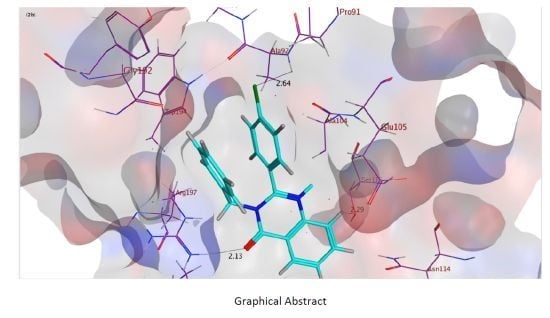
Synthesis, Anti-microbial and Molecular Docking Studies of Quinazolin-4(3H)-one Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
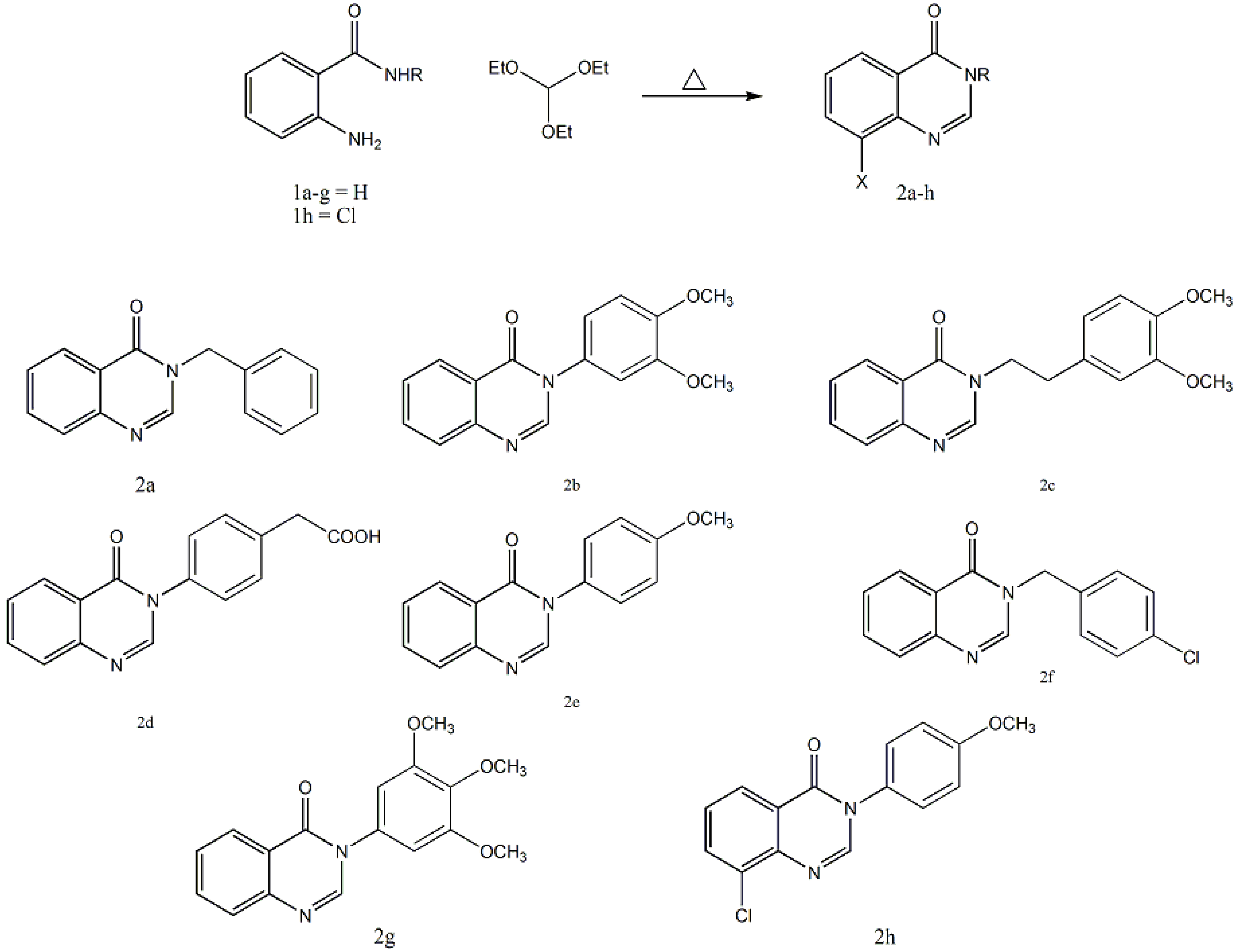
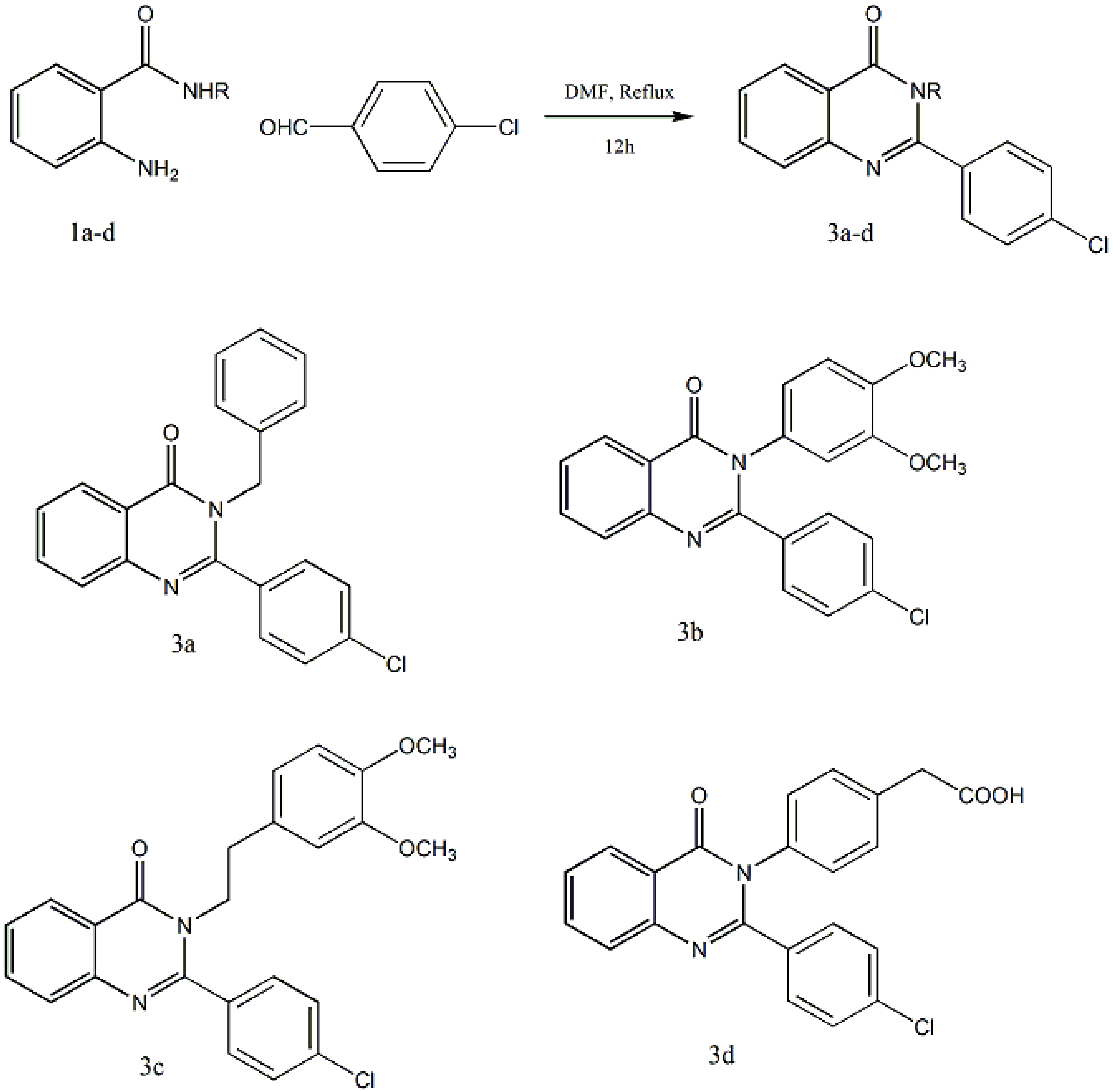
2.2. Antimicrobial Evaluation
| Comp. No. | Gram-Postive Bacteria | Gram-Negative Bacteria | Fungi | ||||
|---|---|---|---|---|---|---|---|
| Staphylococcus aureus | Bacillus subtilis | Pseudomonas aeruginosa | Escherichia coli | Aspergillus fumigatus | Saccharomyces cerevisiae | Candida albicans | |
| 2b | 7.8 ± 0.4 | 9.2 ± 0.3 | 9.7 ± 0.3 | 8.9 ± 0.3 | 9.5 ± 0.4 | 10.3 ± 0.4 | 10.5 ± 0.4 |
| 2c | 12.3 ± 0.3 | 13.1 ± 0.4 | 18.4 ± 0.5 | 15.2 ± 0.4 | 9.4 ± 0.3 | 15.2 ± 0.4 | 13.3 ± 0.4 |
| 2d | 9.8 ± 0.3 | 12.2 ± 0.2 | 14.1 ± 0.4 | 13.8 ± 0.3 | 12.1 ± 0.4 | 13.7 ± 0.3 | 12.8 ± 0.2 |
| 2g | 10.3 ± 0.2 | 11.1 ± 0.4 | 10.9 ± 0.4 | 9.8 ± 0.3 | 11.3 ± 0.5 | 12.2 ± 0.4 | 11.4 ± 0.3 |
| 2h | 9.1 ± 0.4 | 9.6 ± 0.3 | 9.6 ± 0.3 | 9.8 ± 0.3 | 10.4 ± 0.4 | 11.3 ± 0.4 | 12.2 ± 0.4 |
| 3a | 25.6 ± 0.5 | 24.3 ± 0.4 | 30.1 ± 0.6 | 25.1 ± 0.5 | 18.3 ± 0.6 | 23.1 ± 0.4 | 26.1 ± 0.5 |
| 3b | 10.4 ± 0.3 | 11.3 ± 0.3 | 11.1 ± 0.2 | 10.8 ± 0.3 | 9.8 ± 0.2 | 10.8 ± 0.4 | 10.3 ± 0.5 |
| clotrimazole | 18.3 ± 0.6 | 23.1 ± 0.4 | 26.1 ± 0.5 | ||||
| streptomycin | 25.6 ± 0.5 | 24.3 ± 0.4 | 30.1 ± 0.6 | 25.1 ± 0.5 | |||
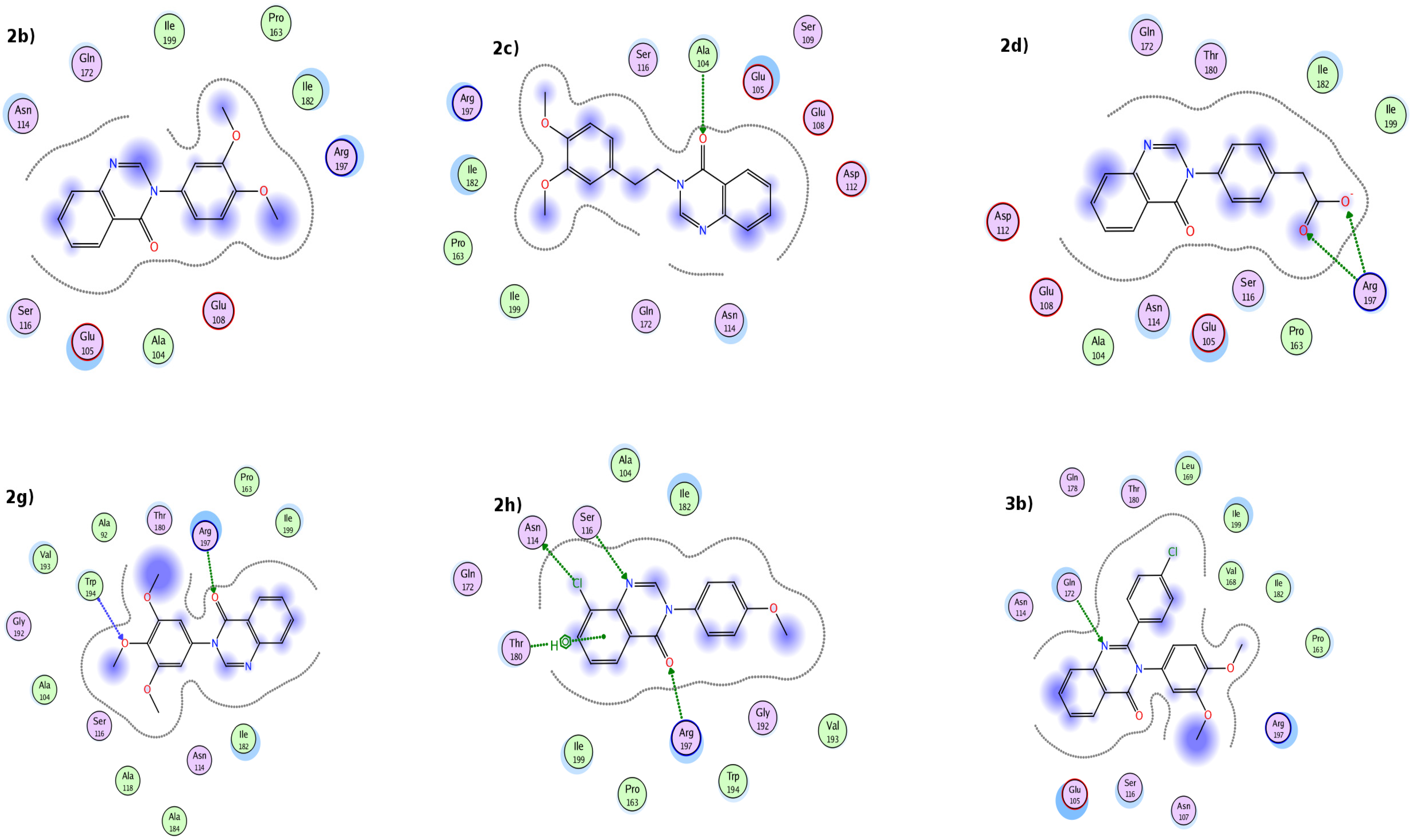
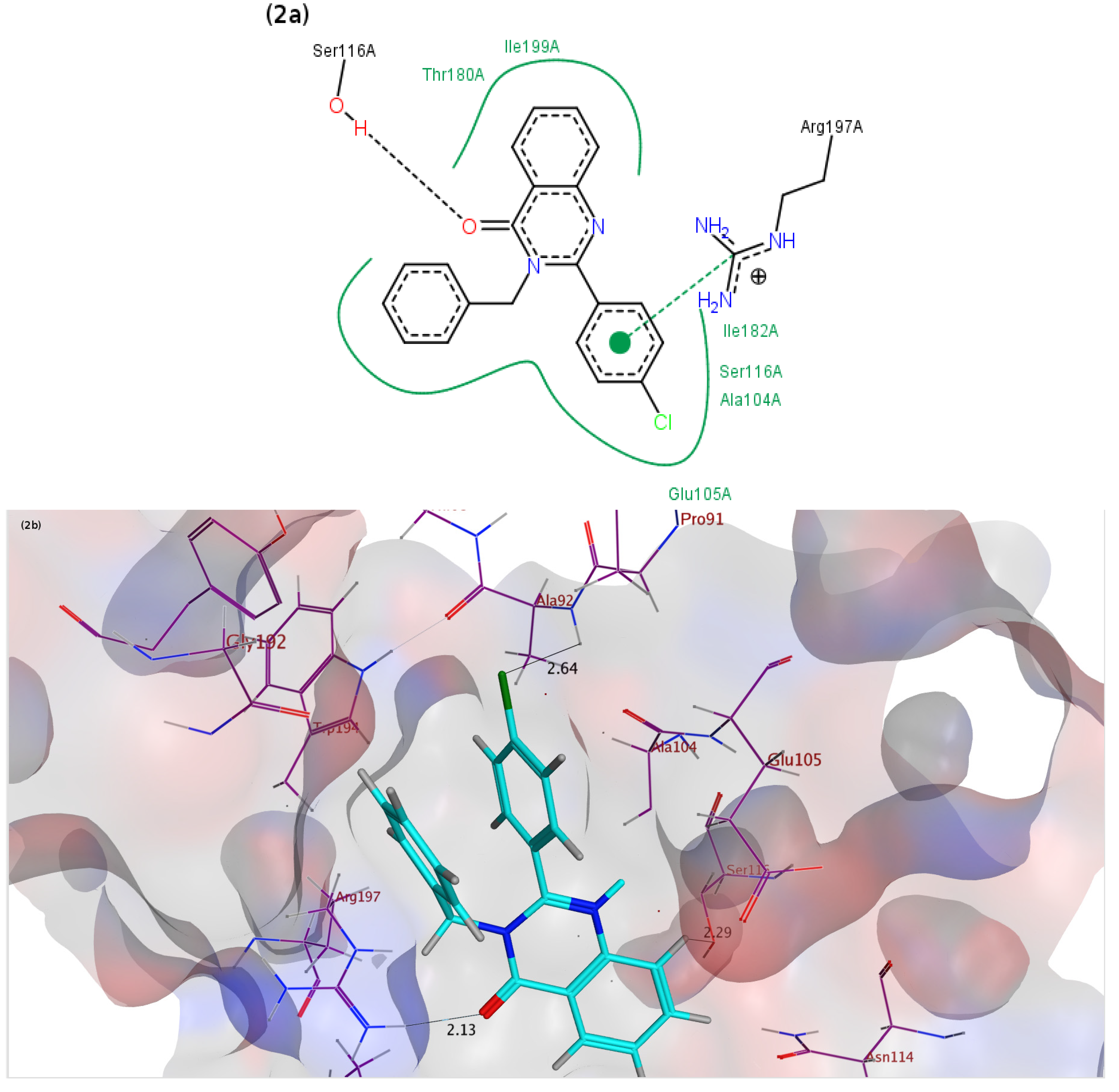
2.3. Molecular Docking Studies
