Effects of Echium plantagineum L. Bee Pollen on Basophil Degranulation: Relationship with Metabolic Profile
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification and Quantification of Organic Acids by HPLC-UV
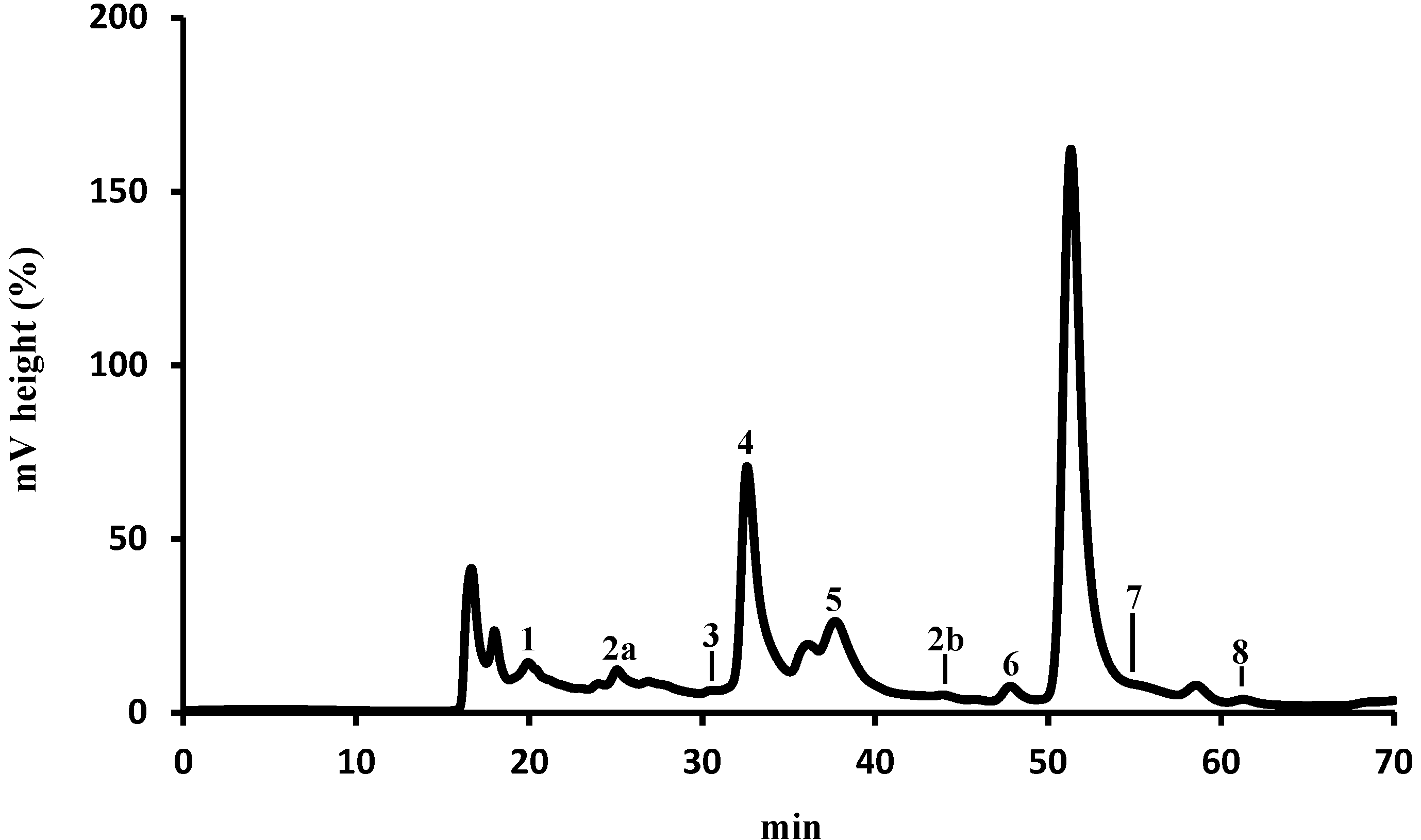
| Organic Acid | mg/kg (Dry Pollen) a | % | |
|---|---|---|---|
| 1 | Oxalic | 51.05 ± 0.98 | 0.50 |
| 2a + 2b | Aconitic | 6.89 ± 0.26 | 0.07 |
| 3 | Citric | 10.24 ±0.05 | 0.10 |
| 4 | Pyruvic | 890.27 ± 0.85 | 8.69 |
| 5 | Malonic | 8152.87 ± 0.37 | 79.57 |
| 6 | Shikimic | 2.20 ± 0.43 | 0.02 |
| 7 | Acetic | 1132.02 ± 111.33 | 11.05 |
| 8 | Fumaric | 0.48 ± 0.02 | 0.005 |
| Total | 10245.54 ± 114.29 |
2.2. Identification and Quantification of Fatty Acids by GC-IT/MS
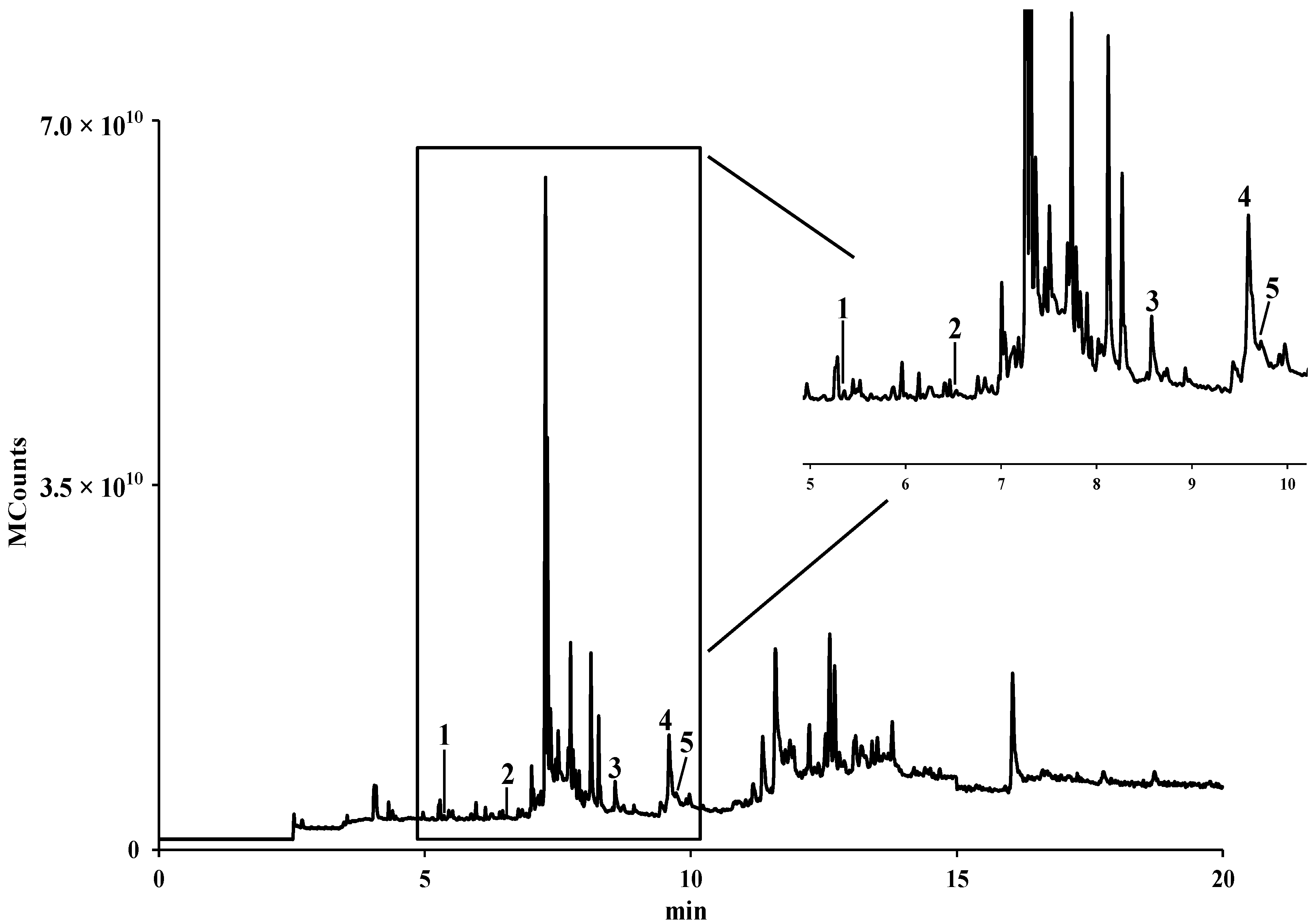
| Fatty Acid | mg/kg (Dry Pollen) a | % | |
|---|---|---|---|
| 1 | Capric (C10:0) | 53.0 ± 1.7 | 7.67 |
| 2 | Lauric (C12:0) | 27.1 ± 2.6 | 3.93 |
| 3 | Palmitic (C16:0) | 391.6 ± 19.3 | 56.72 |
| 4 | α-Linolenic (C18:3) | 183.9 ± 18.6 | 26.64 |
| 5 | Stearic (C18:0) | 34.8 ± 2.8 | 5.04 |
| Total | 690.4 ± 45.0 |
2.3. Effect on RBL-2H3 Cells Viability and Degranulation

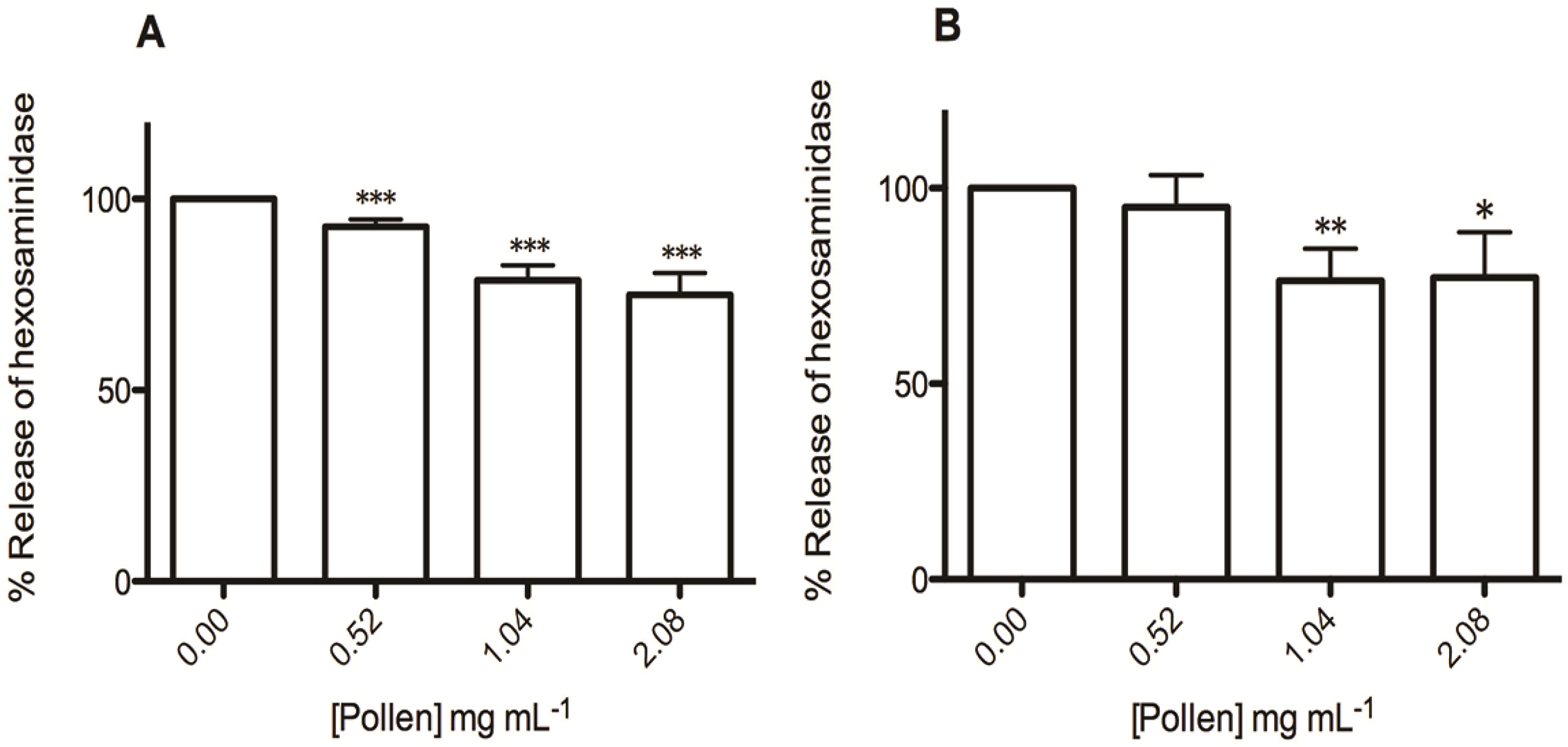
2.4. Effect on Soybean Lipoxygenase Activity
3. Experimental Section
3.1. Standards and Reagents
3.2. Sampling
3.3. Extract Preparation
3.4. Preparation of Standard Solutions
3.5. HPLC-UV Analysis of Organic Acids
3.6. Fatty Acids Purification and Derivatization
3.7. GC-IT/MS Analysis of Fatty Acids
3.8. Cell Culture and Treatments
3.9. MTT Reduction Assay
3.10. Quantification of Released β-Hexosaminidase
3.11. β-Hexosaminidase Inhibitory Activity
3.12. Lipoxygenase Inhibition Assay
3.13. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Singh, A.; Holvoet, S.; Mercenier, A. Dietary polyphenols in the prevention and treatment of allergic diseases. Clin. Exp. Allergy 2011, 41, 1346–1359. [Google Scholar] [CrossRef]
