Synthesis of A New Class of Pyridazin-3-one and 2-Amino-5-arylazopyridine Derivatives and Their Utility in the Synthesis of Fused Azines
Abstract
:1. Introduction
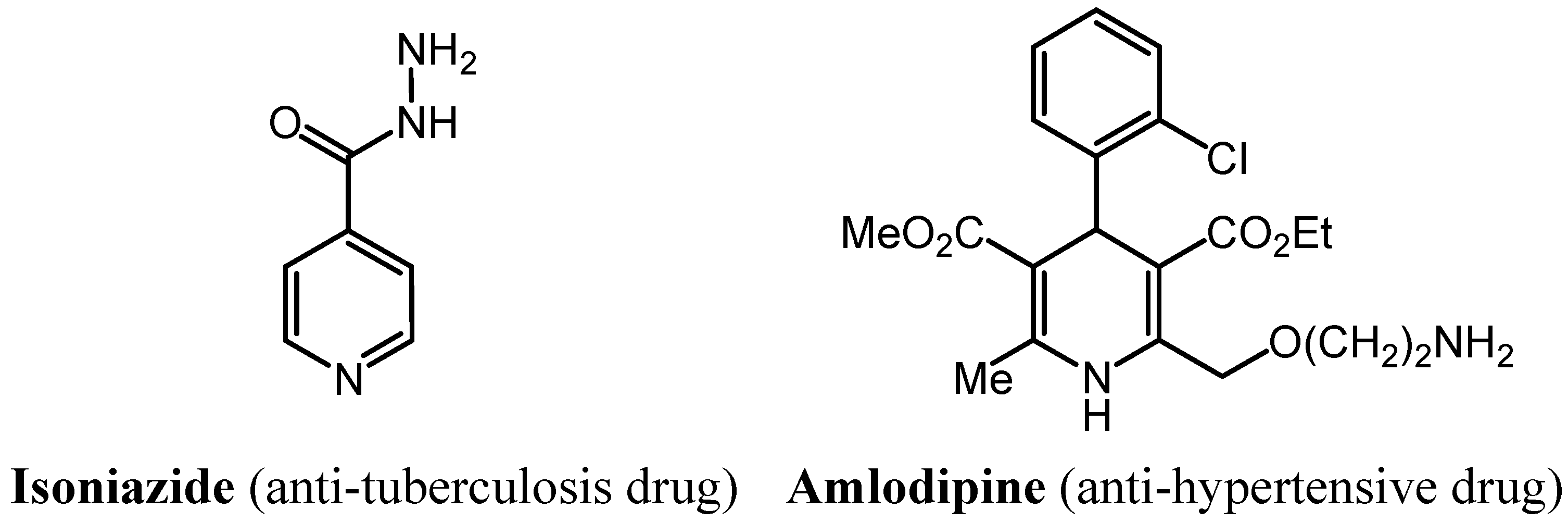
