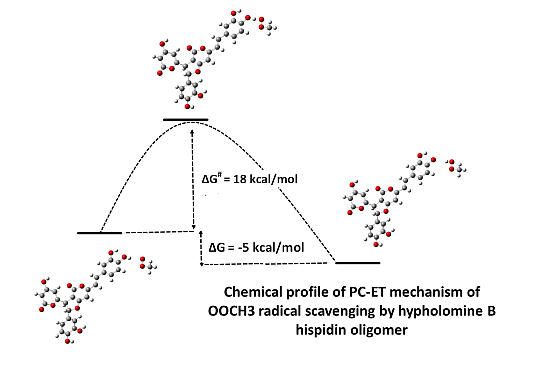
Antioxidant Activity of Hispidin Oligomers from Medicinal Fungi: A DFT Study
Abstract
:1. Introduction
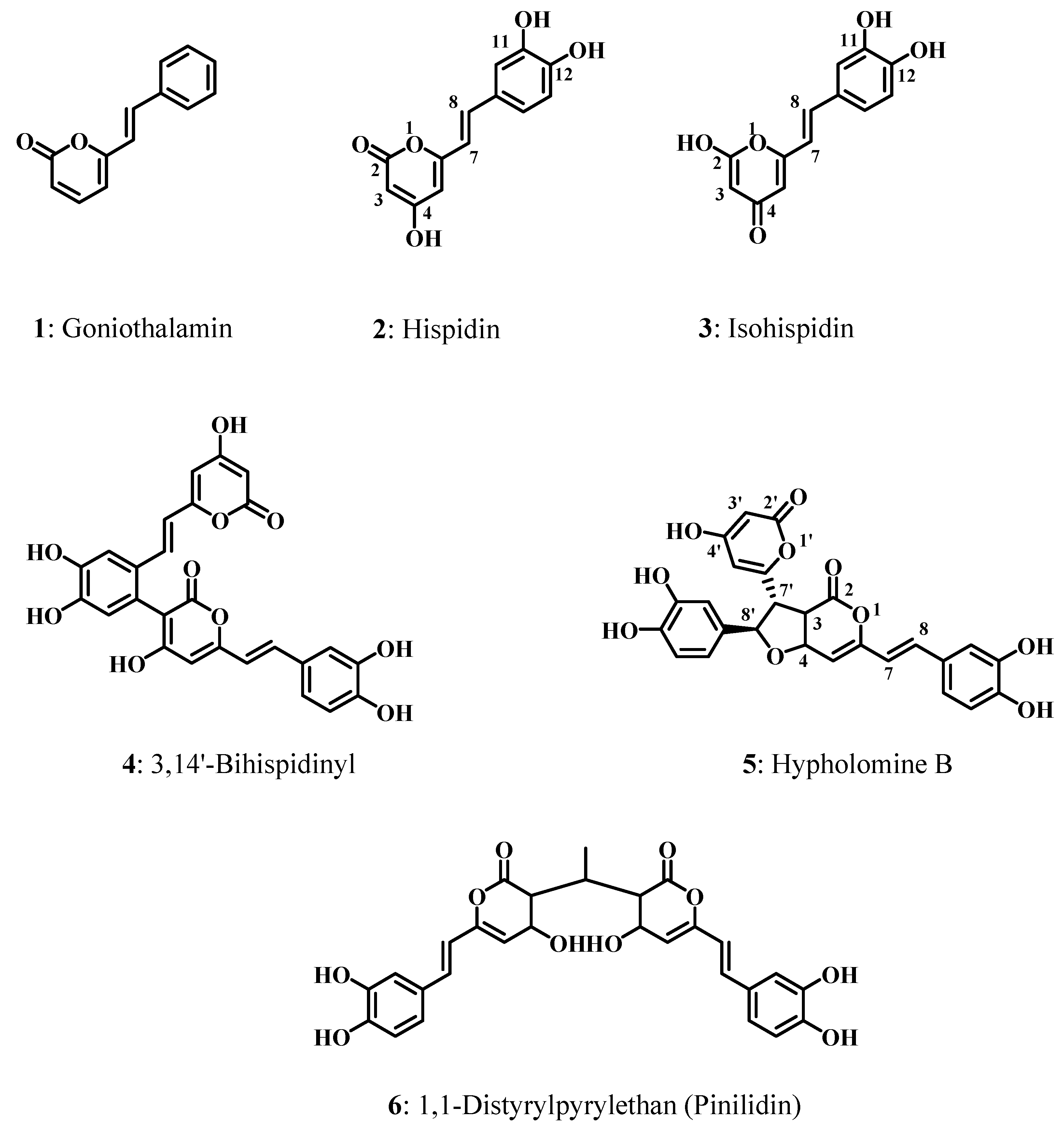
2. Results and Discussion
2.1. Hispidin Dimerization
2.2. Structure-Antioxidant Activity Relationships
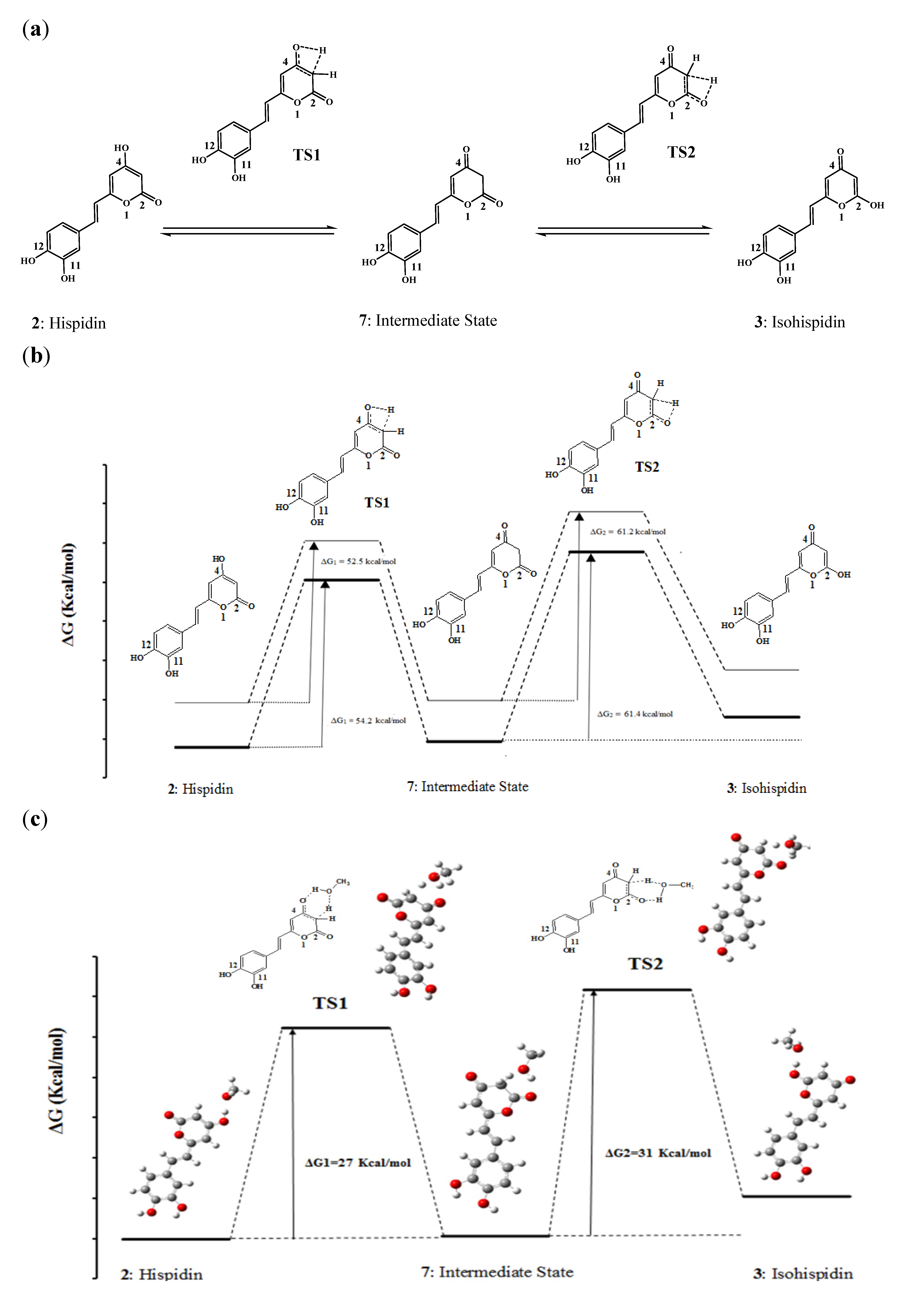
2.2.1. Tautomerism Effect
| Compounds | Gas | PCM | ||
|---|---|---|---|---|
| B3P86/6-31+G(d,p) | B3P86/6-311+G(d,p) | B3P86/6-31+G(d,p) | B3P86/6-311+G(d,p) | |
| BDE (kcal/mol) | ||||
| Hispidin | ||||
| 4-OH | 90.0 | 88.2 | 90.6 | 89.5 |
| 11-OH | 78.9 | 78.7 | 80.3 | 80.0 |
| 12-OH | 75.0 | 74.6 | 76.2 | 75.9 |
| Isohispidin | ||||
| 2-OH | 79.2 | 79.0 | 80.9 | 80.6 |
| 11-OH | 79.0 | 78.9 | 80.3 | 80.0 |
| 12-OH | 75.2 | 74.9 | 76.5 | 76.1 |
| IP(eV) | ||||
| Hispidin | 7.75 | 7.82 | 6.24 | 6.30 |
| Isohispidin | 7.96 | 8.10 | 6.39 | 6.39 |
2.2.2. Isomerism Effect
| Compounds | BDEs (kcal/mol) | IP (eV) | IC50 (µmol/L) | |||||
|---|---|---|---|---|---|---|---|---|
| 4-OH | 11-OH | 12-OH | 4'-OH | 11'-OH | 12'-OH | |||
| (a) Gas phase | ||||||||
| Hispidin (trans) | 90.0 | 78.9 | 75.0 | - | - | - | 7.75 | 1.31 ± 0.81 |
| Hispidin (cis) | 89.1 | 78.2 | 76.0 | - | - | - | 7.95 | 1.31 ± 0.81 |
| 3,14'-Bihispidinyl | 83.6 | 78.9 | 74.8 | 89.2 | 77.1 | 74.6 | 7.08 | 0.90 ± 0.61 |
| Hypholomine B (syn) | - | 77.4 | 73.9 | 89.9 | 77.5 | 77.5 | 7.7 | 0.31 ± 0.22 |
| Hypholomine B (anti) | - | 88.6 | 75.4 | 91.2 | 77.8 | 77.7 | 7.7 | 0.31 ± 0.22 |
| 1,1-Distyrylpyrylethan | 101.2 | 78.8 | 74.6 | 99.2 | 88.6 | 74.7 | 7.2 | 0.37 ± 0.15 |
| (b) PCM | ||||||||
| Hispidin (trans) | 90.6 | 80.3 | 76.2 | - | - | - | 6.24 | 1.31 ± 0.81 |
| Hispidin (cis) | 90.9 | 79.3 | 77.0 | - | - | - | 6.30 | 1.31 ± 0.81 |
| 3,14'-Bihispidinyl | 84.8 | 80.4 | 76.3 | 90.2 | 79.3 | 76.6 | 6.10 | 0.90 ± 0.61 |
| Hypholomine B (syn) | - | 79.1 | 75.7 | 92.8 | 79.4 | 79.2 | 6.3 | 0.31 ± 0.22 |
| Hypholomine B (anti) | - | 84.7 | 76.3 | 92.7 | 79.9 | 79.7 | 6.3 | 0.31 ± 0.22 |
| 1,1-Distyrylpyrylethan | 97.2 | 79.7 | 76.1 | 94.4 | 85.4 | 76.2 | 6.2 | 0.37 ± 0.15 |

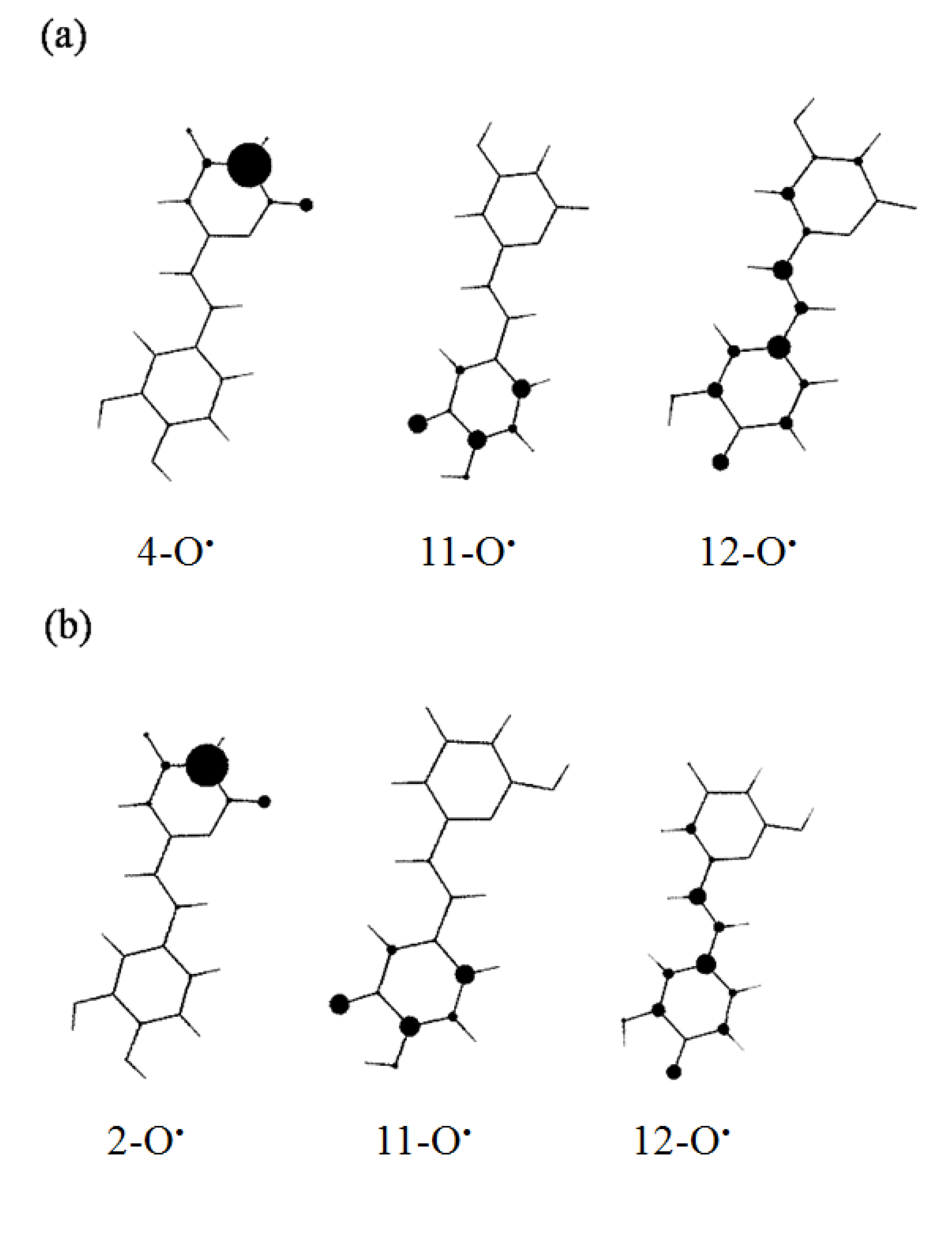
2.2.3. BDEs Analysis

2.2.4. Double BDEs Analysis
| Compounds | BDEd (kcal/mol) | IPd (eV) | IC50 (µmol/L) | ||||