- Medeiros, K.C.P.; Figueiredo, C.A.V.; Figueiredo, T.B.; Freire, K.R.L.; Santos, F.A.R.; Alcantara-Neves, N.M.; Silva, T.M.S.; Piuvezam, M.R. Anti-allergic effect of bee pollen phenolic extract and myricetin in ovalbumin-sensitized mice. J. Ethopharmacol. 2008, 119, 41–46. [Google Scholar] [CrossRef]
- Rossenwasser, L.J. Mechanisms of IgE inflammation. Curr. Allergy Asthma Rep. 2011, 11, 178–183. [Google Scholar] [CrossRef]
- Broide, D.H. Molecular and cellular mechanisms of allergic disease. J. Allergy Clin. Immunol. 2001, 108, 565–571. [Google Scholar] [CrossRef]
- Chan, T.K.; Ng, D.S.W.; Cheng, C.; Guan, S.P.; Koh, H.M.; Wong, W.S.F. Anti-allergic actions of rottlerin from Mallotus philippinensis in experimental mast cell-mediated anaphylactic models. Phytomedicine 2013, 20, 853–860. [Google Scholar] [CrossRef]
- Valenta, R. The future of antigen-specific immunotherapy of allergy. Nat. Rev. Immunol. 2002, 2, 446–453. [Google Scholar]
- Vlaisavljevic, S.; Kaurinovic, B.; Popovic, M.; Djurendic-Brenesel, M.; Vasiljevic, B.; Cvetkovic, D.; Vasiljevic, S. Trifolium pratense L. as a potential natural antioxidant. Molecules 2014, 19, 713–725. [Google Scholar] [CrossRef]
- Ferreres, F.; Pereira, D.M.; Valentão, P.; Andrade, P.B. First report of non-coloured flavonoids in Echium plantagineum bee pollen: Differentiation of isomers by liquid chromatography/iontrap mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 801–806. [Google Scholar] [CrossRef]
- Moita, E.; Gil-Izquierdo, A.; Sousa, C.; Ferreres, F.; Silva, L.R.; Valentão, P.; Domínguez-Perles, R.; Baenas, N.; Andrade, P.B. Integrated analysis of COX-2 and iNOS derived inflammatory mediators in LPS-stimulated RAW macrophages pre-exposed to Echium plantagineum L. bee pollen extract. PLoSOne 2013, 8, e59131. [Google Scholar]