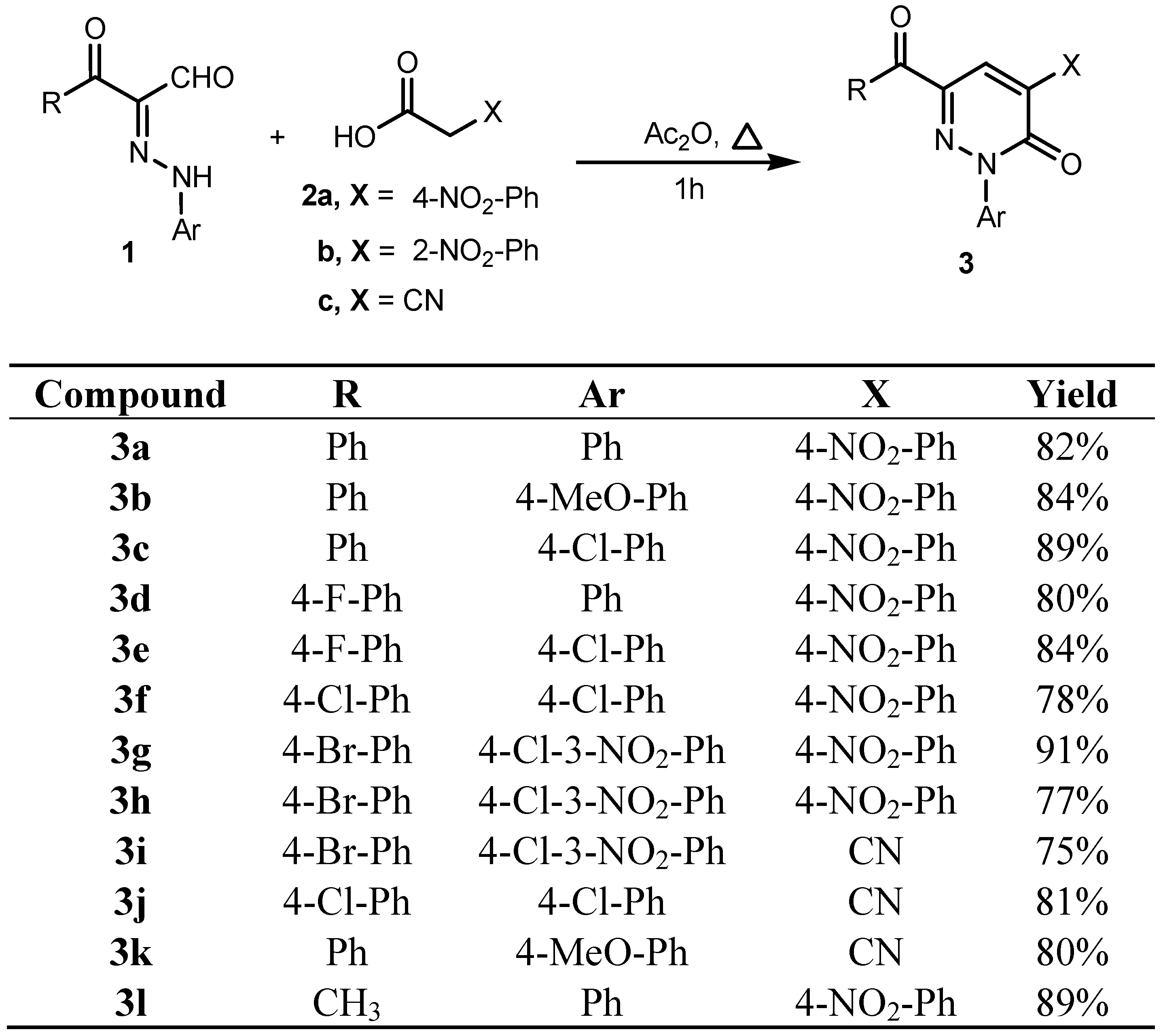
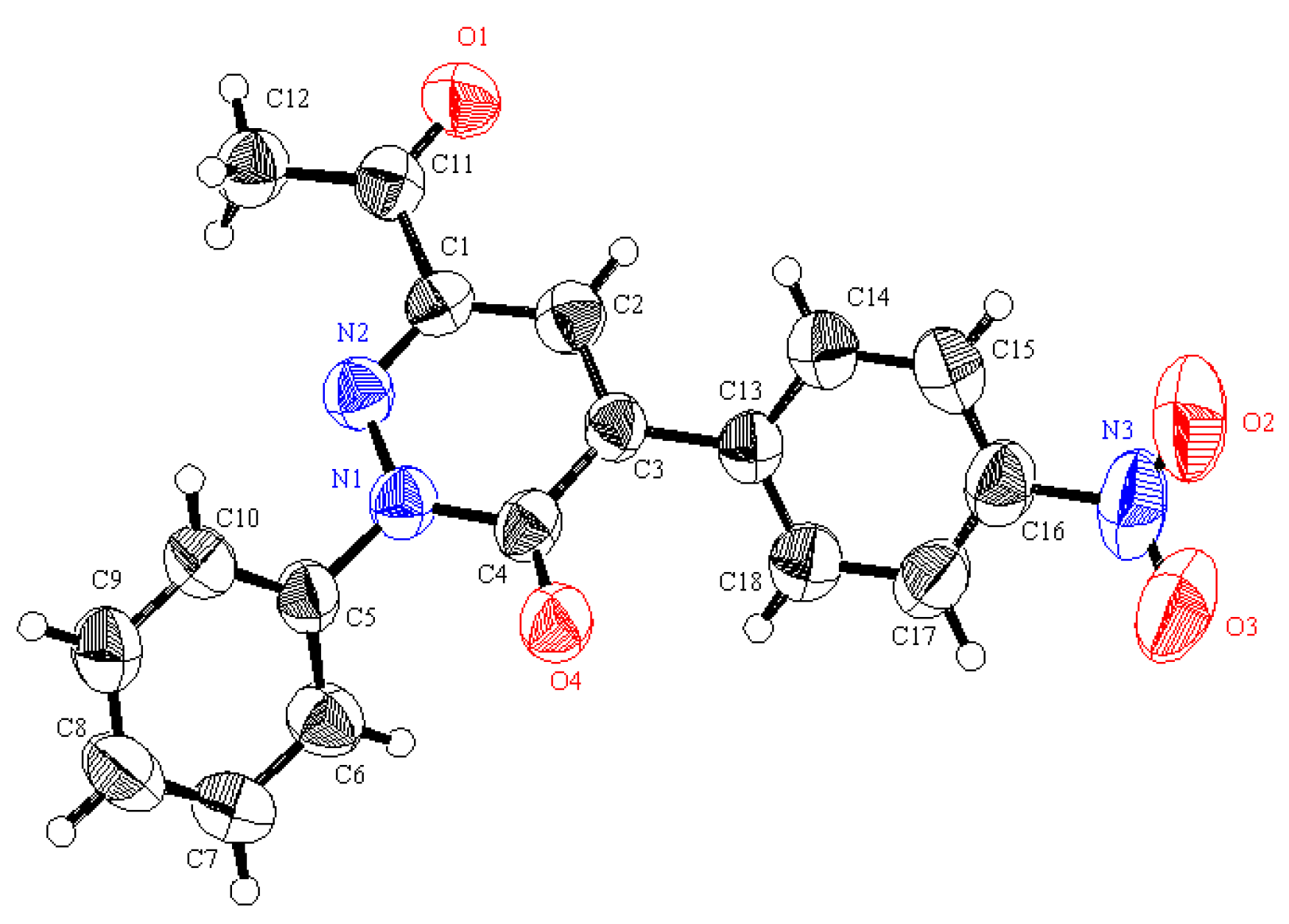
