GSK3β Regulates Milk Synthesis in and Proliferation of Dairy Cow Mammary Epithelial Cells via the mTOR/S6K1 Signaling Pathway
Abstract
:1. Introduction
2. Results and Discussion
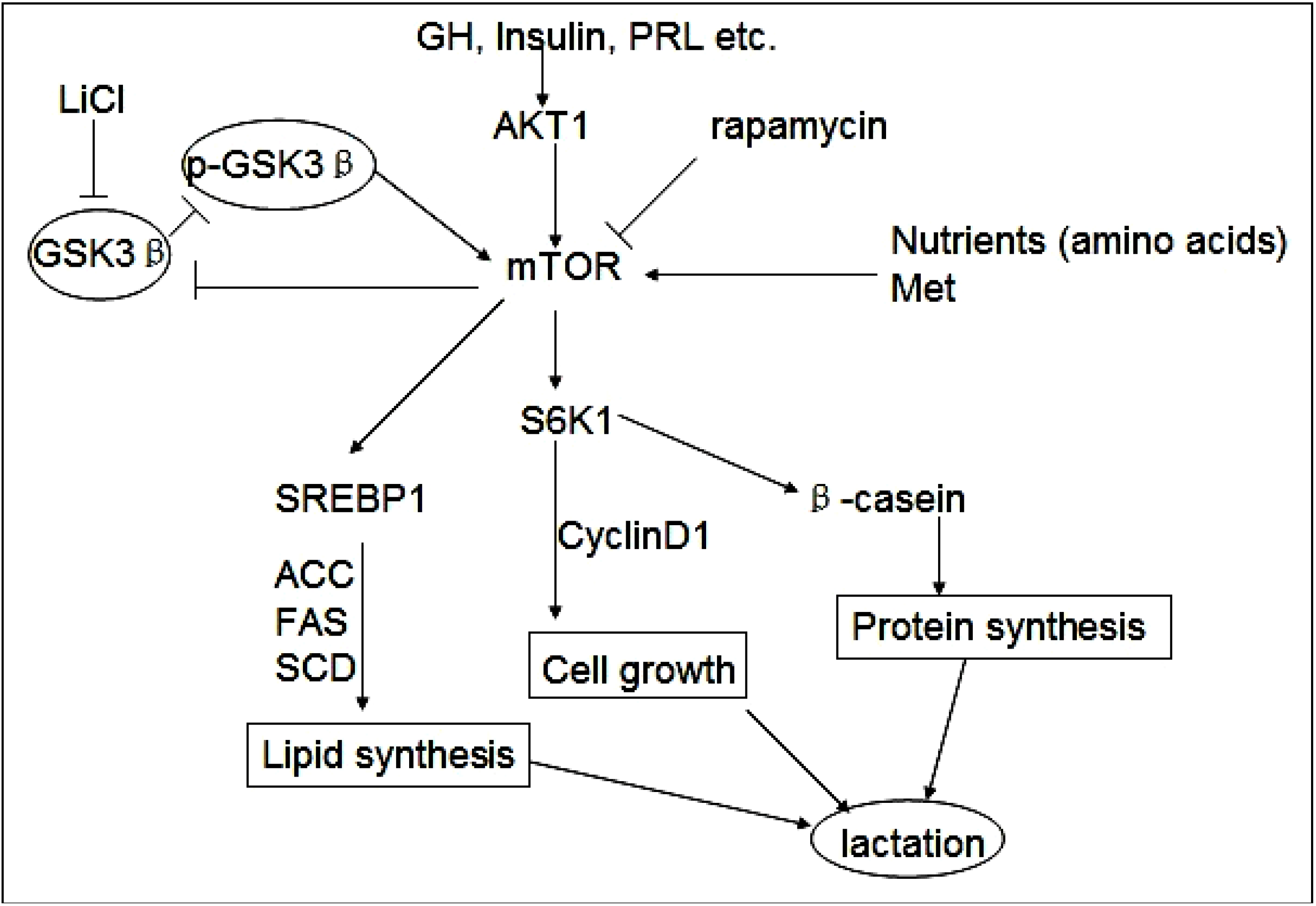
2.1. Overexpression of GSK3β Suppresses GSK3β Phosphorylation and the mTOR/S6K1 Signaling Pathway Leading to Milk Synthesis, and Proliferation of DCMECs
2.2. GSK3β Inhibition Enhances GSK3β Phosphorylation and the mTOR/S6K1 Signaling Pathway Leading to Milk Synthesis, and Increases the Proliferation of DCMECs


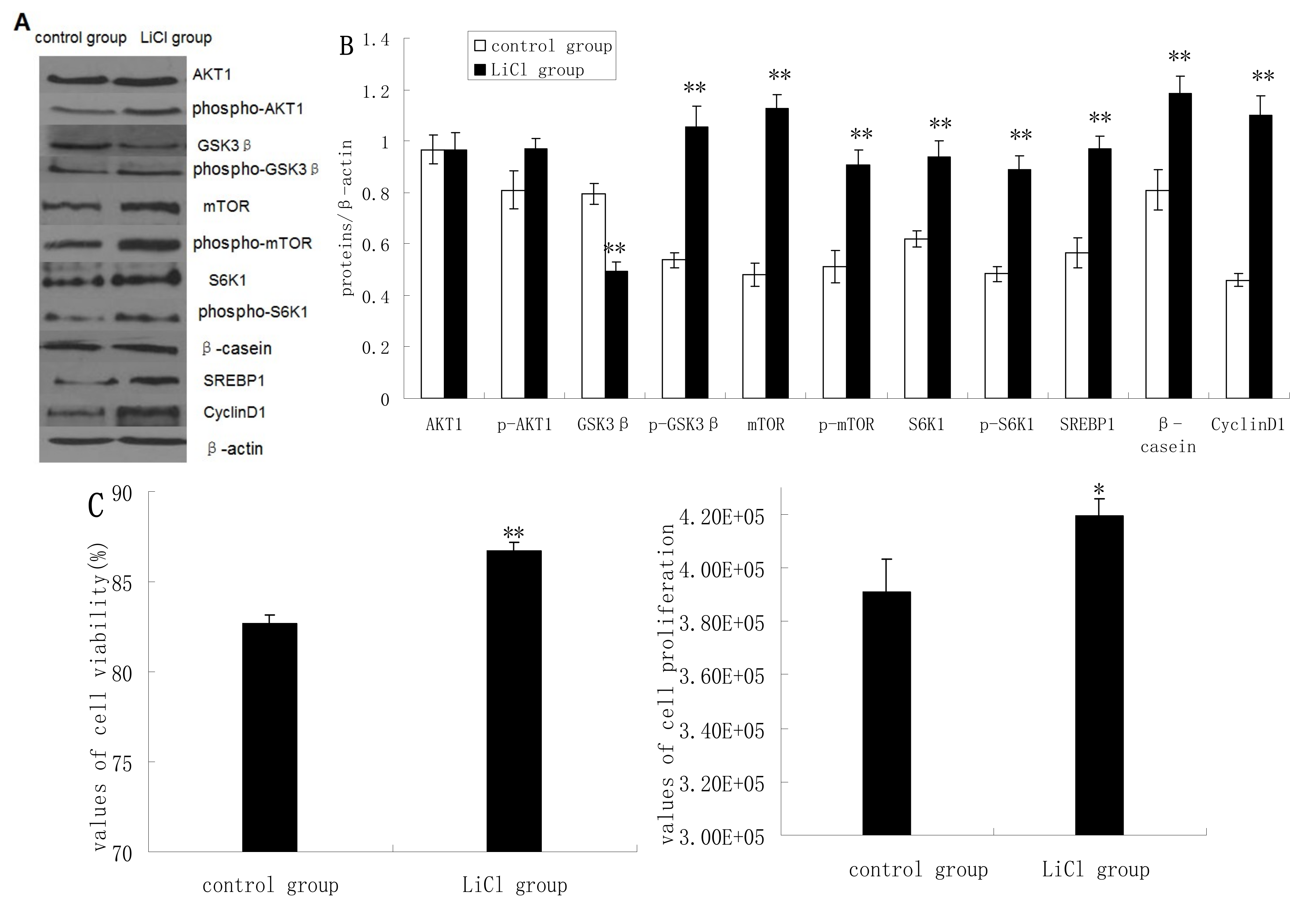
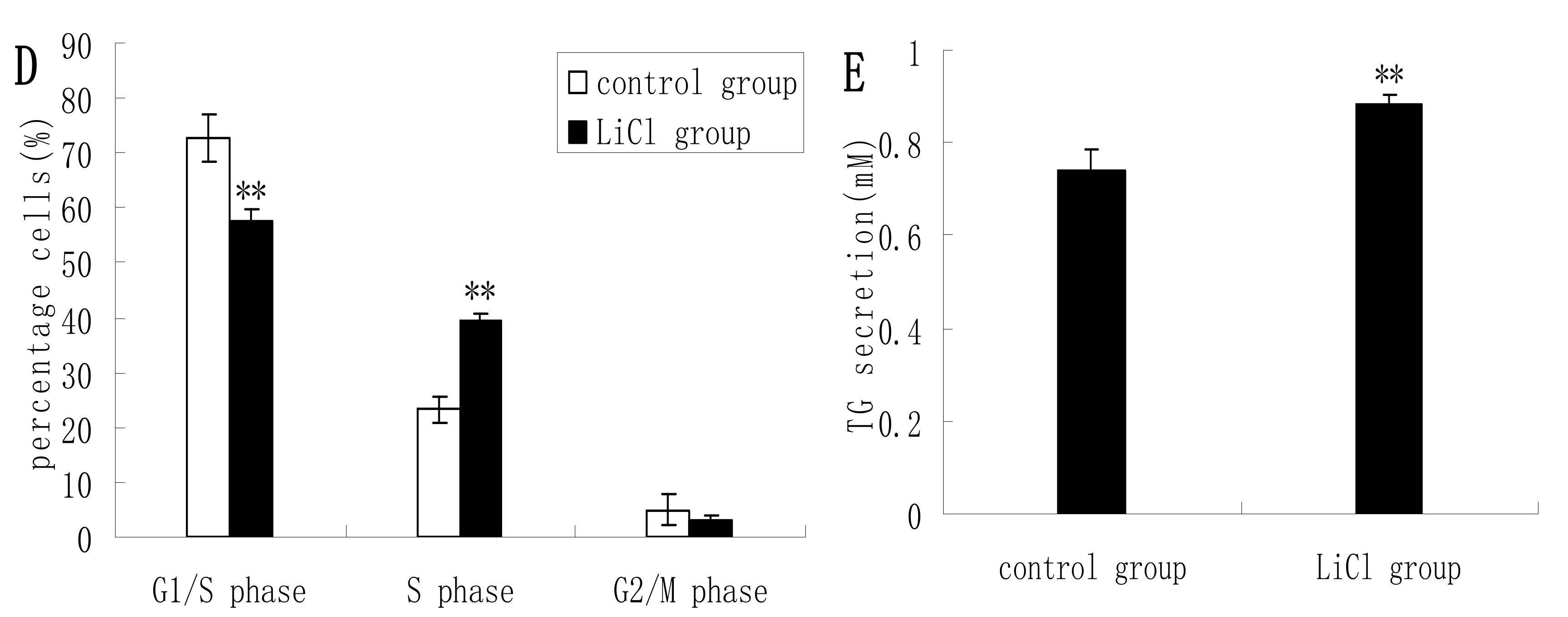
2.3. Inhibition of the mTOR/S6K1 Signaling Pathway by Rapamycin Blocks the Effects of LiCl on GSK3β
2.4. Methionine Activates the mTOR/S6K1 Signaling Pathway and Reverses the Blocking Effect of Rapamycin on GSK3β Phosphorylation by LiCl
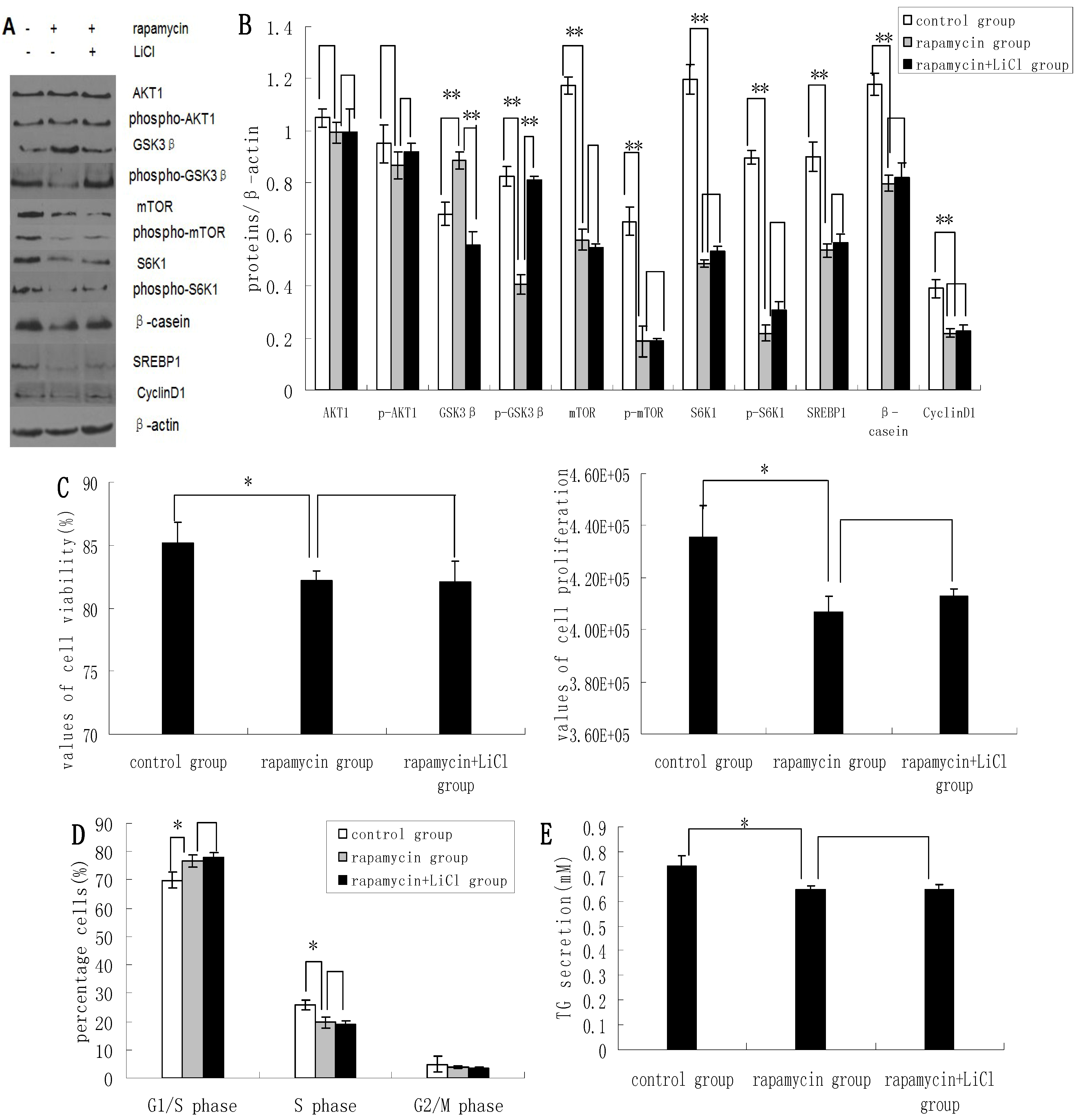
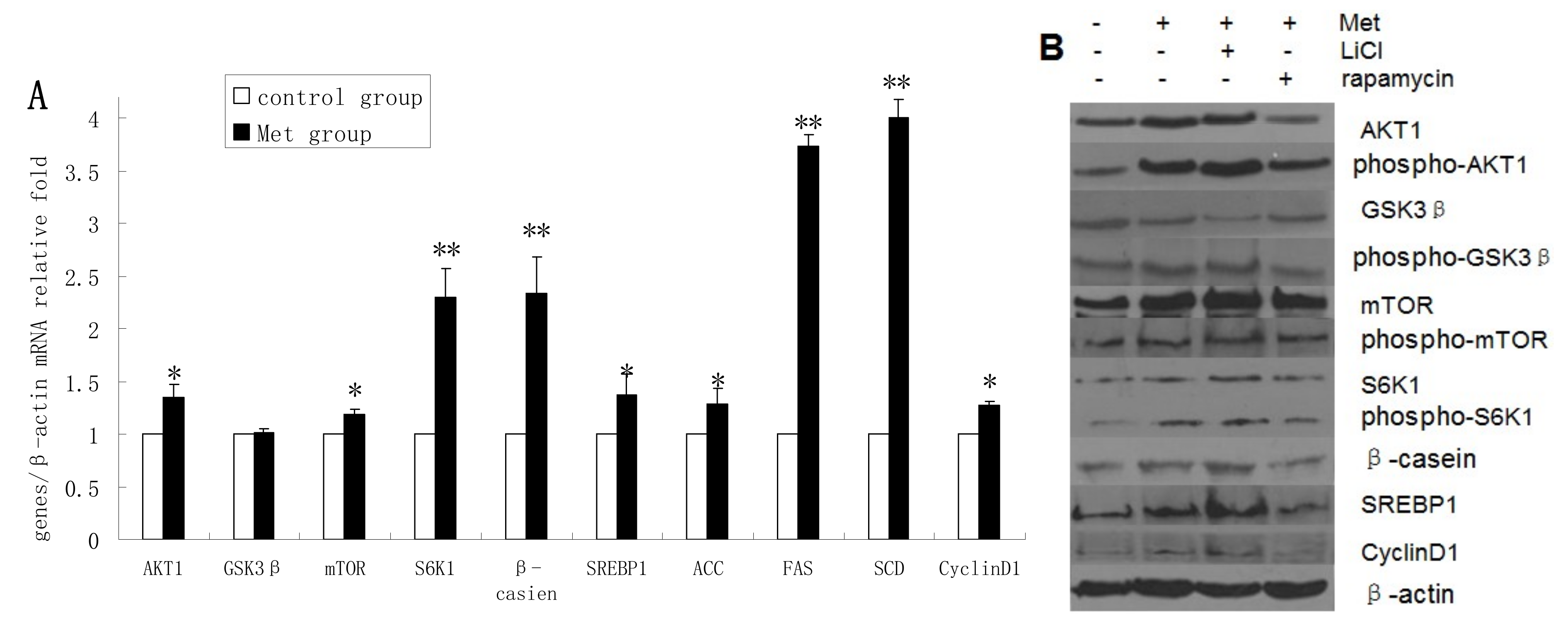

2.5. Discussion
3. Experimental Section
3.1. Instruments and Materials
3.2. Cell Preparation and Treatments
3.3. Inhibition of GSK3β and mTOR
3.4. pGCMV-IRES-EGFP-GSK3β Vector Construction and Transfection
3.5. Treatment of DCMECs with Methionine
3.6. Cell Viability and Proliferation Assessments
3.7. Cell Cycle Assessment
3.8. Detection of Triglyceride Secretion
3.9. RNA Extraction and Quantitative Real Time PCR
| Primer Name | Primer Sequences (5' → 3') | |
|---|---|---|
| Forward Primer | Reverse Primer | |
| β-actin | AAGGACCTCTACGCCAACACG | TTTGCGGTGGACGATGGAG |