3. Experimental Section
3.1. General Procedure for the Preparation of Qquinazolin-4(3H)-one Derivatives 2a–h
3.2. General Method for Preparation of Compounds Derivatives 3a–o
3.3. Antimicrobial Evaluation [30]
3.3.1. Antifungal Activity
3.3.2. Antifungal Assay
3.3.3. Antibacterial Activity
3.3.4. Material and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 2007, 24, 223–246. [Google Scholar] [CrossRef]
- Mhaske, S.B.; Argade, N.P. The chemistry of recently isolated naturally occurring quinazolinone alkaloids. Tetrahedron 2006, 62, 9787–9826. [Google Scholar] [CrossRef]
- Tseng, M.-C.; Yang, H.-Y.; Chu, Y.-H. Total synthesis of asperlicin C, circumdatin F, demethylbenzomalvin A, demethoxycircumdatin H, sclerotigenin, and other fused quinazolinones. Org. Biomol. Chem. 2010, 8, 419–427. [Google Scholar] [CrossRef]
- Kubicova, L.; Kudelova, P.; Dostal, H.; Waisser, K. Antialgal activity of 3-Phenyl-2H-1,3-benzoxazine-2,4(3H)-diones and 3-Phenyl-3H-quinazoline-4-ones against Chlorella Vulgaris. Folia Pharm. Univ. Carol. 2000, 25, 81–87. [Google Scholar]
- Hanusek, J. Synthesis, kinetics, and biological activity of 2-Phenylquinazoline-4-thiones. Chem. List. 2001, 95, 811–813. [Google Scholar]
- William, R.; Detlef, M.; Stephen, E.; Bert, K.; Thomas, S.; Manfred, M. Protein kinase inhibitors of the quinazoline class exert anti-cytomegaloviral activity in vitro and in vivo. Antivir. Res. 2008, 79, 49–61. [Google Scholar] [CrossRef]