|---|---|---|---|---|---|---|---|
| 4-OH | 11-OH | 4'-OH | 11'-OH | 12'-OH | |||
| (a) Gas phase | |||||||
| Hispidin | 99.3 | 81.8 | - | - | - | 7.9 | 1.31 ± 0.81 |
| 3,14'-Bihispidinyl | 89.4 | 82.2 | 106.9 | 82.1 | 87.6 | 7.4 | 0.90 ± 0.61 |
| Hypholomine B ( anti) | - | 81.2 | 111.0 | 96.2 | 95.7 | 7.9 | 0.31 ± 0.22 |
| 1,1-Distyrylpyrylethan | 108.4 | 81.7 | 100.8 | 97.2 | 90.6 | 7.4 | 0.37 ± 0.15 |
| (b) PCM | |||||||
| Hispidin | 93.9 | 78.2 | - | - | - | 6.3 | 1.31 ± 0.81 |
| 3,14'-Bihispidinyl | 89.4 | 78.9 | 99.0 | 84.5 | 89.0 | 6.2 | 0.90 ± 0.61 |
| Hypholomine B ( anti) | - | 77.4 | 101.1 | 97.6 | 97.0 | 6.3 | 0.31 ± 0.22 |
| 1,1-Distyrylpyrylethan | 101.3 | 78.8 | 98.5 | 97.5 | 91.2 | 6.2 | 0.37 ± 0.15 |
2.2.5. Effect of the Number of OH Groups and Conjugation of the Catechol Moiety
2.3. Thermodynamical and Kinetic Study
2.3.1. Thermodynamic of the PC-ET Mechanism

| ΔGPC−ET | ΔGET | |||||||
|---|---|---|---|---|---|---|---|---|
| DPPH | CH3OO• | DPPH | CH3OO• | |||||
| Gas | PCM | Gas | PCM | Gas | PCM | Gas | PCM | |
| (a) B3P86//First PC-ET | ||||||||
| Hispidin | −4.27 | −0.12 | −8.76 | −8.13 | 88.50 | 22.20 | 143.63 | 41.55 |
| 3,14'−Bihispidinyl | −4.10 | 0.09 | −8.59 | −7.91 | 74.25 | 19.45 | 129.38 | 38.80 |
| Hypholomine B | −3.29 | −0.28 | −7.78 | −8.28 | 87.50 | 22.63 | 142.63 | 41.98 |
| Pinilidin | −4.54 | −0.15 | −9.02 | −8.16 | 75.65 | 20.21 | 130.78 | 39.56 |
| (b) B3P86//Second PC-ET | ||||||||
| Hispidin | 4.04 | 3.42 | −0.45 | −4.58 | 93.84 | 25.88 | 148.97 | 45.22 |
| 3,14'-Bihispidinyl | 2.41 | 2.06 | −2.07 | −5.94 | 81.36 | 22.98 | 136.49 | 42.32 |
| Hypholomine B | 1.67 | 1.13 | −2.82 | −6.88 | 92.45 | 25.64 | 147.59 | 44.99 |
| Pinilidin | 4.09 | 2.04 | −0.40 | −5.97 | 82.16 | 23.00 | 137.29 | 42.34 |
| (c) MPWB1K//First PC-ET | ||||||||
| Hispidin | −2.26 | 1.34 | −5.78 | −5.07 | 43.94 | 26.24 | 163.06 | 46.97 |
| 3,14'-Bihispidinyl | −2.00 | 1.51 | −5.33 | −4.90 | 81.13 | 24.85 | 146.25 | 45.58 |
| Hypholomine B | −1.18 | 1.22 | −4.71 | −5.19 | 97.51 | 27.24 | 162.63 | 47.96 |
| Pinilidin | −2.36 | 1.39 | −5.89 | −4.41 | 85.23 | 25.53 | 150.35 | 123.55 |
| (d) MPWB1K//Second PC-ET | ||||||||
| Hispidin | 18.25 | 17.15 | 0.13 | −3.66 | 109.41 | 40.95 | 163.91 | 50.20 |
