In Vitro Inhibitory Effects of Scutellarin on Six Human/Rat Cytochrome P450 Enzymes and P-glycoprotein
Abstract
:1. Introduction
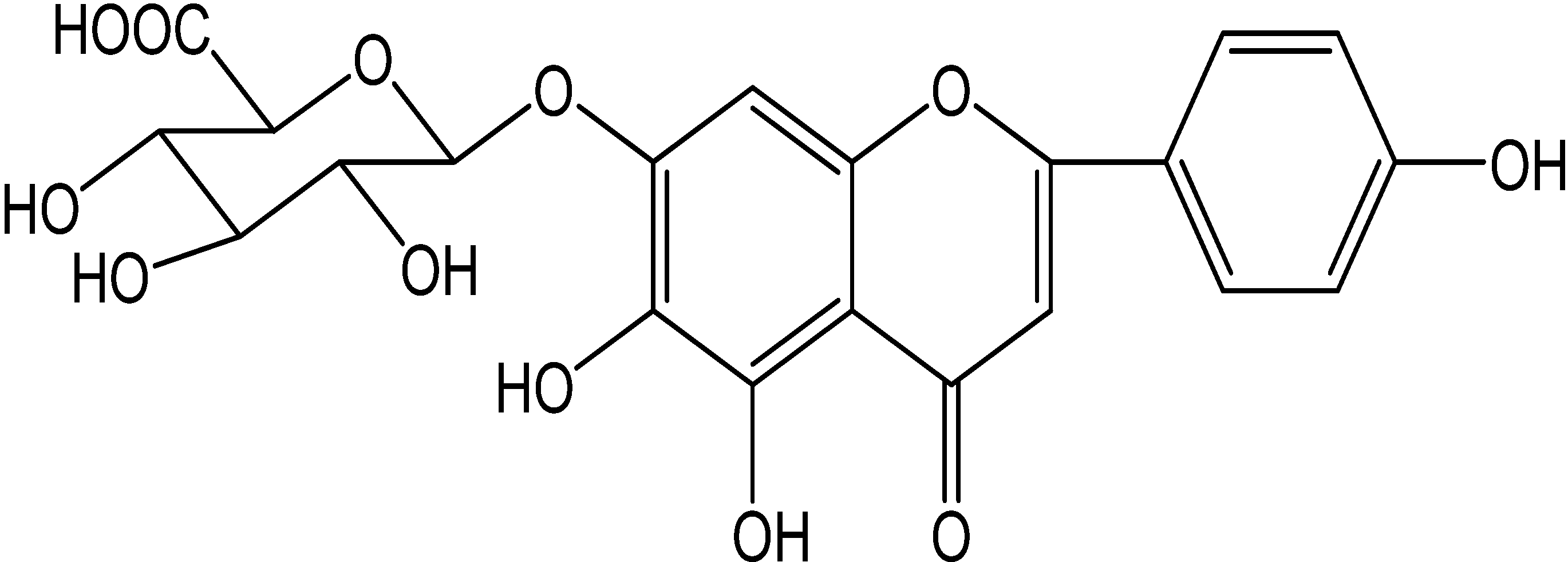
2. Results and Discussion
| Isoenzyme | Substrate | Metabolites | IC50 for HLM | IC50 for RLM |
|---|---|---|---|---|
| (μM) | (μM) | |||
| CYP1A2 | Phenacetin | Acetaminophen | >100 | >100 |
| CYP2C8 | Paclitaxel | 6α-Hydroxypaclitaxel | >100 | 63.1[95% CI: 39.3–101.3] |
| CYP2C9 | Diclofenac | 4-Hydroxydiclofenac | >100 | >100 |
| CYP2C19 | S-Mephenytoin | 4-Hydroxymephenytoin | 63.8 [95% CI: 38.5–105.7] | 85.6[95% CI: 43.5–168.4] |
| CYP2D6 | Dextromethorphan | Dextrorphan | >100 | >100 |
| CYP3A4 | Midazolam | 1-Hydroxymidazolam | >100 | >100 |
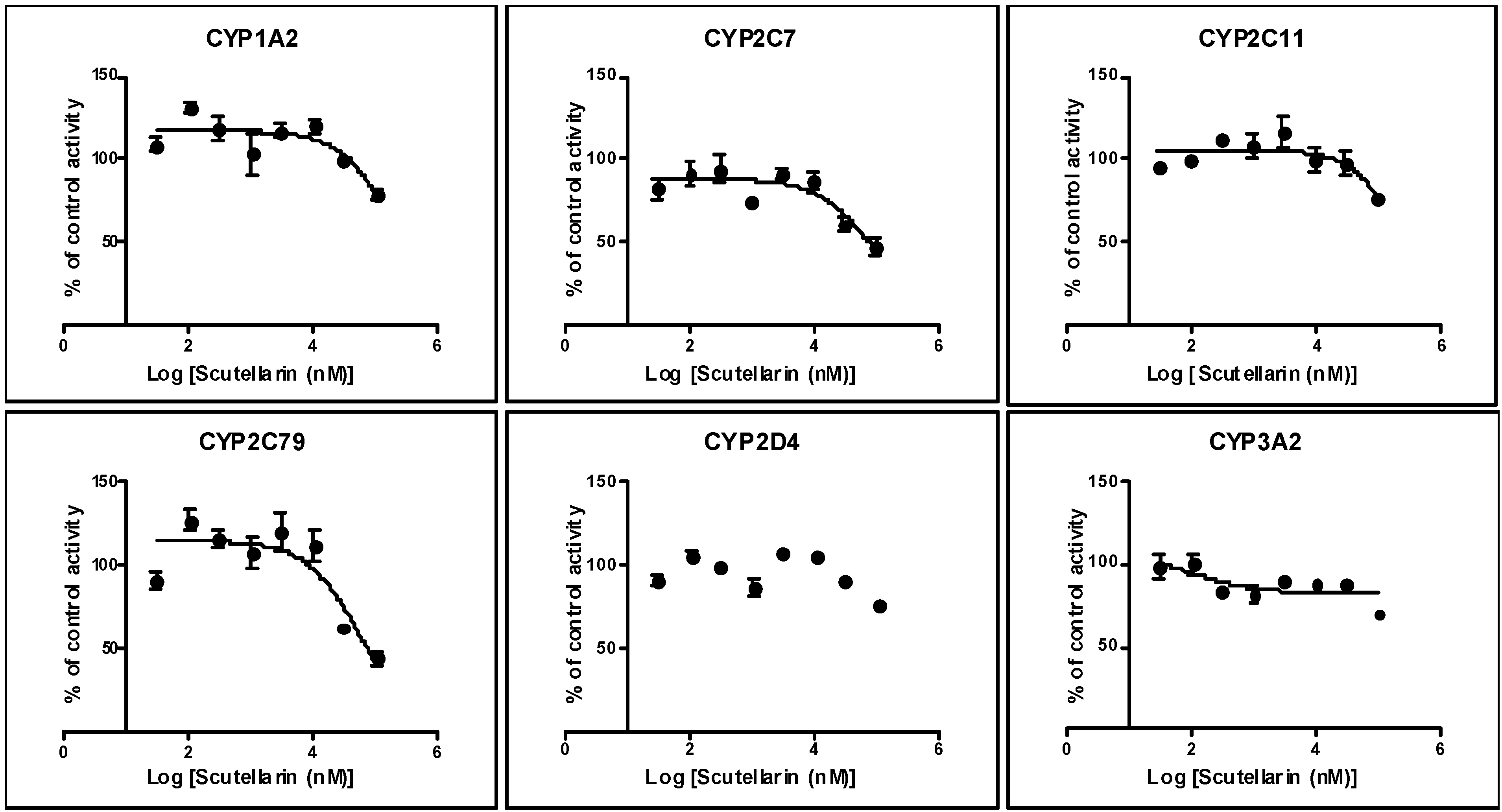
2.1. Inhibition by Scutellarin on Six CYPs in HLM
2.2. Inhibition by Scutellarin on Six CYPs in RLM

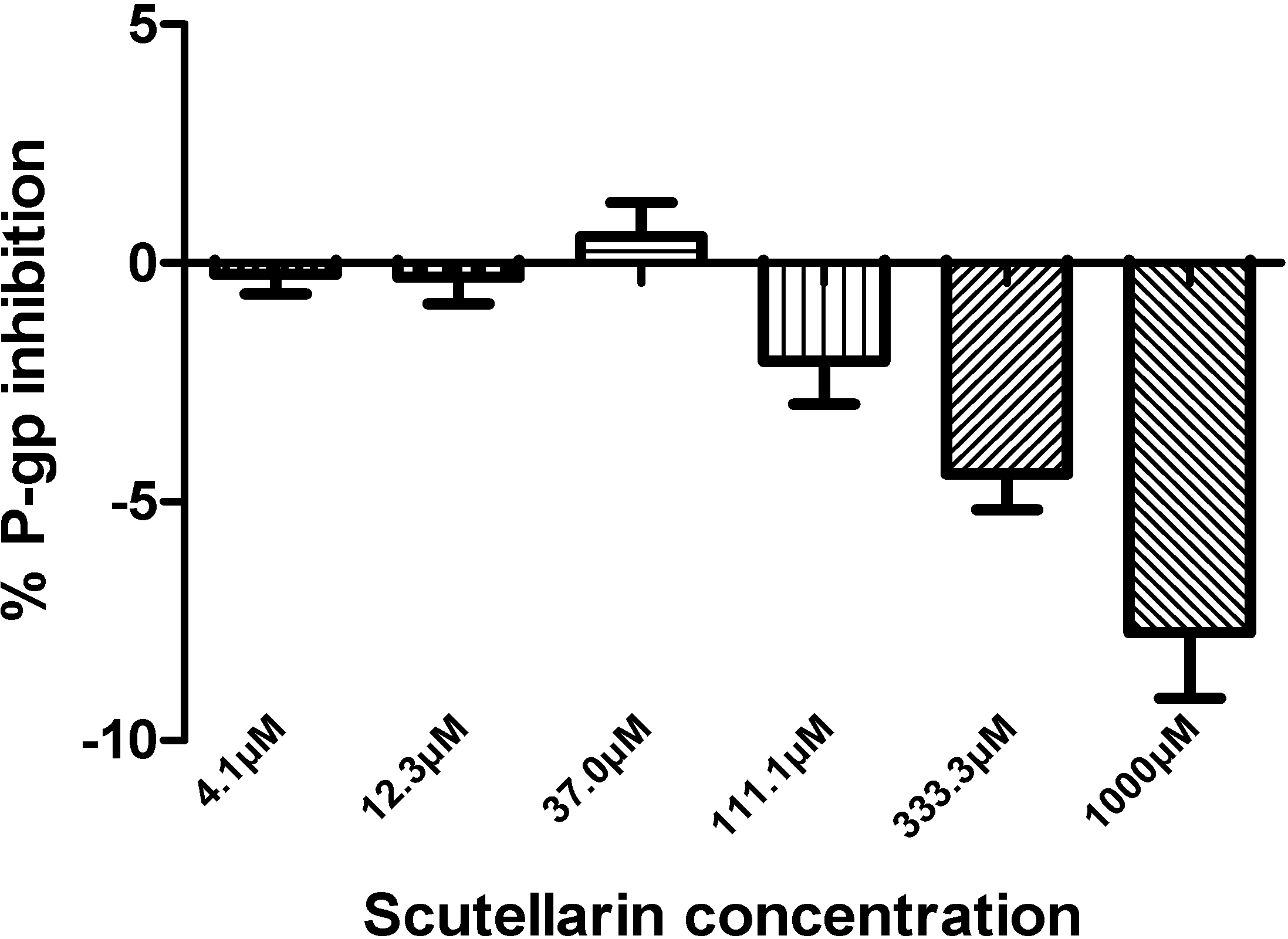
2.3. Inhibition by Scutellarin on P-glycoprotein
3. Experimental
3.1. Materials
| Unit | Gender | Age (From 21 to 77 Years) | Race | |||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | ≤39 | 40~49 | 50~59 | ≥60 | Caucasian | African Ameracian | |
| Number | 27 | 23 | 8 | 15 | 13 | 14 | 47 | 3 |
3.2. Instrument
3.3. CYP450 Assay
3.3.1. Microsomal Incubations and Treatment Protocol
3.3.2. LC-MS/MS Analysis
3.4. P-glycoprotein Assay [49]
3.5. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McGraw, J.; Waller, D. Cytochrome P450 variations in different ethnic populations. Expert. Opin. Drug Met. 2012, 8, 371–382. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Ambudkar, S.V.; Kimchi-Sarfaty, C.; Sauna, Z.E.; Gottesman, M.M. P-glycoprotein: From genomics to mechanism. Oncogene 2003, 22, 7468–7485. [Google Scholar] [CrossRef]