- Chan, J.H.; Hong, J.S.; Kuyper, L.F.; Baccanari, D.P.; Joyner, S.S.; Tansik, R.L.; Boytos, C.M.; Rudolph, S.K. Selective inhibitors of candida albicans dihydrofolate reductase: Activity and selectivity of 5-(Arylthio)-2,4-diaminoquinazolines. J. Med. Chem. 1995, 38, 3608–3616. [Google Scholar] [CrossRef]
- Castaldo, R.A.; Gump, D.W.; McCormack, J.J. Activity of 2,4-Diaminoquinazoline Compounds against Candida species. J. Antimicrob. Agents Chemother. 1979, 15, 81–86. [Google Scholar] [CrossRef]
- Harushia, K.; Kiesuke, Y.; Seiko, H.; Shingo, H.; Ryota, K.; Norimitsu, H.; Makoto, M.; Yoshiteru, O. Exploration of a new type of antimalarial compounds based on febrifugine. J. Med. Chem. 2006, 49, 4698–4706. [Google Scholar] [CrossRef]
- Yen, M.H.; Sheu, J.R.; Peng, I.H.; Lee, Y.M.; Chern, J.W. Pharmacological Activity of DC-015, a Novel Potent and Selective α1-Adrenoceptor Antagonist. J. Pharm. Pharmacol. 1996, 48, 90–95. [Google Scholar] [CrossRef]
- Yasutaka, T.; Takao, S.; Nobuhisa, W.; Hideyuki, A.; Shigeru, S.; Isao, S. Cyclic GMP phosphodiesterase inhibitors. 2. Requirement of 6-substitution of quinazoline derivatives for potent and selective inhibitory activity. J. Med. Chem. 1994, 37, 2106–2111. [Google Scholar] [CrossRef]
- Marianne, D.; Fredric, P.; Olivier, C.; Jean-Claude, T.; Jean-Pierre, C.; Yves, B. Synthesis and in vitro cytotoxic evaluation of new derivatives of pyrido[1,2-a]benzimidazolic ring system: The pyrido[1',2':1,2]imidazo[4,5-h]quinazolines. Chem. Pharm. Bull. 2001, 49, 1061–1065. [Google Scholar] [CrossRef]