- Lee, E.J.; Kim, J.S.; Kim, H.P.; Lee, J-H; Kang, S.S. Phenolic constituents from the flower buds of Lonicera japonica and their 5-lipoxygenase inhibitory activities. Food Chem. 2010, 120, 134–139. [Google Scholar] [CrossRef]
- López-Bucio, J.; Nieto-Jacobo, M.F.; Ramírez-Rodríguez, V.; Herrera-Estrella, L. Organic acid metabolism in plants: From adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci. 2000, 160, 1–13. [Google Scholar] [CrossRef]
- Guimarães, L.; Barros, M.; Dueñas, M.; Carvalho, A.M.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterisation of phenolic compounds in wild fruits from Northeastern Portugal. Food Chem. 2013, 141, 3721–3730. [Google Scholar] [CrossRef]
- Almeida-Muradian, L.B.; Pamplona, L.C.; Coimbra, S.; Barth, O.M. Chemical composition and botanical evaluation of dried bee pollen pellets. J. Food Compos. Anal. 2005, 18, 105–111. [Google Scholar] [CrossRef]
- Yang, Q.; O’Shea, T.M. Dietary Echium oil increases tissue (n-3) long-chain polyunsaturated fatty acids without elevating hepatic lipid concentrations in premature neonatal rats. J. Nutr. 2009, 139, 1353–1359. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; García-Maroto, F.; Campra-Madrid, P.; Gómez-Mercado, F. Occurrence and characterization of oils rich in γ-linolenic acid Part II: Fatty acids and squalene from Macaronesian Echium leaves. Phytochemistry 2000, 54, 525–529. [Google Scholar] [CrossRef]
- Simpoulos, A.P. The importance ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Luckasen, R.; Whiteand, J.G.; Hersey, J.H. Mitogenic properties of a calcium ionophore, A23187. Proc. Natl. Acad. Sci. USA 1974, 71, 5088–5090. [Google Scholar] [CrossRef]