| AKT1 | TAAAGAAGGAGGTCATCGTGG | CGGGACAGGTGGAAGAAAA |
| GSK3β | CCATCCTTATTCCTCCTC | TTGGTCTGTCCACGGTCT |
| mTOR | ATGCTGTCCCTGGTCCTTATG | GGGTCAGAGAGTGGCCTTCAA |
| S6K1 | AAATGCTGCTTCTCGTCT | GTTCTTCCCAGTTAATATGTCT |
| β-casein | AACAGCCTCCCACAAAAC | AGCCATAGCCTCCTTCAC |
| SREBP1 | AGTAGCAGCGGTGGAAGT | GCAGCGGCTCTGGATT |
| ACC | AGACAAACAGGGACCATT | AGGGACTGCCGAAACAT |
| FAS | CCACGGCTGTCGGTAAT | CGCTCCCACTCATCCTG |
| SCD | CTGTGGAGTCACCGAACC | TAGCGTGGAACCCTTTT |
| CyclinD1 | CCGTCCATGCGGAAGATC | CAGGAAGCGGTCCAGGTAG |
3.10. Western Blotting Analysis
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jacobs, K.M.; Bhave, S.R.; Ferraro, D.J.; Jaboin, J.J.; Hallahan, D.E. GSK-3 β: A bifunctional role in cell death pathways. Int. J. Cell. Biol. 2012. doi:org/10.1155/2012/930710. [Google Scholar]
- Aparicio, I.M.; Garcia, H.M.; Fair, T.; Lonergan, P. Identification and regulation of glycogen synthase kinase-3 during bovine embryo development. Reproduction 2010, 140, 83–92. [Google Scholar] [CrossRef]
- Pap, M.; Cooper, G.M. Role of translation initiation factor 2B in control of cell survival by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3β signaling pathway. Mol. Cell. Biol. 2002, 22, 578–586. [Google Scholar] [CrossRef]
- Stein, J.; Milewski, W.M.; Hara, M.; Steiner, D.F.; Dey, A. GSK-3 inactivation or depletion promotes beta-cell replication via down regulation of the CDK inhibitor, p27 (Kip1). Islets 2011, 3, 21–34. [Google Scholar] [CrossRef]