- Lin, J.H.; Yamazaki, M. Role of P-glycoprotein in pharmacokinetics: Clinical implications. Clin. Pharmacokinet. 2003, 42, 59–98. [Google Scholar] [CrossRef]
- Balayssac, D.; Authier, N.; Cayre, A.; Coudore, F. Does inhibition of P-glycoprotein lead to drug-drug interactions? Toxicol. Lett. 2005, 156, 319–329. [Google Scholar] [CrossRef]
- Zhang, L.; Reynolds, K.S.; Zhao, P.; Huang, S.M. Drug interactions evaluation: An integrated part of risk assessment of therapeutics. Toxicol. Appl. Pharmacol. 2010, 243, 134–145. [Google Scholar] [CrossRef]
- Song, M.; Hong, M.; Lee, M.; Jee, J.; Lee, Y.M.; Bae, J.S.; Jeong, T.C.; Lee, S. Selective inhibition of the cytochrome P450 isoform by hyperoside and its potent inhibition of CYP2D6. Food Chem. Toxicol. 2013, 59, 549–553. [Google Scholar]
- Tian, X.; Cheng, Z.Y.; He, J.; Jia, L.J.; Qiao, H.L. Concentration-dependent inhibitory effects of baicalin on the metabolism of dextromethorphan, a dual probe of CYP2D and CYP3A, in rats. Chem. Biol. Interact. 2013, 203, 522–529. [Google Scholar] [CrossRef]
- Tian, X.; Cheng, Z.Y.; Jin, H.; Gao, J.; Qiao, H.L. Inhibitory effects of baicalin on the expression and activity of CYP3A induce the pharmacokinetic changes of midazolam in rats. Evid-Based Compl. Alt. 2013, 2013. [Google Scholar] [CrossRef]
- Wongwanakul, R.; Vardhanabhuti, N.; Siripong, P.; Jianmongkol, S. Effects of rhinacanthin-C on function and expression of drug efflux transporters in Caco-2 cells. Fitoterapia 2013, 89, 80–85. [Google Scholar] [CrossRef]
- Qiang, F.; Lee, B.J.; Ha, I.; Kang, K.W.; Woo, E.R.; Han, H.K. Effect of maceligan on the systemic exposure of paclitaxel: In vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2010, 41, 226–231. [Google Scholar] [CrossRef]