- Mani Chandrica, P.; Yakaiah, T.; Raghu Ram Rao, A.; Narsaiah, B.; ChakraReddy, N.; Sridhar, V.; Rao, J.V. Synthesis of novel 4,6-disubstituted quinazoline derivatives, their anti-inflammatory and anti-cancer activity (cytotoxic) against U937 leukemia cell lines. Eur. J. Med. Chem. 2008, 43, 846–852. [Google Scholar] [CrossRef]
- Hyao, S.; Mvera, M.J.; Strycker, W.; Leipzi, T.; Klup, R.; Hartzler, H. New sedative and hypotensive 3-substituted 2,4(1H,3H)-quinazolinediones. J. Med. Chem. 1965, 8, 807–811. [Google Scholar] [CrossRef]
- Cohen, E.; Klarberg, E.; James Vaughan, R., Jr. Quinazolinone sulfonamides. A new class of diuretic agentss. J. Am. Chem. Soc. 1960, 82, 2731–2735. [Google Scholar] [CrossRef]
- Kenji, M.; Junko, U.; Takashi, S.; Michio, I.; Neil, A.G.; Jin-Chen, Y.; Shusuke, T.; Shoji, O.; Yuji, N. Potent and selective inhibitors of platelet-derived growth factor receptor phosphorylation. 3. Replacement of quinazoline moiety and improvement of metabolic polymorphism of 4-[4-(N-substituted (thio)carbamoyl)-1-piperazinyl]-6,7-dimethoxyquinazoline derivatives. J. Med. Chem. 2003, 46, 4910–4925. [Google Scholar] [CrossRef]
- Joachim, R.; William, P.E.; Stephen, O.; Philip, D.G.C.; Philip, L.W.; Michael, B.; Donald, E.B.; Brian, T.B.; Georgiy, B.; Libing, C.; et al. Quinazolinone derivatives as orally available ghrelin receptor antagonists for the treatment of diabetes and obesity. J. Med. Chem. 2007, 50, 5202–5216. [Google Scholar] [CrossRef]