- Kaidanovich, B.O.; Woodgett, J.R. GSK-3: Functional insights from cell biology and animal models. Front. Mol. Neurosci. 2011, 4, 1–25. [Google Scholar]
- Chen, Y.; Patel, V.; Bang, S.; Cohen, N.; Millar, J. Maturation and activity of sterol regulatory element binding protein 1 is inhibited by acyl-coa binding domain containing 3. PLoS One 2012, 7, e49906. [Google Scholar]
- Shimura, T.; Kakuda, S.; Ochiai, Y.; Nakagawa, H.; Kuwahara, Y. Acquired radioresistance of human tumor cells by DNA-PK/AKT/GSK3β-mediated CyclinD1 overexpression. Oncogene 2010, 29, 4826–4837. [Google Scholar] [CrossRef]
- Sundqvist, A.; Bengoechea, M.T.; Ye, X.; Lukiyanchuk, V.; Jin, J. Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCF Fbw7. Cell. MeTable 2005, 1, 379–391. [Google Scholar] [CrossRef]
- Inoki, K.; Ouyang, H.; Zhu, T.; Lindvall, C.; Wang, Y. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 2006, 126, 955–968. [Google Scholar] [CrossRef]
- Oppi, W.C.; Suagee, J.; Corl, B. Regulation of lipid synthesis by liver X receptor α and sterol regulatory element-binding protein 1 in mammary epithelial cells. J. Dairy Sci. 2011, 96, 112–121. [Google Scholar] [CrossRef]
- Anderson, S.M.; Rudolph, M.C.; McManaman, J.L.; Neville, M.C. Secretory activation in the mammary gland: It’s not just about milk protein synthesis! Breast Cancer Res. 2007, 9, 204–217. [Google Scholar]
- Dong, J.; Peng, J.; Zhang, H.; Mondesire, W.H.; Jian, W. Role of glycogen synthase kinase 3β in rapamycin-mediated cell cycle regulation and chemosensitivity. Cancer Res. 2005, 65, 1961–1972. [Google Scholar] [CrossRef]