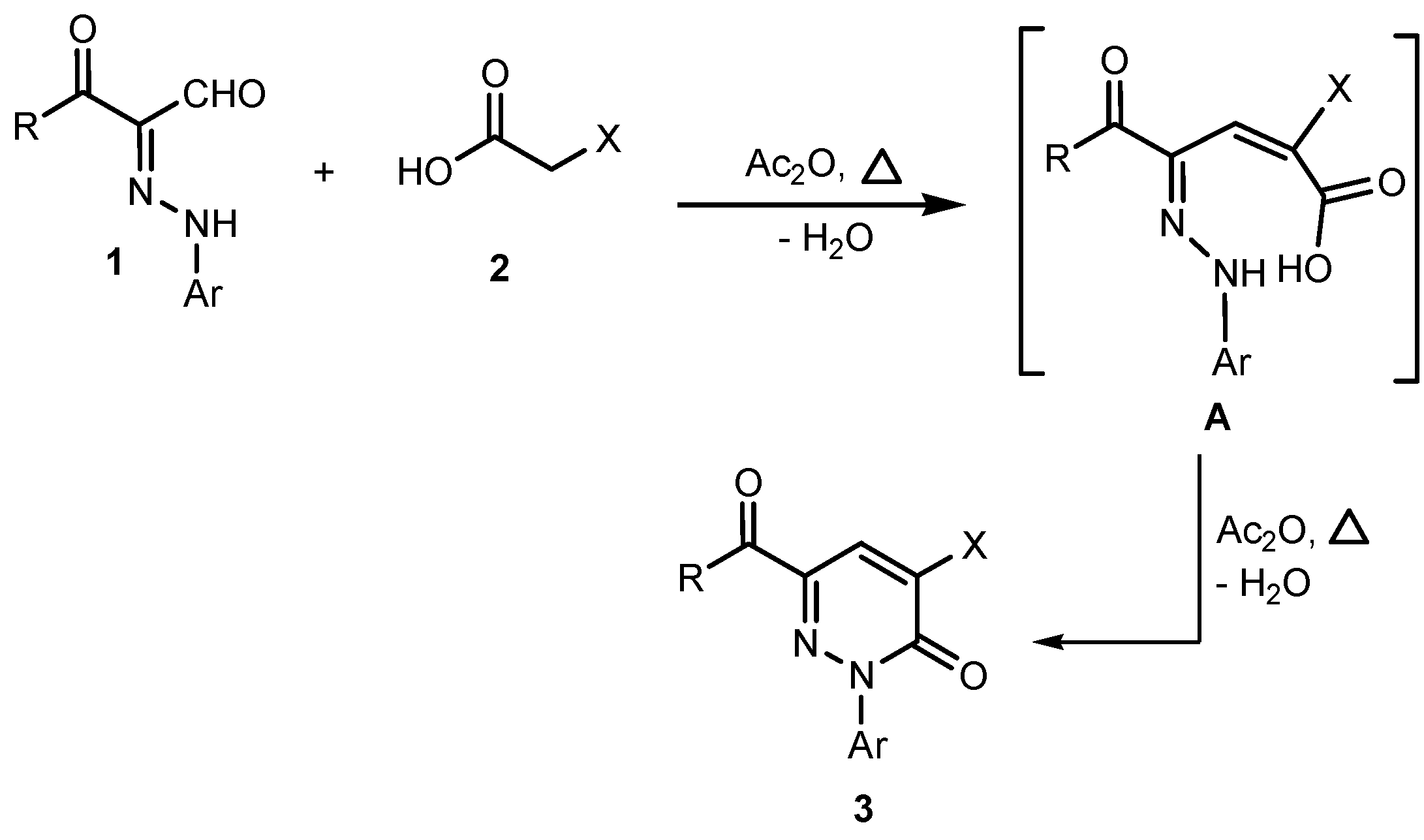
2. Results and Discussion