| 3,14'-Bihispidinyl | 16.92 | 15.79 | 13.10 | 9.38 | 96.93 | 38.05 | 162.05 | 50.87 |
| Hypholomine B | 15.88 | 14.85 | 12.35 | 8.44 | 108.2 | 40.72 | 173.14 | 61.44 |
| Pinilidin | 18.30 | 15.76 | 14.77 | 9.35 | 97.73 | 38.07 | 162.85 | 58.79 |
2.3.2. Kinetics of the Reaction Styrylpyrone + CH3OO• → [styrylpyrone-H]• + CH3OOH
| ΔG | ΔG# | KTST | KTST/W | KTST/ST | |
|---|---|---|---|---|---|
| (a) B3P86//Gas phase | |||||
| Hispidin + R• → [Hispidin-H]• + RH | −8.76 | 4.89 | 1.61*109 | 4.29*109 | 1.59*1010 |
| 3,14'-Bihispidinyl + R• → [3,14'-Bihispidinyl -H]• + RH | −8.59 | 5.15 | 1.04*109 | 2.73*109 | 3.63*109 |
| Hypholomine B + R• → [Hypholomine B -H]• + RH | −7.78 | 6.00 | 2.49*109 | 6.93*108 | 2.74*109 |
| Pinilidin + R• → [Pinilidin -H]• + RH | −9.02 | 4.89 | 1.61*109 | 4.08*109 | 1.28*1010 |
| (b) B3P86//PCM | |||||
| Hispidin + R• → [Hispidin-H]• + RH | −8.13 | 8.55 | 3.32*106 | 1.52*107 | 1.20*107 |
| 3,14'-Bihispidinyl + R• → [3,14'-Bihispidinyl -H]• + RH | −7.91 | 11.30 | 3.20*104 | 1.62*105 | 1.02*105 |
| Hypholomine B + R• → [Hypholomine B -H]• + RH | −8.28 | 8.22 | 5.87*106 | 2.66*107 | 1.84*107 |
| Pinilidin + R• → [Pinilidin -H]• + RH | −8.16 | 5.66 | 1.62*109 | 1.28*109 | 1.80*1010 |
| (c) MPWB1K//Gas phase | |||||
| Hispidin + R• → [Hispidin-H]• + RH | −5.78 | 16.96 | 2.36 | 6.30 | 2.68*101 |
| 3,14'-Bihispidinyl + R• → [3,14'-Bihispidinyl -H]• + RH | −5.33 | 17.20 | 1.58 | 4.12 | 7.35 |
| Hypholomine B + R• → [Hypholomine B -H]• + RH | −4.71 | 18.02 | 3.96*10−1 | 1.10 | 5.66 |
| Pinilidin + R• → [Pinilidin -H]• + RH | −5.89 | 17.24 | 1.47 | 3.73 | 13.31 |
| (d) MPWB1K//PCM | |||||
| Hispidin + R• → [Hispidin-H]• + RH | −5.07 | 17.64 | 7.40*10−1 | 3.38 | 1.47*102 |
| 3,14'-Bihispidinyl + R• → [3,14'-Bihispidinyl -H]• + RH | −4.9 | 20.40 | 7.03*10−3 | 3.56*10−2 | 2.41 |
| Hypholomine B + R• → [Hypholomine B -H]• + RH | −5.19 | 17.82 | 5.46*10−1 | 2.48 | 1.49*102 |
| Pinilidin + R• → [Pinilidin -H]• + RH | −4.41 | 16.02 | 1.14*101 | 5.10*101 | 3.51*103 |
3. Methodology
- (i)