- Stambolic, V.; Ruel, L.; Woodgett, J.R. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 1996, 6, 1664–1669. [Google Scholar] [CrossRef]
- Freland, L.; Beaulieu, J.M. Inhibition of GSK3 by lithium, from single molecules to signaling networks. Mol. Neurosci. 2012, 5, 14. [Google Scholar]
- Porstmann, T.; Santos, C.R.; Griffiths, B.; Cully, M.; Wu, M. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008, 8, 224–236. [Google Scholar] [CrossRef]
- Papkoff, J.; Aikawa, M. WNT-1 and HGF regulate GSK3β activity and β-catenin signaling in mammary epithelial cells. Biochemi. Biophys. Res. Commun. 1998, 247, 851–858. [Google Scholar] [CrossRef]
- Lu, L.M.; Li, Q.Z.; Huang, J.G.; Gao, X.J. Proteomic and functional analyses reveal MAPK1 regulates milk protein synthesis. Molecules 2013, 18, 263–275. [Google Scholar]
- Lu, L.M.; Gao, X.J.; Li, Q.Z.; Huang, J.G.; Liu, R. Comparative phosphoproteomics analysis of the effects of L-methionine on dairy cow mammary epithelial cells. Can. J. Anim. Sci. 2012, 92, 433–442. [Google Scholar] [CrossRef]
- Vadlakonda, L.; Pasupuleti, M.; Pallu, R. Role of PI3K-AKT-mTOR and Wnt signaling pathways in transition of G1-S phase of cell cycle in cancer cells. Front. Oncol. 2013, 3, 85. [Google Scholar]
- Liu, H.; Remedi, M.S.; Pappan, K.L.; Kwon, G.; Rohatgi, N. Glycogen synthase kinase-3 and mammalian target of rapamycin pathways contribute to DNA synthesis, cell cycle progression, and proliferation in human islets. Diabetes 2009, 58, 663–672. [Google Scholar]