- National Commission of Chinese Pharmacopoeia. Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2010; pp. 707–708. [Google Scholar]
- Liao, S.G.; Zhang, L.J.; Li, C.B.; Lan, Y.Y.; Wang, A.M.; Huang, Y.; Zhen, L.; Fu, X.Z.; Zhou, W.; Qi, X.L.; et al. Rapid screening and identification of caffeic acid and its esters in Erigeron breviscapus by ultra-performance liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Sp. 2010, 24, 2533–2541. [Google Scholar]
- Han, Y.L.; Li, D.; Ren, B.; Jing, G.P.; Meng, X.L.; Zhou, Z.Y.; Yu, Q.; Li, Y.; Wan, L.L.; Guo, C. Evaluation of impact of Herba Erigerontis injection, a Chinese herbal prescription, on rat hepatic cytochrome P450 enzymes by cocktail probe drugs. J. Ethnopharmacol. 2012, 139, 104–109. [Google Scholar]
- Hong, H.; Liu, G. Protection against hydrogen peroxide-induced cytotoxicity in PC12 cells by scutellarin. Life Sci. 2004, 74, 2959–2973. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Ou, J.; Jiang, J.; Wu, D. Research on scutellarin parenteral solution’s protective effects in rats with severe acute pancreatitis and multiple organ injuries. Inflammation 2012, 35, 1005–1014. [Google Scholar] [CrossRef]
- Luo, P.; Zhang, Z.; Yi, T.; Zhang, H.; Liu, X.; Mo, Z. Anti-inflammatory activity of the extracts and fractions from Erigeron multiradiatus through bioassay-guided procedures. J. Ethnopharmacol. 2008, 119, 232–237. [Google Scholar] [CrossRef]
- Luo, P.; Tan, Z.H.; Zhang, Z.F.; Zhang, H.; Liu, X.F.; Mo, Z.J. Scutellarin isolated from Erigeron multiradiatus inhibits high glucose-mediated vascular inflammation. Yakuga. Zasshi 2008, 128, 1293–1299. [Google Scholar] [CrossRef]