- Baba, A.; Kawamura, N.; Makino, H.; Ohta, Y.; Taketomi, S.; Sohda, T. Studies on disease-modifying antirheumatic drugs: Synthesis of novel quinoline and quinazoline derivatives and their anti-inflammatory effect. J. Med. Chem. 1996, 39, 5176–5182. [Google Scholar] [CrossRef]
- Huang, A.X.; Johanson, M.G.; Li, A.; Liu, J.; Marcus, A.P.; Medina, J.C.; Zhu, L. Cxcr3 antagonists. WO2002083143 A1, 24 October 2002. Chem. Abstr. 2002, 137, 337909. [Google Scholar]
- Yarosh, D.B.; Galvin, J.W.; Nay, S.L.; Peña, A.V.; Canning, M.T.; Brown, D.A. Anti-inflammatory activity in skin by biomimetic of Evodia rutaecarpa extract from traditional Chinese medicine. J. Dermatol. Sci. 2006, 42, 13–21. [Google Scholar]
- Uddin, R.; Lodhi, M.U.; Ul-Haq, Z. Combined pharmacophore and 3D-QSAR study on a series of Staphylococcus aureus Sortase A inhibitors. Chem. Biol. Drug Des. 2012, 80, 300–314. [Google Scholar] [CrossRef]
- Mabkhot, Y.N.; Barakat, A.; Al Majid, A.M.A.; Alshahrani, S.; Yousuf, S.; Choudhary, M.I. Synthesis, reactions and biological activity of some new bis-heterocyclic ring compounds containing sulphur atom. Chem. Cent. J. 2013, 7, 112–120. [Google Scholar] [CrossRef]
- Mabkhot, Y.N.; Barakat, A.; Al Majid, A.M.A.; Choudhary, M.I. Synthesis of thieno [2,3-b] thiophene containing bis-heterocycles-novel pharmacophores. Int. J. Mol. Sci. 2013, 14, 5712–5722. [Google Scholar] [CrossRef]