- Wang, H.; Brown, J.; Gu, Z.; Garcia, C.A.; Liang, R. Convergence of the mammalian target of rapamycin complex 1-and glycogen synthase kinase 3β-signaling pathways regulates the innate inflammatory response. J. Immun. 2011, 186, 5217–5226. [Google Scholar] [CrossRef]
- Beurel, E.; Michalek, S.M.; Jope, R.S. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immun. 2010, 31, 24–31. [Google Scholar] [CrossRef]
- Porstmann, T.; Santos, C.; Lewis, C.; Griffiths, B.; Schulze, A. A new player in the orchestra of cell growth: SREBP activity is regulated by mTORC1 and contributes to the regulation of cell and organ size. Biochem. Soc. Trans. 2009, 37, 278–283. [Google Scholar] [CrossRef]
- Noěl, A.; Barrier, L.; Rinaldi, F.; Hubert, C.; Fauconneau, B. Lithium chloride and staurosporine potentiate the accumulation of phosphorylated glycogen synthase kinase 3β/Tyr216, resulting in glycogen synthase kinase 3β activation in SH-SY5Y human neuroblastoma cell lines. J. Neurol. Res. 2011, 89, 755–763. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.S. The role of GSK3 in glucose homeostasis and the development of insulin resistance. Diabetes Res. Clin. Pract. 2007, 77, S49–S57. [Google Scholar] [CrossRef]
- Bionaz, M.; Loor, J.J. Gene networks driving bovine mammary protein synthesis during the lactation cycle. Bioinform. Biol. Insights 2011, 5, 83–98. [Google Scholar]