- Su, Y.; Liu, W.; Ma, L.; Liu, X.; Liu, Z.; Zhu, B. Scutellarin inhibits translocation of protein kinase C in diabetic thoracic aorta of the rat. Clin. Exp. Pharmacol. Physiol. 2012, 39, 136–140. [Google Scholar] [CrossRef]
- Pan, Z.W.; Zhang, Y.; Mei, D.H.; Zhang, R.; Wang, J.H.; Zhang, X.Y.; Xu, C.Q.; Lu, Y.J.; Yang, B.F. Scutellarin exerts its anti-hypertrophic effects via suppressing the Ca2+-mediated calcineurin and CaMKII signaling pathways. Naunyn Schmiedebergs Arch. Pharmacol. 2010, 381, 137–145. [Google Scholar] [CrossRef]
- Lin, L.L.; Liu, A.J.; Liu, J.G.; Yu, X.H.; Qin, L.P.; Su, D.F. Protective effects of scutellarin and breviscapine on brain and heart ischemia in rats. J. Cardiovasc. Pharmacol. 2007, 50, 327–332. [Google Scholar]
- Zhang, G.; Qiu, S.; Wei, H. Scutellarin blocks sodium current in freshly isolated mouse hippocampal CA1 neurons. Neurochem. Res. 2011, 36, 947–54. [Google Scholar] [CrossRef]
- Lu, K.; Han, M.; Ting, H.L.; Liu, Z.; Zhang, D. Scutellarin from Scutellaria baicalensis suppresses adipogenesis by upregulating PPARα in 3T3-L1 cells. J. Nat. Prod. 2013, 76, 672–678. [Google Scholar] [CrossRef]
- Zhang, G.H.; Wang, Q.; Chen, J.J.; Zhang, X.M.; Tam, S.C.; Zheng, Y.T. The anti-HIV-1 effect of scutellarin. Biochem. Biophys. Res. Commun. 2005, 334, 812–816. [Google Scholar] [CrossRef]
- Bai, X.; Sun, N.; Zhao, X.; Niu, L.; Song, M.; Sun, Y.; Jiang, J.; Guo, J.; Bai, Y.; He, J.; et al. In vitro Screening for compounds derived from traditional Chinese medicines (TCMs) with antiviral activities against porcine reproductive and respiratory syndrome virus. J. Microbiol. Biotechnol. 2013, 23, 1076–1083. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, S.; Tu, J.; Cao, Z.; Pan, Y.; Shang, B.; Liu, R.; Bao, M.; Guo, P.; Zhou, Q. Novel function of scutellarin in inhibiting cell proliferation and inducing cell apoptosis of human Burkitt lymphoma Namalwa cells. Leuk. Lymphoma 2012, 53, 2456–2464. [Google Scholar] [CrossRef]