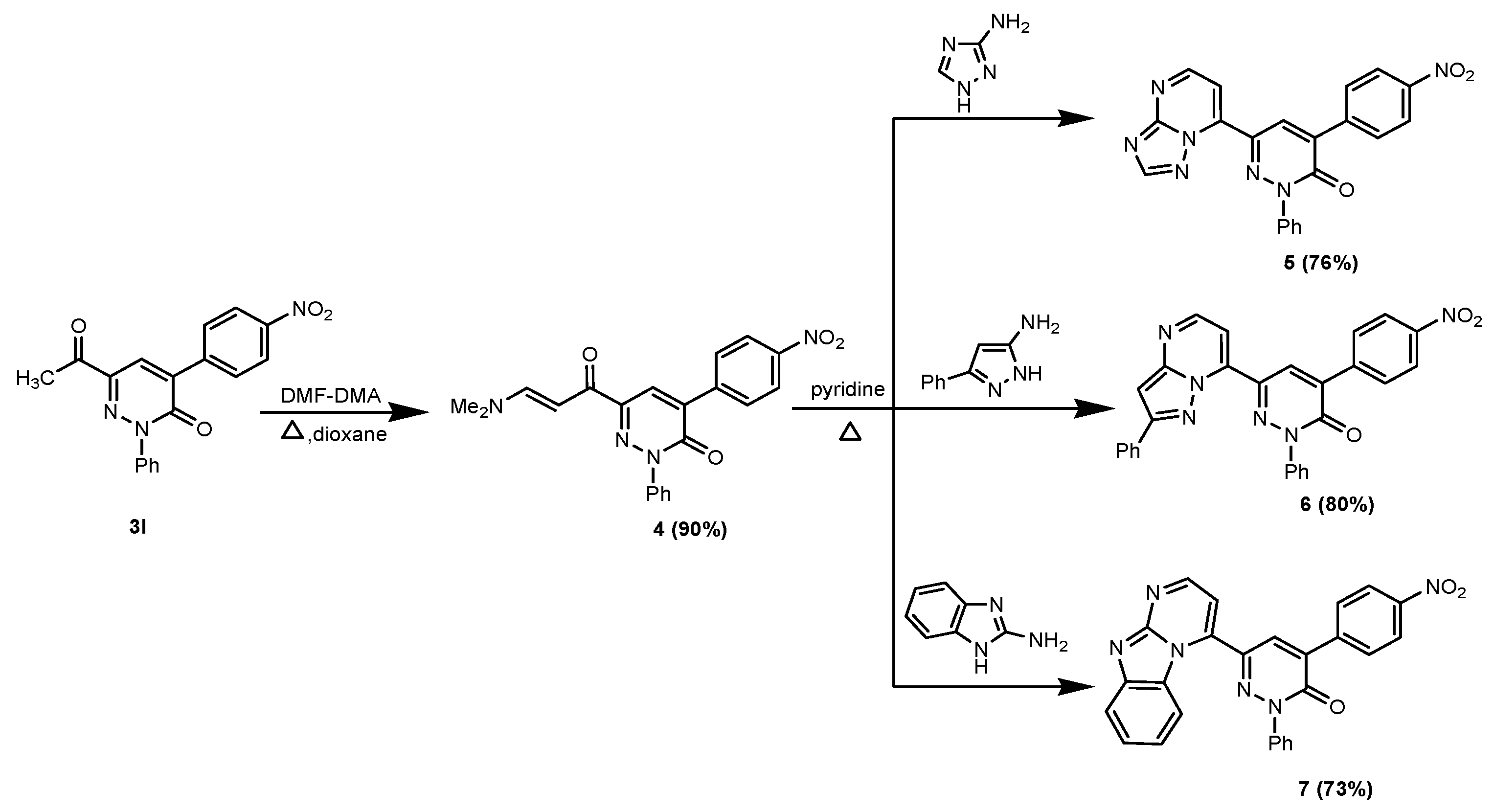
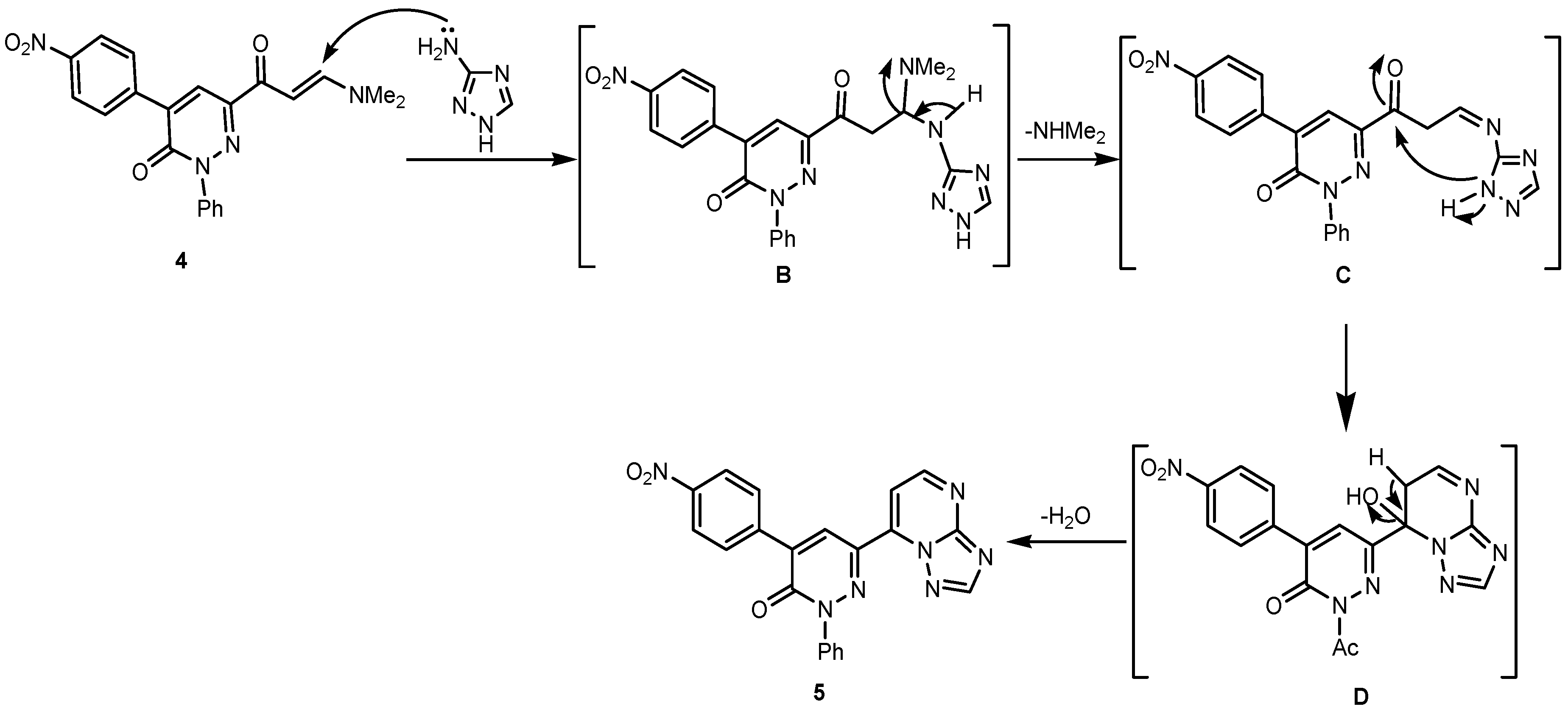