- Shin, S.; Wolgamott, L.; Yoon, S.O. Glycogen synthase kinase (GSK)-3 and mammalian target of rapamycin complex 1 (mTORC1) cooperate to regulate protein S6 kinase 1 (S6K1). Cell. Cycle 2012, 11, 1053–1054. [Google Scholar] [CrossRef]
- Proud, C. Signalling to translation: How signal transduction pathways control the protein synthetic machinery. Biochem. J. 2007, 403, 217–234. [Google Scholar] [CrossRef]
- Hou, X.; Arvisais, E.W.; Davis, J.S. Luteinizing hormone stimulates mammalian target of rapamycin signaling in bovine luteal cells via pathways independent of AKT and mitogen-activated protein kinase: Modulation of glycogen synthase kinase 3 and AMP-activated protein kinase. Endocrinology 2010, 151, 2846–2857. [Google Scholar] [CrossRef]
- Magnuson, B.; Ekim, B.; Fingar, D.C. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem. J. 2012, 441, 1–21. [Google Scholar] [CrossRef]
- Düvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell. 2010, 39, 171–183. [Google Scholar]
- Kim, Y.M.; Shin, H.T.; Seo, Y.H.; Byun, H.O.; Yoon, S.H. Sterol regulatory element-binding protein (SREBP)-1-mediated lipogenesis is involved in cell senescence. J. Biol. Chem. 2010, 285, 29069–29077. [Google Scholar] [CrossRef]
- Bakan, I.; Laplante, M. Connecting mTORC1 signaling to SREBP-1 activation. Curr. Opin. Lipidol. 2012, 23, 226–234. [Google Scholar] [CrossRef]
- Powers, T. Cell growth control: mTOR takes on fat. Mol. Cell. 2008, 31, 775–776. [Google Scholar] [CrossRef]
- Boutinaud, M.; Guinard, F.J.; Helenejammes, J. The number and activity of mammary epithelial cells, determining factors for milk production. Reprod. Nutr. Dev. 2004, 44, 499–508. [Google Scholar] [CrossRef]
