Synthesis and 1,3-Dipolar Cycloaddition Reactions of Chiral Maleimides
Abstract
:Introduction
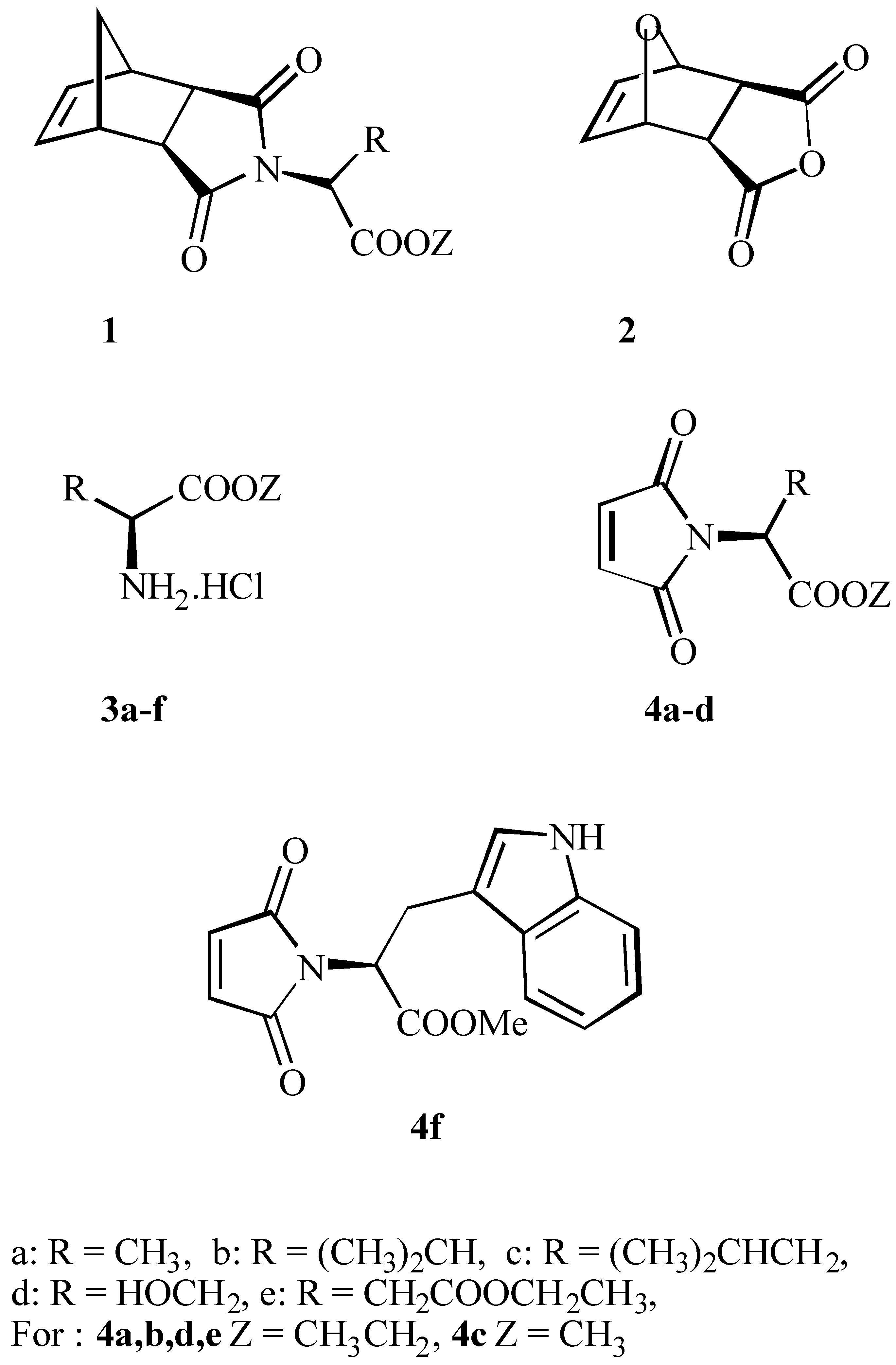
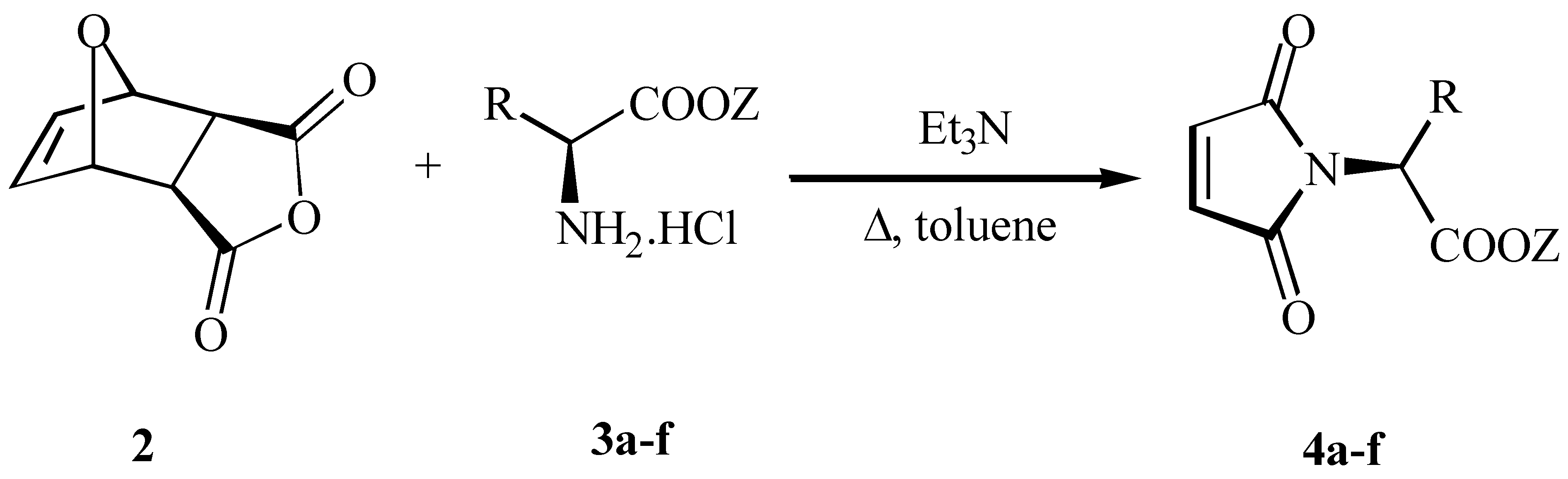
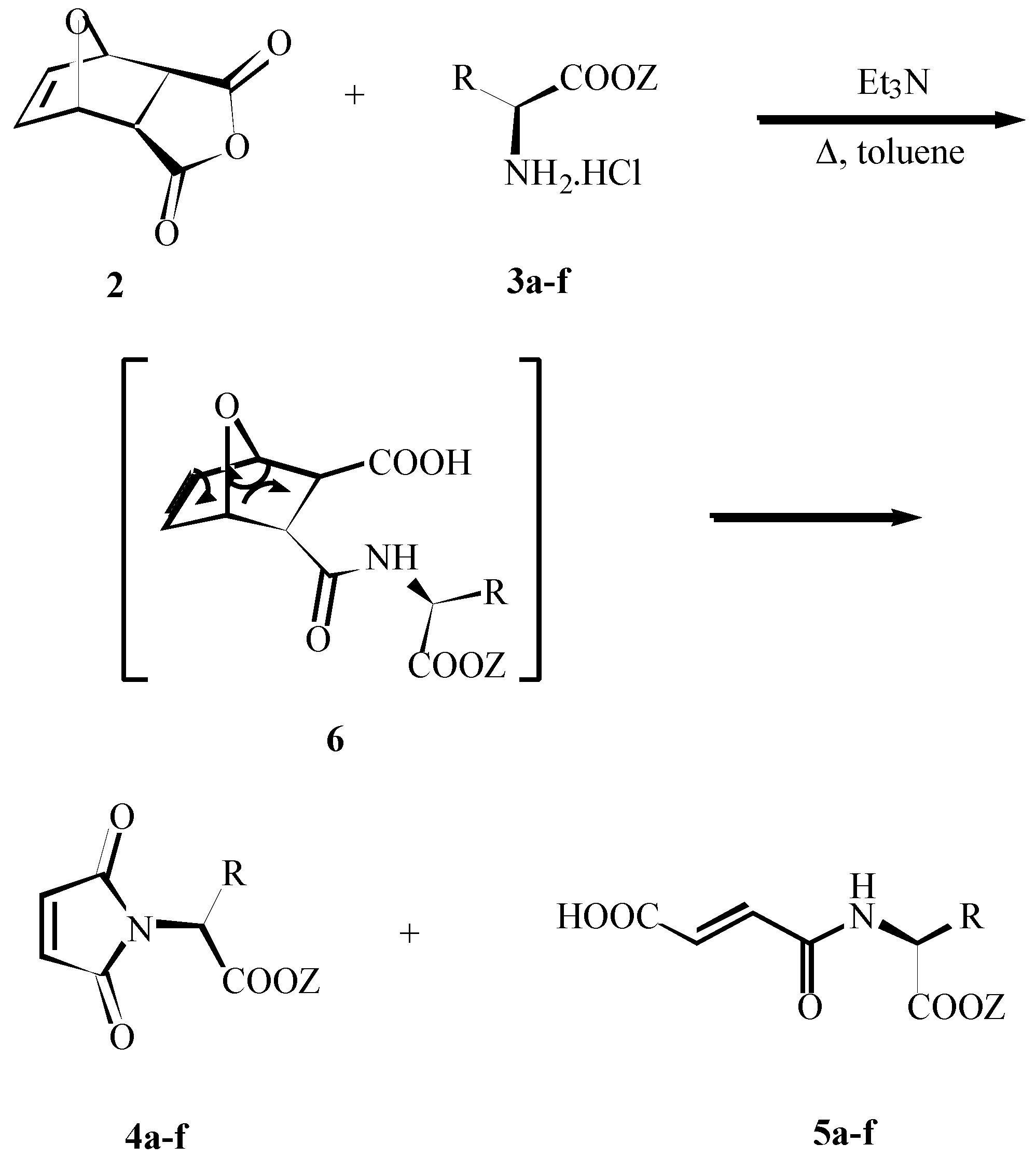
Results and Discussion
Conclusion
Experimental
General
N-Maleonyl-(S)-alanine ethyl ester 4a
Method B
N-Maleonyl-(S)-valine-ethyl ester 4b
Method B
N-Maleonyl-(S)-leucine-methyl ester 4c
Method A
Method B
Method C
N-Maleonyl-(S)-serine- ethyl ester 4d
Method A
N-Maleonyl-(S)-aspartic acid diethyl ester 4e
Method A
Method B
Method C
N-Maleonyl-(S)-tryptophane-methyl ester 4f
Method B
1,3-Dipolar cycloaddition of 2,4,6-trimethylphenylnitrile oxide to maleimide 4a-e
1,3-Dipolar cycloaddition of phenylnitrile oxide to maleimide 4c,e
Acknowledgement
References
- Corrie, J. E. T. J. Chem. Soc. Perkin Trans. 1 1994, 2975.