- Li, H.; Huang, D.; Gao, Z.; Chen, Y.; Zhang, L.; Zheng, J. Scutellarin inhibits the growth and invasion of human tongue squamous carcinoma through the inhibition of matrix metalloproteinase-2 and -9 and αvβ6 integrin. Int. J. Oncol. 2013, 42, 1674–1681. [Google Scholar]
- Li, H.; Fan, H.; Wang, Z.; Zheng, J.; Cao, W. Potentiation of scutellarin on human tongue carcinoma xenograft by low-intensity ultrasound. PLoS One 2013, 8, e59473. [Google Scholar]
- Xu, H.; Zhang, S. Scutellarin-induced apoptosis in HepG2 hepatocellular carcinoma cells via a STAT3 pathway. Phytother. Res. 2013, 27, 1524–1528. [Google Scholar]
- Yang, B.; Zhao, Y.L.; Yang, X.; Liao, X.L.; Yang, J.; Zhang, J.H.; Gao, C.Z. Scutellarin-cyclodextrin conjugates: Synthesis, characterization and anticancer activity. Carbohydr. Polym. 2013, 92, 1308–1314. [Google Scholar] [CrossRef]
- Wu, Y.X.; Sato, E.; Kimura, W.; Miura, N. Baicalin and scutellarin are proteasome inhibitors that specifically target chymotrypsin-like catalytic activity. Phytother. Res. 2013, 27, 1362–1367. [Google Scholar]
- Li, Q.; Wu, J.H.; Guo, D.J.; Cheng, H.L.; Chen, S.L.; Chan, S.W. Suppression of diet-induced hypercholesterolemia by scutellarin in rats. Planta Med. 2009, 75, 1203–1208. [Google Scholar] [CrossRef]
- Gao, Z.X.; Huang, D.Y.; Li, H.X.; Zhang, L.N.; Lv, Y.H.; Cui, H.D.; Zheng, J.H. Scutellarin promotes in vitro angiogenesis in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2010, 400, 151–156. [Google Scholar] [CrossRef]