- Proton Coupled-Electron Transfer (PC-ET) versus Hydrogen atom transfer (HAT)ArOH + R• → ArO• + RHIn the above reaction, the electron and proton are transferred from the active phenolic hydroxyl group to the free radical in a single step. This type of reactions can be subdivided into two distinct subclasses, hydrogen atom transfer (HAT) and proton coupled electron transfer (PC-ET) [14,29,30]. In HAT, the proton and electron are transferred together, as a hydrogen atom. In PC-ET mechanism, the proton and electron are transferred between different sets of orbitals [14]. Depending on the structure of reactants and the environment (e.g., solvent, enzyme), this reaction can proceed via HAT or PC-ET [31]. Various criteria can be used to distinguish between HAT and PC-ET. Tishchenko et al. used electronic adiabacity and nonadiabactity criterion to determine the mechanism involved in the reactivity of phenol with •NH2 and •OOCH3 radicals [31]. With regard to this criterion, HAT is electronically adiabatic, while PC-ET involves electronic nonadiabatic effects [31]. By using orbital-based criterion, Mayer et al. found that the degenerate hydrogen atom exchange reaction between phenoxyl radical (PhO•) and phenol (PhOH) involves a transfer of a proton and an electron between two different sets of molecular orbitals (PC-ET mechanism), while the reaction between benzyl radical and toluene involves the transfer of proton and electron between the same sets of molecular orbitals (HAT mechanism) [14]. As shown by Mayer et al. and Oksana et al. the reactivity of phenols with free radicals such CH3OO• and PhO• involve a PC-ET mechanism [14,31]. Therefore, one can consider that the reactivity of the current series of hispidin oligomers (ArOH) with CH3OO• proceeds through a PC-ET mechanism. The natural bond orbitals (NBO) charges were calculated for the reactants (hispidin oligomers and peroxy radical) and TS structures using both hybrid functionals B3P86 and MPWB1K in gas and solvent. The results showed a slight variation of NBO charges of the abstracted hydrogen atom between the TS and the reactants. For instance, a slight increase of 0.02 was obtained in gas phase with MPWB1K hybrid functional. However, the sum of the oxygen atoms (bold atoms) in the reactants [Ph-O-H + •O-OCH3] and transition state [Ph-O…H…O-OCH3]• are −0.90 and −1.06, respectively. Thus, −0.16 more negative charge resides on the oxygen at the TS than in the reactants. In the PC-ET mechanism of phenoxyl/phenol, Mayer et al. found −0.17 more negative charge residing on oxygen atoms at the TS than in the reactants [14]. From this results, the reactivity of the hispidin oligomers with CH3OO• can be considered as proceeding through a PC-ET mechanism.The O-H bond dissociation is homolytic. Therefore, the bond dissociation enthalpy (BDE) of OH groups is considered as the main parameter that governs this mechanism. BDEs are calculated for each OH group of hispidin oligomers by the following formula:where H is the enthalpy that takes into account temperature-dependent corrections [zero point energy (ZPE), translational, rotational and vibrational energies at 298K]; H (ArOH, 298K) and H (ArO•, 298K) are the enthalpies of the hispidin oligomer and its corresponding radical (obtained after homolytic OH bond dissociation), respectively; and H (H•, 298K) is the enthalpy of hydrogen radical. BDE is an intrinsic parameter that helps to estimate the capacity of a compound to lose an hydrogen atom. The lower is the BDE, the easier is the OH bond dissociation and the more important is its role in the antioxidant reactivity. The PC-ET mechanism [Eq. (1)] depends as well on the reactivity of the antioxidant with the free radical R• (i.e., DPPH, CH3OO•). The reaction is thermodynamically favorable (exergonic) if its Gibbs free energy (ΔG) is negative. The PC-ET mechanism can be followed by a second hydrogen atom transfer from the phenoxyl radical (i.e., radical obtained from the first hydrogen atom transfer). Double bond dissociation enthalpy (BDEd) becomes an important parameter when calculated BDEs are similar. BDEd are calculated by the following formula:BDE = H(ArO•, 298K) + H(H•, 298K) − H(ArOH, 298K),where H ([ArO•− H], 298K) and H (ArO•, 298K) are the enthalpies of phenoxyl radical obtained after the first and second hydrogen atom transfer, respectively. As for BDE, the lower is the BDEd, the easier is the O-H bond rupture of phenoxyl radical, and the more important is its role in the antioxidant reactivity. We have demonstrated in a previous study the significant role of BDEd parameter to rationalize the antioxidant activity of a series of guaiacol oligomers [11].BDEd = H([ArO•− H], 298K) + H(H•, 298K) − H(ArO•, 298K),
- (ii)