- Janda, K. D.; Ashley, J. A.; Jones, T. M.; McLeod, D. A.; Schloeder, D. M.; Weinhouse, M. I. J. Am. Chem. Soc. 1990, 112, 8886.
- Matocsy, G.; Nadasi, M.; Adriska, V. Pesticide Chemistry; Akademiai Kiadó: Budapest, 1988. [Google Scholar]
- Fujinami, A.; Ozaki, T.; Nodera, K.; Tanaka, K. Agric. Biol. Chem. 1972, 36, 318.
- Tottori, N.; Ueda, M.; Kirino, O.; Oba, S.; Fujinami, A.; Kato, T.; Ozaki, T. Japan Kokai 74 124 225. Chem. Abst. 1975, 82, 150501. [Google Scholar]
- Grigg, R.; Surendrakumar, S.; Thianpatanagul, S.; Vipond, D. J. J. Chem. Soc. Perkin Trans. 1 1988, 2693.
- Baldwin, S. W.; Greenspan, P.; Alaimo, C.; McPhail, A.T. Tetrahedron Lett. 1991, 32, 5877.
- Konopíková, M.; Fisera, L.; Prónayová, N. Collect. Czech. Chem. Commun. 1991, 57, 1521.
- Searle, N. E. U. S. Pat. 2 444 536 (1948). Chem. Abst. 1948, 42, 7340. [Google Scholar]
- Biagini, S. C. G.; Bush, S. M.; Gibson, V. C.; Mazzariol, L.; North, M.; Teasdale, W. G.; Williams, C. M.; Zagotto, G.; Zamuner, D. Tetrahedron 1995, 51, 7247.
- Tochtermann, W.; Bruhu, S.; Wolff, C. Tetrahedron Lett. 1994, 35, 1165.
- Grundmann, C.; Dean, M. J. J. Org. Chem. 1965, 30, 2809.
- Sample Availability: Available from the authors.


| Compd. | Method A Yield (%) | Method B Yield (%) | Method C Yield (%) |
|---|---|---|---|
| 4a | < 5 | 28 | < 5 |
| 4b | < 5 | 50 | < 5 |
| 4c | 24 | 44 | 49 |
| 4d | 16 | x | < 5 |
| 4e | 39 | 62 | 55 |
| 4f | x | 50 | Mst |
| Compound | R | Z | Ar | Ratio endo / exo |
|---|---|---|---|---|
| 4a | CH3 | Et | Mst | 75 : 25 |
| 4b | (CH3)2CH | Et | Mst | 70 : 30 |
| 4c | (CH3)2CHCH2 | Me | Mst | 64 : 36 |
| 4c | (CH3)2CHCH2 | Me | Ph | 59 : 41 |
| 4d | CH2OH | Et | Mst | 59 : 41 |
| 4e | CH2COOEt | Et | Mst | 71 : 29 |
| 4e | CH2COOEt | Et | Ph | 72 : 28 |
| 4f | 3-Indolylmethyl | Me | Mst | 60 : 40 |
© 1997 MDPI. All rights reserved.
Share and Cite
Ondrus, V.; Fisera, L. Synthesis and 1,3-Dipolar Cycloaddition Reactions of Chiral Maleimides. Molecules 1997, 2, 49-56. https://doi.org/10.3390/feb97p5
Ondrus V, Fisera L. Synthesis and 1,3-Dipolar Cycloaddition Reactions of Chiral Maleimides. Molecules. 1997; 2(2):49-56. https://doi.org/10.3390/feb97p5
Chicago/Turabian StyleOndrus, Vladimir, and Lubor Fisera. 1997. "Synthesis and 1,3-Dipolar Cycloaddition Reactions of Chiral Maleimides" Molecules 2, no. 2: 49-56. https://doi.org/10.3390/feb97p5




