidepressant like effects of natural food flavour (E)-methyl isoeugenol. Food Funct. 2014, 5, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Marques, T.H.C.; Marques, M.L.B.G.C.B.; Lima, D.S.; Siqueira, H.D.S; Nogueira Neto, J.D.; Branco, M.S.B.G.C.; Araujo de Souza, A.; de Sousa, P.S. Evaluation of the neuropharmacological properties of nerol in mice. World J. Neurosci. 2013, 3, 32–38. [Google Scholar] [CrossRef]
- Radulović, N.S.; Miltojević, A.B.; Randjelović, P.J.; Stojanović, N.M.F. Effects of methyl and isopropyl N-methylanthranilates from Choisya ternata Kunth (Rutaceae) on experimental anxiety and depression in mice. Phytother. Res. 2013, 27, 1334–1338. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.I.; de Aquino, N.M.R.; Teixeira, N.P.F.; Moura, B.A.; do Amaral, J.F.; de Sousa, D.P.; Vasconcelos, S.M.; de Sousa, F.C. Central nervous system activity of acute administration of isopulegol in mice. Pharmacol. Biochem. Behav. 2007, 88, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Lima, N.G.; de Sousa, D.P.; Pimenta, F.C.; Alves, M.F.; de Souza, F.S.; Macedo, R.O.; Cardoso, R.B.; de Morais, L.C.; Melo Diniz, M.F.; de Almeida, R.N. Anxiolytic-like activity and GC-MS analysis of (R)-(+)-limonene fragrance, a natural compound found in foods and plants. Pharmacol. Biochem. Behav. 2013, 103, 450–454. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.A.; de Carvalho, R.B.; Silva, O.A.; de Sousa, D.P.; de Freitas, R.M. Potential antioxidant and anxiolytic effects of (+)-limonene epoxide in mice after marble-burying test. Pharmacol. Biochem. Behav. 2014, 118, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Linck, V.M.; da Silva, A.L.; Figueiró, M.; Caramão, E.B.; Moreno, P.R.; Elisabetsky, E. Effects of inhaled Linalool in anxiety, social interaction and aggressive behavior in mice. Phytomedicine 2010, 17, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Souto-Maior, F.N.; de Carvalho, F.L.; de Morais, L.C.; Netto, S.M.; de Sousa, D.P.; de Almeida, R.N. Anxiolytic-like effects of inhaled linalool oxide in experimental mouse anxiety models. Pharmacol. Biochem. Behav. 2011, 100, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.R.; Salvadori, M.G.; de Almeida, A.A.; de Sousa, D.P.; Jordán, J.; Satyal, P.; de Freitas, R.M.; de Almeida, R.N. Anxiolytic-like effects and mechanism of (−)-myrtenol: A monoterpene alcohol. Neurosci. Lett. 2014, 579, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.P.; de Oliveira, G.A.L.; de Almeida, A.A.C.; Islam, M.T.; de Sousa, D.P.; de Freitas, R.M. Anxiolytic-like effects of phytol: Possible involvement of GABAergic transmission. Brain Res. 2014, 1547, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira, N.S.; de Oliveira-Silva, G.; de Freitas, L.B.; da Silva Prado, L.C.; Bispo-da-Silva, L.B. The aversive, anxiolytic-like, and verapamil-sensitive psychostimulant effects of pulegone. Biol. Pharm. Bull. 2014, 37, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Lábaque, M.C.; Kembro, J.M.; Luna, A.; Marin, R.H. Effects of thymol feed supplementation on female Japanese quail (Coturnix coturnix) behavioral fear response. Anim. Feed Sci. Technol. 2013, 183, 67–72. [Google Scholar] [CrossRef]
- Bhagwat, V.; Chowta, M.N.; Shoeb, A.; Maskeri, R.; Venkatesh, V.; Rai, A. Evaluation of anxiolytic activity of vanillin in wistar albino rats. Int. J. Nut. Pharmacol. Neurol. Dis. 2013, 3, 96–101. [Google Scholar]
- Satou, T.; Kasuya, H.; Takahashi, M.; Murakami, S.; Hayashi, S.; Sadamoto, K.; Koike, K. Relationship between duration of exposure and anxiolytic-like effects of essential oil from Alpinia zerumbet. Flavour Frag. J. 2011, 26, 180–185. [Google Scholar] [CrossRef]