- Sousa, C.; Pontes, H.; Carmo, H.; Dinis-Oliveira, R.J.; Valentão, P.; Andrade, P.B.; Remião, F.; Bastos, M.L.; Carvalho, F. Water extracts of Brassica oleracea var. costata potentiate paraquat toxicity to rat hepatocytes in vitro. Toxicol. In Vitro 2009, 23, 1131–1138. [Google Scholar] [CrossRef]
- Tang, J.-M.; Liu, J.; Wu, W. Studies on the degranulation of RBL-2H3 cells induced by traditional Chinese medicine injections. Chin. Med. 2012, 3, 200–208. [Google Scholar] [CrossRef]
- Gründemann, C.; Papagiannopoulos, M.; Lamy, E.; Mersch-Sundermann, V.; Huber, R. Immunomodulatory properties of a lemon-quince preparation (Gencydo®) as an indicator of anti-allergic potency. Phytomedicine 2011, 18, 760–768. [Google Scholar] [CrossRef]
- Yodsaoue, O.; Cheeprache, S.; Karla, C.; Ponglimanont, C.; Tewtrakul, S. Anti-allergic activity of principles from the roots and heartwood of Caesalpinia sappan on antigen induced β-hexosaminidase release. Phytother. Res. 2009, 23, 1028–1031. [Google Scholar] [CrossRef]
- Stevens, R.L.; Austen, K.F. Recent advances in the cellular and molecular biology of mast cells. Immunol. Today 1989, 10, 381–386. [Google Scholar] [CrossRef]
- Cheung, K.L.; Chen, H.; Chen, Q.; Wang, J.; Ho, H.P.; Wong, C.K.; Kong, S.K. CTAB-coated gold nanorods elicit allergic response through degranulation and cell death in human basophils. Nanoscale 2012, 4, 4447–4449. [Google Scholar] [CrossRef]
- Kawakami, T.; Galli, J.J. Regulation of mast-cell and basophil function and survival by IgE. Nat. Rev. Immunol. 2002, 2, 773–786. [Google Scholar] [CrossRef]
- Kepley, C.L.; Lauer, F.T.; Oliver, J.M.; Burchiel, S.W. Environmental polycyclic aromatic hydrocarbons, benzo(a)pyrene (BaP) and BaP-quinones, enhance IgE-mediated histamine release and IL-4 production in human basophils. Clin. Immunol. 2003, 107, 10–19. [Google Scholar] [CrossRef]