- Electron Transfer-Proton Transfer (ET-PT)ArOH + R• → ArOH+•+ R− → ArO• + RHThe ET-PT mechanism consists of two steps. In the first step, an electron transfer (ET) from the styrylpyrone to the free radical leads to the formation of radical cation ArOH+•. In the second step, a heterolytic O-H bond dissociation of the radical cation (i.e., proton loss) leads to the formation of a phenoxyl radical. This mechanism is governed by the ionization potential (IP) of the radical cation and the Gibbs free energy reaction of the first step. The lower is the IP value, the easier is the electron transfer and the higher is the antioxidant activity.
- (iii)
- Sequential Proton Loss Electron Transfer (SPLET)ArOH → ArO− + H+ArO− + R• → ArO• + R−R− + H+ → RHThe SPLET mechanism consists of three steps. In the first step, a heterolytic bond dissociation of a phenolic hydroxyl group leads to the formation of a phenoxyl anion and the release of a proton. In the second step, an electron transfer from the phenoxyl anion to the free radical leads to the formation of a phenoxyl radical and an anion (R−). In the last step, the protonation of R− leads to the formation of RH. This mechanism is strongly favored under alkaline conditions (e.g., high pH), which may help in the proton of the first step [32,33].
- (iv)
- Adduct formation (AF)ArOH + R• → [ArOH-R]• → stable adductsThe AF mechanism is more specific and is observed between (a) carbon centered radicals and double bonds; or (b) hydroxyl radicals and aromatic rings. Numerous side reactions may occur that lead to stable adducts from [ArOH-R]•. In the present study, we focused on the first two mechanisms PC-ET and ET-PT. The identification of the major mechanism was carried out in two stages, which were (i) determination of the most active OH group (i.e., those with low BDEs); and (ii) thermodynamic and kinetic studies of the reactivity of the active OH groups with free radicals (e.g., CH3OO• and DPPH). Both mechanisms lead to the formation of the same final products and thus possess the same free Gibbs reaction energy. Consequently, they are a similar from a thermodynamic point of view (ΔGPC−ET = ΔGET−PT). The favored mechanism was determined kinetically by calculating the activation free energies of PC-ET and ET-PT mechanisms (ΔG#).