- Diehl, J.A.; Cheng, M.; Roussel, M.F.; Sherr, C.J. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998, 12, 3499–3511. [Google Scholar] [CrossRef]
- George, B.; Vollenbrǒker, B.; Saleem, M.A.; Huber, T.B.; Pavenstǎdt, H. GSK3β inactivation in podocytes results in decreased phosphorylation of p70S6K accompanied by cytoskeletal rearrangements and inhibited motility. Am. J. Physiol. Renal Physiol. 2011, 300, F1152–F1162. [Google Scholar] [CrossRef]
- Appuhamy, J.A.; Knoebel, N.A.; Nayananjalie, W.A.; Escobar, J.; Hanigan, M.D. Isoleucine and leucine independently regulate mTOR signaling and protein synthesis in MAC-T cells and bovine mammary tissue slices. J. Nutr. 2013, 142, 484–491. [Google Scholar]
- Huang, Y.L.; Zhao, F.; Luo, C.C.; Zhang, X.; Si, Y. SOCS3-mediated blockade reveals major contribution of JAK2/STAT5 signaling pathway to lactation and proliferation of dairy cow mammary epithelial cells in vitro. Molecule 2013, 18, 12987–13002. [Google Scholar] [CrossRef]
- Parisi, F.; Riccardo, S.; Daniel, M.; Saqcena, M.; Kundu, N. Drosophila insulin and target of rapamycin (TOR) pathways regulate GSK3 beta activity to control Myc stability and determine Myc expression in vivo. BMC Biol. 2011, 9, 1–13. [Google Scholar] [CrossRef]
- Tong, H.L.; Gao, X.J.; Li, Q.Z.; Liu, J.; Li, N. Metabolic regulation of mammary gland epithelial cells of dairy cow by galactopoietic compound isolated from vaccariae segetalis. Agric. Sci. China 2011, 10, 1106–1116. [Google Scholar] [CrossRef]