- Gueck, T.; Seidel, A.; Fuhrmann, H. Effects of essential fatty acids on mediators of mast cells in culture. Essent. Fatty Acids 2003, 68, 317–322. [Google Scholar] [CrossRef]
- Lumia, M.; Luukkainen, P.; Tapanaimen, H.; Kaila, M.; Erkkola, M.; Uusitalo, L.; Niinisto, S.; Kenward, M.G.; Ilonen, J.; Simell, O.; et al. Dietary fatty acid composition during pregnancy and the risk of asthma in the offspring. Pediatr. Allergy Immunol. 2011, 22, 827–835. [Google Scholar] [CrossRef]
- Kawai, M.; Hirano, T.; Higa, S.; Arimitsu, J.; Maruta, M.; Kuwahara, Y.; Ohkawara, T.; Hagihara, K.; Yamadori, T.; Shima, Y.; et al. Flavonoids and related compounds as anti-allergic substances. Allergol. Int. 2007, 56, 113–123. [Google Scholar] [CrossRef]
- Pergola, C.; Werz, O. 5-Lipoxygenase inhibitors: A review of recent developments and patents. Expert Opin. Ther. Patents 2010, 20, 355–375. [Google Scholar] [CrossRef]
- Prigge, S.T.; Boyington, J.C.; Gaffney, B.J.; Amzel, L.M. Structure conservation in lipoxygenases: Structural analysis of soybean lipoxygenase-1 and modeling of human lipoxygenases. Protein Struct. Funct. Genet. 1996, 24, 275–291. [Google Scholar] [CrossRef]
- Pereira, D.M.; Faria, J.; Gaspar, L.; Valentão, P.; Andrade, P.B. Boerhaavia diffusa: Metabolite profiling of a medicinal plant from Nyctaginaceae. Food Chem. Toxicol. 2009, 47, 2142–2149. [Google Scholar] [CrossRef]
- Pereira, D.M.; Vinholes, J.; Guedes de Pinho, P.; Valentão, P.; Mouga, T.; Teixeira, N.; Andrade, P.B. A gas chromatography-mass spectrometry multi-target method for the simultaneous analysis of three classes of metabolites in marine organisms. Talanta 2012, 100, 391–400. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. Available online: http://webbook.nist.gov/chemistry/name-ser.html (accessed on 6 January 2014).