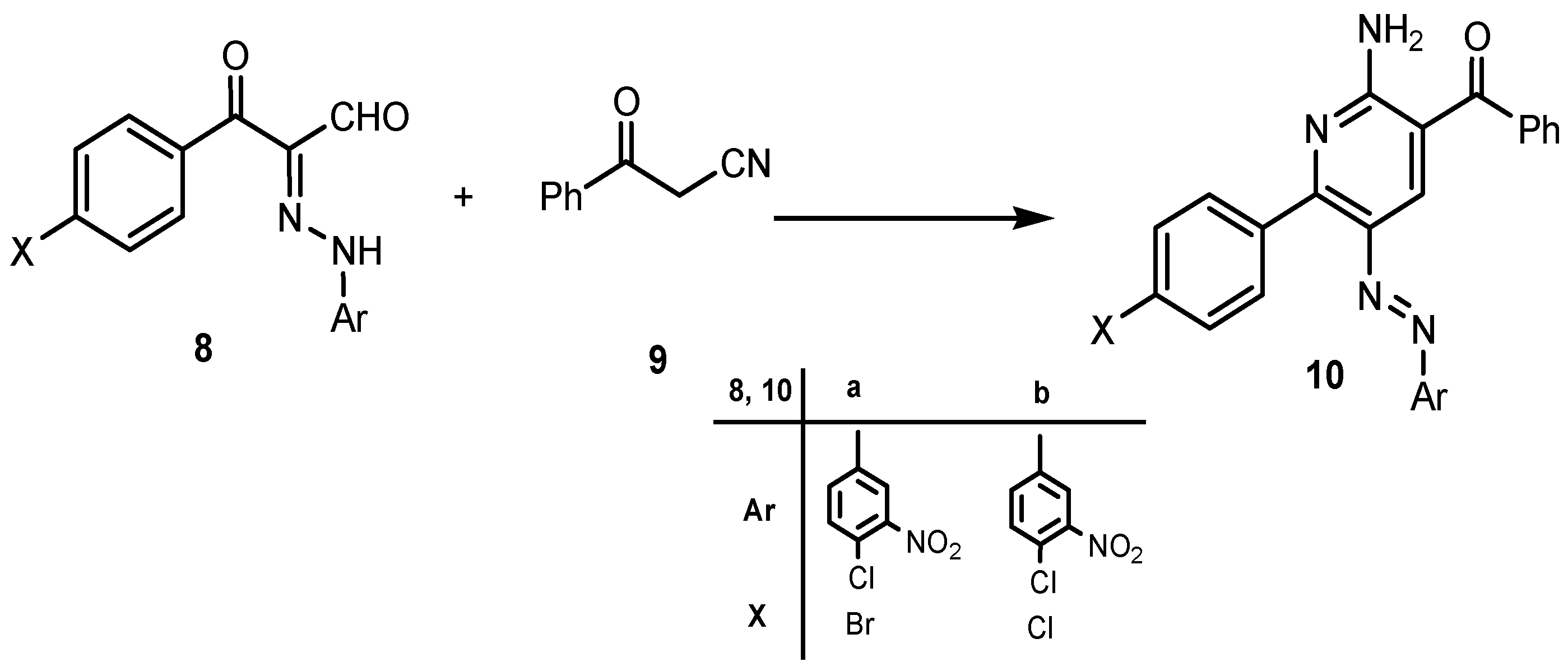