- Min, L.; Chen, S.W.; Li, W.J.; Wang, R.; Li, Y.L.; Wang, W.J.; Mi, X.J. The effects of angelica essential oil in social interaction and hole-board tests. Pharmacol. Biochem. Behav. 2005, 81, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Umezu, T. Anticonflict effects of plant-derived essential oils. Pharmacol. Biochem. Behav. 1999, 64, 35–40. [Google Scholar] [CrossRef]
- Ni, C.H.; Hou, W.H.; Kao, C.C.; Chang, M.L.; Yu, L.F.; Wu, C.C.; Chen, C. The anxiolytic effect of aromatherapy on patients awaiting ambulatory surgery, a randomized controlled trial. Evid. Based Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Morrone, L.A.; Rombolà, L.; Pelle, C.; Corasaniti, M.T.; Zappettini, S.; Paudice, P.; Bonanno, G.; Bagetta, G. The essential oil of bergamot enhances the levels of amino acid neurotransmitters in the hippocampus of rat: implication of monoterpene hydrocarbons. Pharmacol. Res. 2007, 55, 255–262. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, J.S.; Céspedes, I.C.; Abrão, R.O.; Dos Santos, T.B.; Diniz, L.; Britto, L.R.; Spadari-Bratfisch, R.C.; Ortolani, D.; Melo-Thomas, L.; et al. Chronic unpredictable mild stress alters an anxiety-related defensive response, Fos immunoreactivity and hippocampal adult neurogenesis. Behav. Brain Res. 2013, 250, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Goes, T.C.; Antunes, F.D.; Alves, P.B.; Teixeira-Silva, F. Effect of sweet orange aroma on experimental anxiety in humans. J. Altern. Complement. Med. 2012, 18, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Silenieks, L.B.; Koch, E.; Higgins, G.A. Silexan, an essential oil from flowers of Lavandula angustifolia, is not recognized as benzodiazepine-like in rats trained to discriminate a diazepam cue. Phytomedicine 2013, 20, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Woelk, H.; Schläfke, S. A multi-center, double-blind, randomised study of the Lavender oil preparation Silexan in comparison to Lorazepam for generalized anxiety disorder. Phytomedicine 2010, 17, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Kasper, S.; Gastpar, M.; Müller, W.E.; Volz, H.P.; Möller, H.J.; Schläfke, S.; Dienel, A. Lavender oil preparation Silexan is effective in generalized anxiety disorder-a randomized, double-blind comparison to placebo and paroxetine. Int. J. Neuropsychopharmacol. 2014, 17, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Baldinger, P.; Höflich, A.S.; Mitterhauser, M.; Hahn, A.; Rami-Mark, C.; Spies, M.; Wadsak, W.; Lanzenberger, R.; Kasper, S. Effects of Silexan on the serotonin-1A receptor and microstructure of the human brain: A randomized, placebo-controlled, double-blind, cross-over study with molecular and structural neuroimaging. Int. J. Neuropsychopharmacol. 2015, 18. [Google Scholar] [CrossRef] [PubMed]
- Heldwein, C.G.; Silva, L.L.; Reckziegel, P.; Barros, F.M.; Bürger, M.E.; Baldisserotto, B.; Mallmann, C.A.; Schmidt, D.; Caron, B.O.; Heinzmann, B.M. Participation of the GABAergic system in the anesthetic effect of Lippia alba (Mill.) N.E. Brown essential oil. Braz. J. Med. Biol. Res. 2012, 45, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Aoshima, H.; Hamamoto, K. Potentiation of GABAA Receptors expressed in Xenopus oocytes by perfume and phytoncid. Biosci. Biotechnol. Biochem. 1999, 63, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Lehrner, J.; Eckersberger, C.; Walla, P.; Pötsch, G.; Deecke, L. Ambient odor of orange in a dental office reduces anxiety and improves mood in female patients. Physiol. Behav. 2000, 71, 83–86. [Google Scholar] [CrossRef]