- Al-Nahary, T.T.; El-Ries, M.A.N.; Mohamed, G.G.; Attia, A.K.; Mabkhot, Y.N.; Harone, M.; Barakat, A. Multiclass analysis on repaglinide, flubendazole, robenidine hydrochloride and danofloxacin drugs. Arab. J. Chem. 2013, 6, 131–144. [Google Scholar] [CrossRef]
- Mabkhot, Y.N.; Barakat, A.; Al Majid, A.M.A.; Alamary, A.S. A novel and expedient approach to new heterocycles containing thiazole, thiazolo[3,2-a]pyridine, dihydrothiophene, and hydrazonothieno[2,3-b]thiophene moieties. Int. J. Mol. Sci. 2012, 13, 5035–5047. [Google Scholar] [CrossRef]
- Mabkhot, Y.N.; Barakat, A.; Al Majid, A.M.A.; Alshahrani, S.A. Comprehensive and facile Synthesis of some functionalized bis-heterocyclic compounds containing a thieno[2,3-b]thiophene motif. Int. J. Mol. Sci. 2012, 13, 2263–2275. [Google Scholar] [CrossRef]
- Mabkhot, Y.N.; Barakat, A.; Al Majid, A.M.A.; Al-Othman, Z.A.; Alamary, S.A. A facileand convenient synthesis of some novel hydrazones, schiff’s base and pyrazoles incorporating thieno [2,3-b]thiophenes. Int. J. Mol. Sci. 2011, 12, 7824–7834. [Google Scholar] [CrossRef]
- Mabkhot, Y.N.; Al Majid, A.M.A.; Barakat, A.; Alshahrani, S.; Siddiqui, Y. 1,1'-(3-Methyl-4-phenylthieno[2,3-b]thiophene-2,5-diyl)diethanone as building block in heterocyclic synthesis. Novel synthesis of some pyrazoles, and pyrimidines derivatives. Molecules 2011, 16, 6502–6511. [Google Scholar] [CrossRef]