- Wan, Z.Y.; Tong, H.L.; Li, Q.Z.; Gao., X.J. Influence on cellular signal transduction pathway in dairy cow mammary gland epithelial cells by galactopoietic compound isolated from vaccariae segetalis. Agric. Sci. China 2011, 10, 619–630. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhao, F.; Si, Y.; Huang, Y.; Yu, C.; Luo, C.; Zhang, N.; Li, Q.; Gao, X. GSK3β Regulates Milk Synthesis in and Proliferation of Dairy Cow Mammary Epithelial Cells via the mTOR/S6K1 Signaling Pathway. Molecules 2014, 19, 9435-9452. https://doi.org/10.3390/molecules19079435
Zhang X, Zhao F, Si Y, Huang Y, Yu C, Luo C, Zhang N, Li Q, Gao X. GSK3β Regulates Milk Synthesis in and Proliferation of Dairy Cow Mammary Epithelial Cells via the mTOR/S6K1 Signaling Pathway. Molecules. 2014; 19(7):9435-9452. https://doi.org/10.3390/molecules19079435
Chicago/Turabian StyleZhang, Xia, Feng Zhao, Yu Si, Yuling Huang, Cuiping Yu, Chaochao Luo, Na Zhang, Qingzhang Li, and Xuejun Gao. 2014. "GSK3β Regulates Milk Synthesis in and Proliferation of Dairy Cow Mammary Epithelial Cells via the mTOR/S6K1 Signaling Pathway" Molecules 19, no. 7: 9435-9452. https://doi.org/10.3390/molecules19079435
APA StyleZhang, X., Zhao, F., Si, Y., Huang, Y., Yu, C., Luo, C., Zhang, N., Li, Q., & Gao, X. (2014). GSK3β Regulates Milk Synthesis in and Proliferation of Dairy Cow Mammary Epithelial Cells via the mTOR/S6K1 Signaling Pathway. Molecules, 19(7), 9435-9452. https://doi.org/10.3390/molecules19079435