- Pan, Z.; Feng, T.; Shan, L.; Cai, B.; Chu, W.; Niu, H.; Lu, Y.; Yang, B. Scutellarin-induced endothelium-independent relaxation in rat aorta. Phytother. Res. 2008, 22, 1428–1433. [Google Scholar] [CrossRef]
- Tan, Z.H.; Yu, L.H.; Wei, H.L.; Liu, G.T. The protective action of scutellarin against immunological liver injury induced by concanavalin A and its effect on pro-inflammatory cytokines in mice. J. Pharm. Pharmacol. 2007, 59, 115–121. [Google Scholar] [CrossRef]
- Chai, L.; Guo, H.; Li, H.; Wang, S.; Wang, Y.L.; Shi, F.; Hu, L.M.; Liu, Y.; Adah, D. Scutellarin and caffeic acid ester fraction, active components of Dengzhanxixin injection, upregulate neurotrophins synthesis and release in hypoxia/reoxygenation rat astrocytes. J. Ethnopharmacol. 2013, 150, 100–107. [Google Scholar] [CrossRef]
- Guo, H.; Hu, L.M.; Wang, S.X.; Wang, Y.L.; Shi, F.; Li, H.; Liu, Y.; Kang, L.Y.; Gao, X.M. Neuroprotective effects of scutellarin against hypoxic-ischemic-induced cerebral injury via augmentation of antioxidant defense capacity. Chin. J. Physiol. 2011, 54, 399–405. [Google Scholar]
- Guo, L.L.; Guan, Z.Z.; Wang, Y.L. Scutellarin protects against Aβ-induced learning and memory deficits in rats: Involvement of nicotinic acetylcholine receptors and cholinesterase. Acta Pharm. Sin. 2011, 32, 1446–1453. [Google Scholar] [CrossRef]
- Guo, L.L.; Guan, Z.Z.; Huang, Y.; Wang, Y.L.; Shi, J.S. The neurotoxicity of β-amyloid peptide toward rat brain is associated with enhanced oxidative stress, inflammation and apoptosis, all of which can be attenuated by scutellarin. Exp. Toxicol. Pathol. 2013, 65, 579–584. [Google Scholar] [CrossRef]