- Sheperd, T.; Dobson, G.; Verrall, S.R.; Conner, S.; Griffiths, D.W.; McNicol, J.W.; Davies, H.V.; Stewart, D. Potato metabolomics by GC-MS: What are the limiting factors? Metabolomics 2007, 3, 475–488. [Google Scholar] [CrossRef]
- Pinho, B.R.; Sousa, C.; Valentão, P.; Oliveira, J.M.A.; Andrade, P.B. Modulation of basophils’ degranulation and allergy-related enzymes by monomeric and dimeric naphthoquinones. PLoS One 2014, 9, e90122. [Google Scholar]
- Sample Availability: Samples of E. plantagineum bee pollen are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moita, E.; Sousa, C.; Andrade, P.B.; Fernandes, F.; Pinho, B.R.; Silva, L.R.; Valentão, P. Effects of Echium plantagineum L. Bee Pollen on Basophil Degranulation: Relationship with Metabolic Profile. Molecules 2014, 19, 10635-10649. https://doi.org/10.3390/molecules190710635
Moita E, Sousa C, Andrade PB, Fernandes F, Pinho BR, Silva LR, Valentão P. Effects of Echium plantagineum L. Bee Pollen on Basophil Degranulation: Relationship with Metabolic Profile. Molecules. 2014; 19(7):10635-10649. https://doi.org/10.3390/molecules190710635
Chicago/Turabian StyleMoita, Eduarda, Carla Sousa, Paula B. Andrade, Fátima Fernandes, Brígida R. Pinho, Luís R. Silva, and Patrícia Valentão. 2014. "Effects of Echium plantagineum L. Bee Pollen on Basophil Degranulation: Relationship with Metabolic Profile" Molecules 19, no. 7: 10635-10649. https://doi.org/10.3390/molecules190710635
APA StyleMoita, E., Sousa, C., Andrade, P. B., Fernandes, F., Pinho, B. R., Silva, L. R., & Valentão, P. (2014). Effects of Echium plantagineum L. Bee Pollen on Basophil Degranulation: Relationship with Metabolic Profile. Molecules, 19(7), 10635-10649. https://doi.org/10.3390/molecules190710635