4. Conclusions
Acknowledgments
Author Contributions
Conflictts of Interest
References
- Jewers, K.; Davis, J.B.; Dougan, J.; Manchanda, A.H.; Blunden, G.; Kyi, A.; Wetchapinan, S. Goniothalamin and its distribution in four Goniothalamus species. Phytochemistry 1972, 11, 2025–2030. [Google Scholar] [CrossRef]
- Fiasson, J.-L. Distribution of styrylpyrones in the basidiocarps of various Hymenochaetaceae. Biochem. Syst. Ecol. 1982, 10, 289–296. [Google Scholar] [CrossRef]
- Zhu, T.; Guo, J.; Collins, L.; Kelly, J.; Xiao, Z.J.; Kim, S.H.; Chen, C.Y. Phellinus linteus activates different pathways to induce apoptosis in prostate cancer cells. Br. J. Cancer 2007, 96, 583–590. [Google Scholar] [CrossRef]
- Saar, M. Fungi in khanty folk medicine. J. Ethnopharmacol. 1991, 31, 175–179. [Google Scholar] [CrossRef]
- Huang, N.L. A mysterious medicinal fungus in Russia: Inonotus obliquus. Edible Fungi China 2002, 21, 7–8. [Google Scholar]
- Margaill, I.; Plotkine, M.; Lerouet, D. Antioxidant strategies in the treatment of stroke. Free Radic. Biol. Med. 2005, 39, 429–443. [Google Scholar] [CrossRef]
- Lemanska, K.; Szymusiak, H.; Tyrakowska, B.; Zielinski, R.; Soffers, A.E.; Rietjens, I.M. The influence of pH on antioxidant properties and the mechanism of antioxidant action of hydroxyflavones. Free Radic. Biol. Med. 2001, 31, 869–881. [Google Scholar] [CrossRef]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Kozlowski, D.; Marsal, P.; Steel, M.; Mokrini, R.; Duroux, J.-L.; Lazzaroni, R.; Trouillas, P. Theoretical investigation of the formation of a new series of antioxidant depsides from the radiolysis of flavonoid compounds. Radiat. Res. 2007, 168, 243–252. [Google Scholar] [CrossRef]
- Kozlowski, D.; Trouillas, P.; Calliste, C.; Marsal, P.; Lazzaroni, R.; Duroux, J.-L. Density functional theory study of the conformational, electronic, and antioxidant properties of natural chalcones. J. Phys. Chem. A 2007, 111, 1138–1145. [Google Scholar] [CrossRef]
- Anouar, E.; Calliste, C.A.; Kosinova, P.; di Meo, F.; Duroux, J.L.; Champavier, Y.; Marakchi, K.; Trouillas, P. Free radical scavenging properties of guaiacol oligomers: A combined experimental and quantum study of the guaiacyl-moiety role. J. Phys. Chem. A 2009, 113, 13881–13891. [Google Scholar] [CrossRef]
- Trouillas, P.; Marsal, P.; Siri, D.; Lazzaroni, R.; Duroux, J.-L. A DFT study of the reactivity of OH groups in quercetin and taxifolin antioxidants: The specificity of the 3-OH site. Food Chem. 2006, 97, 679–688. [Google Scholar] [CrossRef]
- Trouillas, P.; Marsal, P.; Svobodova, A.; Vostalova, J.; Gazak, R.; Hrbac, J.; Sedmera, P.; Kren, V.; Lazzaroni, R.; Duroux, J.-L.; et al. Mechanism of the Antioxidant Action of Silybin and 2,3-Dehydrosilybin Flavonolignans: A Joint Experimental and Theoretical Study. J. Phys. Chem. A 2008, 112, 1054–1063. [Google Scholar] [CrossRef]
- Mayer, J.M.; Hrovat, D.A.; Thomas, J.L.; Borden, W.T. Proton-coupled electron transfer versus hydrogen atom transfer in benzyl/toluene, methoxyl/methanol, and phenoxyl/phenol self-exchange reactions. J. Am. Chem. Soc. 2002, 124, 11142–11147. [Google Scholar] [CrossRef]
- Wang, L.-F.; Zhang, H.-Y. Unexpected role of 5-OH in DPPH radical-scavenging activity of 4-thiaflavans. Revealed by theoretical calculations. Bioorg. Med. Chem. Lett. 2004, 14, 2609–2611. [Google Scholar] [CrossRef]
- Luzhkov, V.B. Mechanisms of antioxidant activity: The DFT study of hydrogen abstraction from phenol and toluene by the hydroperoxyl radical. Chem. Phys. 2005, 314, 211–217. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Bayat, A.; Fattahi, A. Investigation of the scavenging mechanism of tyrosyl radical by hydroxybenzohydroxamic acid derivatives: A DFT study. Comp. Theor. Chem. 2013, 1018, 35–44. [Google Scholar]
- Jung, J.Y.; Lee, I.K.; Seok, S.J.; Lee, H.J.; Kim, Y.H.; Yun, B.S. Antioxidant polyphenols from the mycelial culture of the medicinal fungi Inonotus xeranticus and Phellinus linteus. J. Appl. Microbiol. 2008, 104, 1824–1832. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Lee, I.-K.; Yun, B.-S. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J. Antibiot. 2011, 64, 349–359. [Google Scholar] [CrossRef]
- Emamian, S.R.; Domingo, L.R.; Tayyari, S.F. Tautomerism in pyridazin-3 (2H)-one: A theoretical study using implicit/explicit solvation models. J. Mol. Graph. Model. 2014, 49, 47–54. [Google Scholar] [CrossRef]
- Chahkandi, B.; Tayyari, S.F.; Bakhshaei, M.; Chahkandi, M. Investigation of simple and water assisted tautomerism in a derivative of 1, 3, 4-oxadiazole: A DFT study. J. Mol. Graph. Model. 2013, 44, 120–128. [Google Scholar] [CrossRef]