- Li, J.H.; Lu, J.; Zhang, H. Functional recovery after scutellarin treatment in transient CEREBRAL ischemic rats: A pilot study with 18 F-fluorodeoxyglucose micropet. Evid. Based Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Xu, W.; Zha, R.P.; Wang, W.Y.; Wang, Y.P. Effects of scutellarin on PKCgamma in PC12 cell injury induced by oxygen and glucose deprivation. Acta Pharm. Sin. 2007, 28, 1573–1579. [Google Scholar]
- Zhu, J.; Choi, R.; Li, J.; Xie, H.; Bi, C.; Cheung, A.; Dong, T.; Jiang, Z.; Chen, J.; Tsim, K. Estrogenic and neuroprotective properties of scutellarin from Erigeron breviscapus: A drug against postmenopausal symptoms and Alzheimer's disease. Planta Med. 2009, 75, 1489–1493. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Guo, H.; Kang, L.; Gao, X.; Hu, L. Neuroprotection of scutellarin is mediated by inhibition of microglial inflammatory activation. Neuroscience 2011, 185, 150–160. [Google Scholar] [CrossRef]
- Cui, M.Y.; Tian, C.C.; Ju, A.X.; Zhang, C.T.; Li, Q.H. Pharmacokinetic interaction between scutellarin and valsartan in rats. Acta Pharm. Sin. 2013, 48, 541–546. [Google Scholar]
- Ma, G.Y.; Cao, Y.F.; Hu, C.M.; Fang, Z.Z.; Sun, X.Y.; Hong, M.; Zhu, Z.T. Comparison of Inhibition Capability of Scutellarein and Scutellarin Towards Important Liver UDP-Glucuronosyltransferase (UGT) Isoforms. Phytother. Res. 2014, 28, 382–386. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Li, Y.; Bai, L.; Xue, M. Acute and subacute toxicological evaluation of scutellarin in rodents. Regul. Toxicol. Pharm. 2011, 60, 106–111. [Google Scholar] [CrossRef]
- Guidance for Industry: Drug interaction studies-study design, data analysis, and implications for dosing and labelling recommendations. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM292362.pdf (accessed on 4 December 2013).