- Cline, M.; Taylor, J.E.; Flores, J.; Bracken, S.; McCall, S.; Ceremuga, T.E. Investigation of the anxiolytic effects of linalool, a lavender extract, in the male Sprague-Dawley rat. AANA J. 2008, 76, 47–52. [Google Scholar] [PubMed]
- Hossain, S.J.; Hamamoto, K.; Aoshima, H.; Hara, Y. Effects of tea components on the response of GABA(A) receptors expressed in Xenopus oocytes. J. Agric. Food Chem. 2002, 50, 3954–3960. [Google Scholar] [CrossRef] [PubMed]
- Brum, L.F.; Elisabetsky, E.; Souza, D. Effects of linalool on [3H]MK801 and [3H]muscimol binding in mouse cortical membranes. Phytother. Res. 2001, 15, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Einat, H. Modelling facets of mania—New directions related to the notion of endophenotypes. J. Psychopharmacol. 2006, 20, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Kritsidima, M.; Newton, T.; Asimakopoulou, K. The effects of lavender scent on dental patient anxiety levels: A cluster randomised-controlled trial. Community Dent. Oral Epidemiol. 2010, 38, 83–87. [Google Scholar] [CrossRef]
- Lister, R.G. Ethologically-based animal models of anxiety disorders. Pharmacol. Ther. 1990, 46, 321–340. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Johnson, N.J. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol. Biochem. Behav. 1995, 52, 297–303. [Google Scholar] [CrossRef]
- Bourin, M.; Hascoët, M. The mouse light/dark box test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- Carobrez, A.P.; Bertoglio, L.J. Ethological and temporal analyses of anxiety-like behavior: The elevated plus-maze model 20 years on. Neurosci. Biobehav. Rev. 2005, 29, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; González, M.I.; Wilson, C.A.; File, S.E. Factor analysis shows that female rat behavior is characterized primarily by activity, male rats are driven by sex and anxiety. Pharmacol. Biochem. Behav. 1999, 64, 731–738. [Google Scholar] [CrossRef]
- Lamprea, M.R.; Cardenas, F.P.; Setem, J.; Morato, S. Thigmotactic responses in an open-field. Braz. J. Med. Biol. Res. 2008, 41, 135–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robin, O.; Alaoui-Ismaïli, O.; Dittmar, A.; Vernet-Maury, E. Basic emotions evoked by eugenol odor differ according to the dental experience. A neurovegetative analysis. Chem. Senses 1999, 24, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Soudry, Y.; Lemogne, C.; Malinvaud, D.; Consoli, S.M.; Bonfils, P. Olfactory system and emotion: Common substrates. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds not are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Sousa, D.P.; Hocayen, P.D.A.S.; Andrade, L.N.; Andreatini, R. A Systematic Review of the Anxiolytic-Like Effects of Essential Oils in Animal Models. Molecules 2015, 20, 18620-18660. https://doi.org/10.3390/molecules201018620
De Sousa DP, Hocayen PDAS, Andrade LN, Andreatini R. A Systematic Review of the Anxiolytic-Like Effects of Essential Oils in Animal Models. Molecules. 2015; 20(10):18620-18660. https://doi.org/10.3390/molecules201018620
Chicago/Turabian StyleDe Sousa, Damião Pergentino, Palloma De Almeida Soares Hocayen, Luciana Nalone Andrade, and Roberto Andreatini. 2015. "A Systematic Review of the Anxiolytic-Like Effects of Essential Oils in Animal Models" Molecules 20, no. 10: 18620-18660. https://doi.org/10.3390/molecules201018620
APA StyleDe Sousa, D. P., Hocayen, P. D. A. S., Andrade, L. N., & Andreatini, R. (2015). A Systematic Review of the Anxiolytic-Like Effects of Essential Oils in Animal Models. Molecules, 20(10), 18620-18660. https://doi.org/10.3390/molecules201018620