- Mabkhot, Y.N.; Al-Har, M.S.; Barakat, A.; Al-Showiman, S.S. Diversity oriented design of various hydrazides and amides derived from isatoic anhydride and their antimicrobial activity evaluation. Dig. J. Nanomater. Biostruct. 2013, 8, 1345–1355. [Google Scholar]
- Mabkhot, Y.N.; Al-Majid, A.M.; Barakat, A.; Al-Showiman, S.S.; Al-Har, M.S.; Radi, S.; Naseer, M.M.; Hadda, T.B. Synthesis and biological evaluation of 2-Aminobenzamide derivatives as antimicrobial agents: Opening/Closing pharmacophore site. Int. J. Mol. Sci. 2014, 15, 5115–5127. [Google Scholar] [CrossRef]
- Smania, A.; Monache, F.D.; Smania, E.F.A.; Cuneo, R.S. Triterpenes and sterols from ganoderma. australe (Fr.) Pat. (Aphyllophoromycetideae). Int. J. Med. Mushrooms 1999, 1, 325–334. [Google Scholar] [CrossRef]
- Zong, Y.; Bice, T.W.; Ton-That, H.; Schneewind, O.; Narayana, S.V. Crystal structures of Staphylococcus aureus sortase A and its substrate complex. J. Biol. Chem. 2004, 279, 31383–31389. [Google Scholar] [CrossRef]
- Cambridge Crytallographic Data Center. GOLD version 5.2; Cambridge Crytallographic Data Center: Cambridge, UK, 2008. [Google Scholar]
- RCSD PDB (Protein Data Bank) Homepage. Available online: http://www.rcsb.org/pdb (accessed on 1 January 2014).
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open babel: An open chemical toolbox. J. Cheminf. 2011, 3, 1–14. [Google Scholar] [CrossRef]
- Molecular Operating Environment (MOE), 2012. Chemical Computing Group Inc.: 1010 Sherbooke St. West, Suite #910, Montreal, QC, H3A 2R7, Canada.