- Pelkonen, O.; Turpeinen, M.; Hakkola, J.; Honkakoski, P.; Hukkanen, J.; Raunio, H. Inhibition and induction of human cytochrome P450 enzymes: Current status. Arch. Toxicol. 2008, 82, 667–715. [Google Scholar] [CrossRef]
- Walsky, R.L.; Obach, R.S. Verification of the selectivity of (+)N-3-benzylnirvanol as a CYP2C19 inhibitor. Drug Metab. Dispos. 2003, 31, 343. [Google Scholar] [CrossRef]
- Yuan, R.; Madani, S.; Wei, X.X.; Reynolds, K.; Huang, S.M. Evaluation of cytochrome P450 probe substrates commonly used by the pharmaceutical industry to study in vitro drug interactions. Drug Metab. Dispos. 2002, 30, 1311–1319. [Google Scholar] [CrossRef]
- Han, Y.L.; Yu, H.L.; Meng, X.L.; Li, D.; Zhou, Z.Y.; Yu, Q.; Wang, F.J.; Zhang, X.Y.; Guo, C. In vitro inhibition of Huanglian [Rhizoma coptidis (L.)] and its six active alkaloids on six cytochrome P450 isoforms in human liver microsomes. Phytother. Res. 2011, 25, 1660–1665. [Google Scholar] [CrossRef]
- Han, Y.L.; Yu, H.L.; Meng, X.L.; Li, D.; Zhou, Z.Y.; Yu, Q.; Wang, F.J.; Zhang, X.Y.; Guo, C. Inhibitory effects of limonin on six human cytochrome P450 enzymes and P-glycoprotein in vitro. Toxicol. In Vitro 2011, 25, 1828–1833. [Google Scholar] [CrossRef]
- Jian, T.Y.; He, J.C.; He, G.H.; Feng, E.F.; Li, H.L.; Bai, M.; Xu, G.L. Scutellarin inhibits cytochrome P450 isoenzyme 1A2 (CYP1A2) in rats. Phytother. Res. 2013, 26, 1226–1230. [Google Scholar]
- Dierks, E.A.; Stams, K.R.; Lim, H.K.; Cornelius, G.; Zhang, H.; Ball, S.E. A method for the simultaneous evaluation of the activities of seven major human drug-metabolizing cytochrome P450s using an in vitro cocktail of probe substrates and fast gradient liquid chromatography tandem mass spectrometry. Drug Metab. Dispos. 2001, 29, 23–29. [Google Scholar]
- Herzke, D.; Thiel, R.; Rotard, W.D.; Neubert, D. Kinetics and organotropy of some polyfluorinated dibenzo-p-dioxins and dibenzofurans (PFDD/PFDF) in rats. Life Sci. 2002, 71, 1475–1486. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds and materials are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Han, Y.-L.; Li, D.; Yang, Q.-J.; Zhou, Z.-Y.; Liu, L.-Y.; Li, B.; Lu, J.; Guo, C. In Vitro Inhibitory Effects of Scutellarin on Six Human/Rat Cytochrome P450 Enzymes and P-glycoprotein. Molecules 2014, 19, 5748-5760. https://doi.org/10.3390/molecules19055748
Han Y-L, Li D, Yang Q-J, Zhou Z-Y, Liu L-Y, Li B, Lu J, Guo C. In Vitro Inhibitory Effects of Scutellarin on Six Human/Rat Cytochrome P450 Enzymes and P-glycoprotein. Molecules. 2014; 19(5):5748-5760. https://doi.org/10.3390/molecules19055748
Chicago/Turabian StyleHan, Yong-Long, Dan Li, Quan-Jun Yang, Zhi-Yong Zhou, Li-Ya Liu, Bin Li, Jin Lu, and Cheng Guo. 2014. "In Vitro Inhibitory Effects of Scutellarin on Six Human/Rat Cytochrome P450 Enzymes and P-glycoprotein" Molecules 19, no. 5: 5748-5760. https://doi.org/10.3390/molecules19055748
APA StyleHan, Y.-L., Li, D., Yang, Q.-J., Zhou, Z.-Y., Liu, L.-Y., Li, B., Lu, J., & Guo, C. (2014). In Vitro Inhibitory Effects of Scutellarin on Six Human/Rat Cytochrome P450 Enzymes and P-glycoprotein. Molecules, 19(5), 5748-5760. https://doi.org/10.3390/molecules19055748