- Trabolsy, Z.B.K.A.; Anouar, E.H.; Zakaria, N.S.S.; Zulkeflee, M.; Hasan, M.H.; Zin, M.M.; Ahmad, R.; Sultan, S.; Weber, J.-F.F. Antioxidant activity, NMR, X-ray, ECD and UV/visible spectra of (+)-terrein: Experimental and theoretical approaches. J. Mol. Struct. 2014, 1060, 102–110. [Google Scholar]
- Urbaniak, A.; Szeląg, M.; Molski, M. Theoretical investigation of stereochemistry and solvent influence on antioxidant activity of ferulic acid. Comp. Theor. Chem. 2013, 1012, 33–40. [Google Scholar] [CrossRef]
- Amić, A.; Marković, Z.; Dimitrić Marković, J.M.; Stepanić, V.; Lučić, B.; Amić, D. Towards an improved prediction of the free radical scavenging potency of flavonoids: The significance of double PCET mechanisms. Food Chem. 2014, 152, 578–585. [Google Scholar] [CrossRef]
- Rastija, V.; Medić-Šarić, M. QSAR study of antioxidant activity of wine polyphenols. Eur. J. Med. Chem. 2009, 44, 400–408. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, S.; Zhang, H.; Zhang, S. Improvement of antioxidative activity of resveratrol by elongating conjugated chain: A DFT theoretical study. Comp. Theor. Chem. 2013, 1019, 39–47. [Google Scholar] [CrossRef]
- Hammes-Schiffer, S. Proton-coupled electron transfer: Classification scheme and guide to theoretical methods. Energ. Environ. Sci. 2012, 5, 7696–7703. [Google Scholar] [CrossRef]
- Hammes-Schiffer, S. Theoretical perspectives on proton-coupled electron transfer reactions. Acc. Chem. Res. 2001, 34, 273–281. [Google Scholar] [CrossRef]
- Tishchenko, O.; Truhlar, D.G.; Ceulemans, A.; Nguyen, M.T. A unified perspective on the hydrogen atom transfer and proton-coupled electron transfer mechanisms in terms of topographic features of the ground and excited potential energy surfaces as exemplified by the reaction between phenol and radicals. J. Am. Chem. Soc. 2008, 130, 7000–7010. [Google Scholar] [CrossRef]
- Di Meo, F.; Lemaur, V.; Cornil, J.; Lazzaroni, R.; Duroux, J.-L.; Olivier, Y.; Trouillas, P. Free Radical Scavenging by Natural Polyphenols: Atom versus Electron Transfer. J. Phys. Chem. A 2013, 117, 2082–2092. [Google Scholar] [CrossRef]
- Musialik, M.; Litwinienko, G. Scavenging of dpph radicals by vitamin E is accelerated by its partial ionization: The role of sequential proton loss electron transfer. Org. Lett. 2005, 7, 4951–4954. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02 ed; Gaussian, Inc. : Wallingford, CT, USA, 2009. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. Hybrid meta density functional theory methods for thermochemistry, thermochemical kinetics, and noncovalent interactions: The MPW1B95 and MPWB1K models and comparative assessments for hydrogen bonding and van der Waals Interactions. J. Phys. Chem. A 2004, 108, 6908–6918. [Google Scholar] [CrossRef]
- Tejero, I.; González-García, N.; González-Lafont, À.; Lluch, J.M. Tunneling in green tea: Understanding the antioxidant activity of catechol-containing compounds. a variational transition-state theory study. J. Am. Chem. Soc. 2007, 129, 5846–5854. [Google Scholar] [CrossRef]
- Guerra, M.; Amorati, R.; Pedulli, G.F. Water effect on the oh dissociation enthalpy of para-substituted phenols: A DFT study. J. Org. Chem. 2004, 69, 5460–5467. [Google Scholar] [CrossRef]
- McMahon, R.J. Chemical reactions involving quantum tunneling. Science 2003, 299, 833–834. [Google Scholar] [CrossRef]
- Sirjean, B.; Dames, E.; Wang, H.; Tsang, W. Tunneling in hydrogen-transfer isomerization of n-alkyl radicals. J. Phys. Chem. A 2011, 116, 319–332. [Google Scholar] [CrossRef]
- Wigner, E. Calculation of the rate of elementary association reactions. J. Chem. Phys. 1937, 5, 720–725. [Google Scholar] [CrossRef]
- Skodje, R.T.; Truhlar, D.G. Parabolic tunneling calculations. J. Phys. Chem. 1981, 85, 624–628. [Google Scholar]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Anouar, E.H.; Shah, S.A.A.; Hassan, N.B.; Moussaoui, N.E.; Ahmad, R.; Zulkefeli, M.; Weber, J.-F.F. Antioxidant Activity of Hispidin Oligomers from Medicinal Fungi: A DFT Study. Molecules 2014, 19, 3489-3507. https://doi.org/10.3390/molecules19033489
Anouar EH, Shah SAA, Hassan NB, Moussaoui NE, Ahmad R, Zulkefeli M, Weber J-FF. Antioxidant Activity of Hispidin Oligomers from Medicinal Fungi: A DFT Study. Molecules. 2014; 19(3):3489-3507. https://doi.org/10.3390/molecules19033489
Chicago/Turabian StyleAnouar, El Hassane, Syed Adnan Ali Shah, Normahanim Binti Hassan, Najoua El Moussaoui, Rohaya Ahmad, Mohd Zulkefeli, and Jean-Frédéric F. Weber. 2014. "Antioxidant Activity of Hispidin Oligomers from Medicinal Fungi: A DFT Study" Molecules 19, no. 3: 3489-3507. https://doi.org/10.3390/molecules19033489