- OpenEye Scientific Software. OE FILTER version 2.5; OpenEye Scientific Software Inc.: Santa Fe, NM, USA.
- Stierand, K.; Rarey, M. Poseview molecular interaction patterns at a glance. J. Cheminf. 2010, 2, 50. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 2a–h and 3a–d are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mabkhot, Y.N.; Al-Har, M.S.; Barakat, A.; Aldawsari, F.D.; Aldalbahi, A.; Ul-Haq, Z. Synthesis, Anti-microbial and Molecular Docking Studies of Quinazolin-4(3H)-one Derivatives. Molecules 2014, 19, 8725-8739. https://doi.org/10.3390/molecules19078725
Mabkhot YN, Al-Har MS, Barakat A, Aldawsari FD, Aldalbahi A, Ul-Haq Z. Synthesis, Anti-microbial and Molecular Docking Studies of Quinazolin-4(3H)-one Derivatives. Molecules. 2014; 19(7):8725-8739. https://doi.org/10.3390/molecules19078725
Chicago/Turabian StyleMabkhot, Yahia Nasser, Munirah S. Al-Har, Assem Barakat, Fahad D. Aldawsari, Ali Aldalbahi, and Zaheer Ul-Haq. 2014. "Synthesis, Anti-microbial and Molecular Docking Studies of Quinazolin-4(3H)-one Derivatives" Molecules 19, no. 7: 8725-8739. https://doi.org/10.3390/molecules19078725
APA StyleMabkhot, Y. N., Al-Har, M. S., Barakat, A., Aldawsari, F. D., Aldalbahi, A., & Ul-Haq, Z. (2014). Synthesis, Anti-microbial and Molecular Docking Studies of Quinazolin-4(3H)-one Derivatives. Molecules, 19(7), 8725-8739. https://doi.org/10.3390/molecules19078725