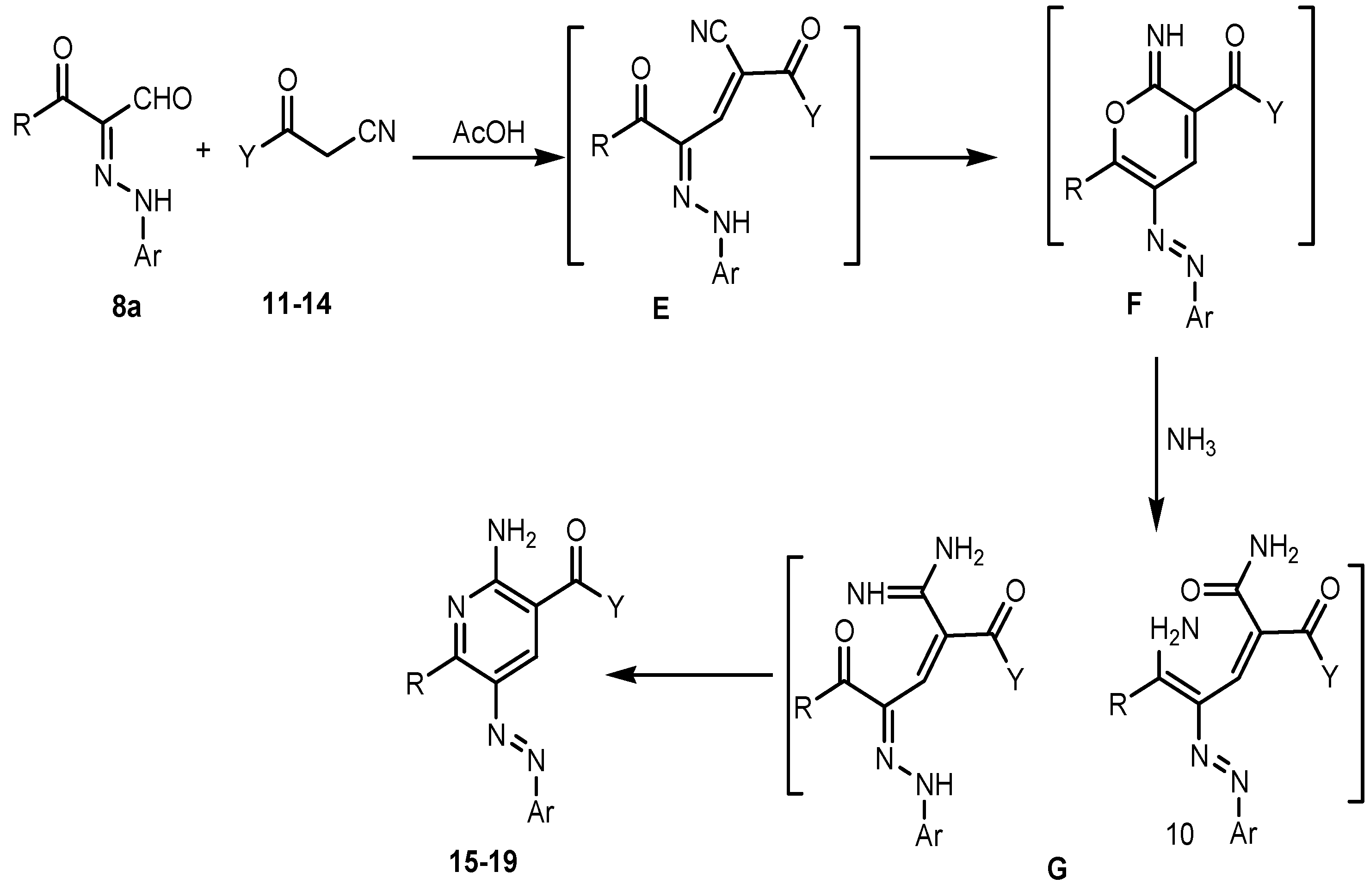
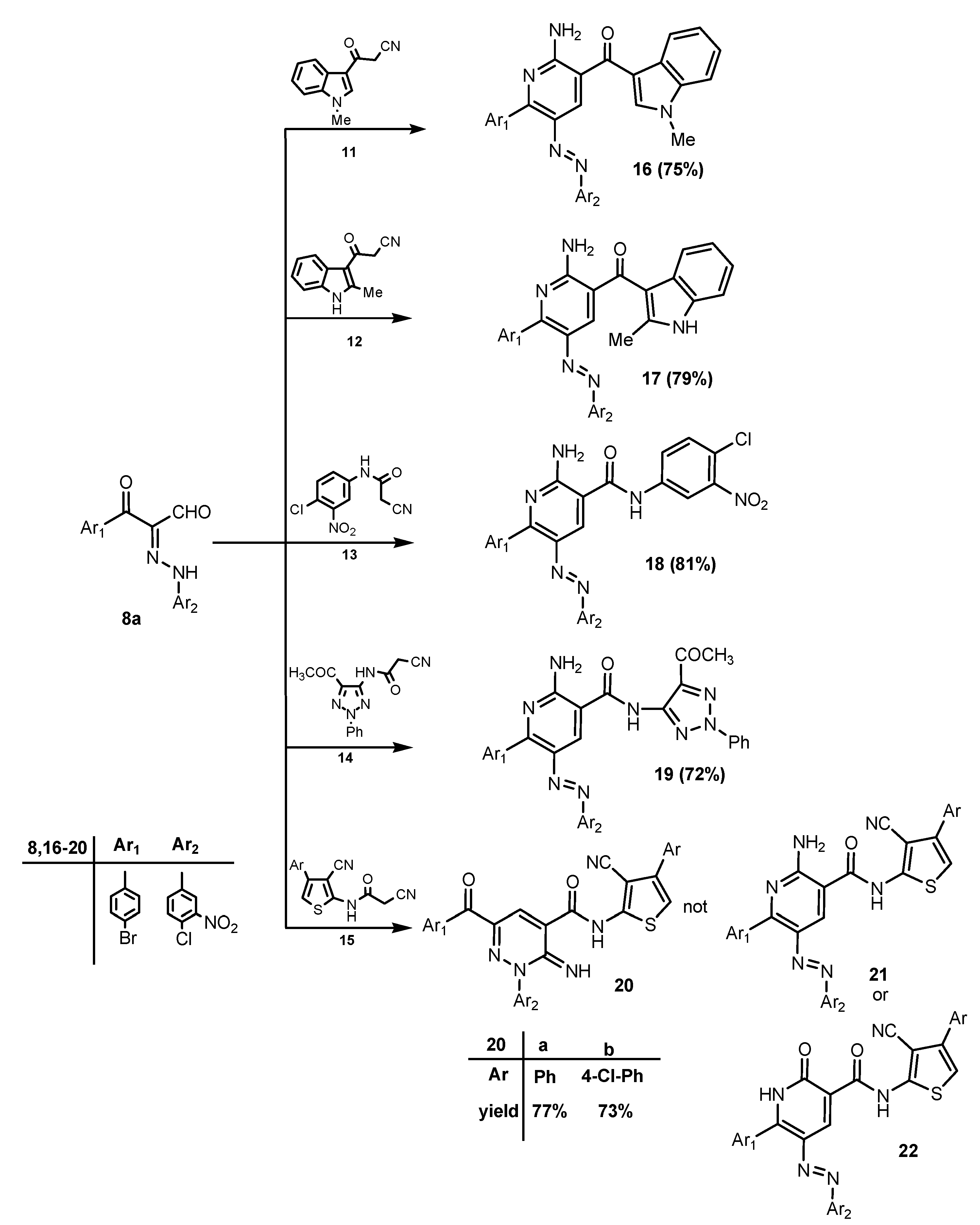
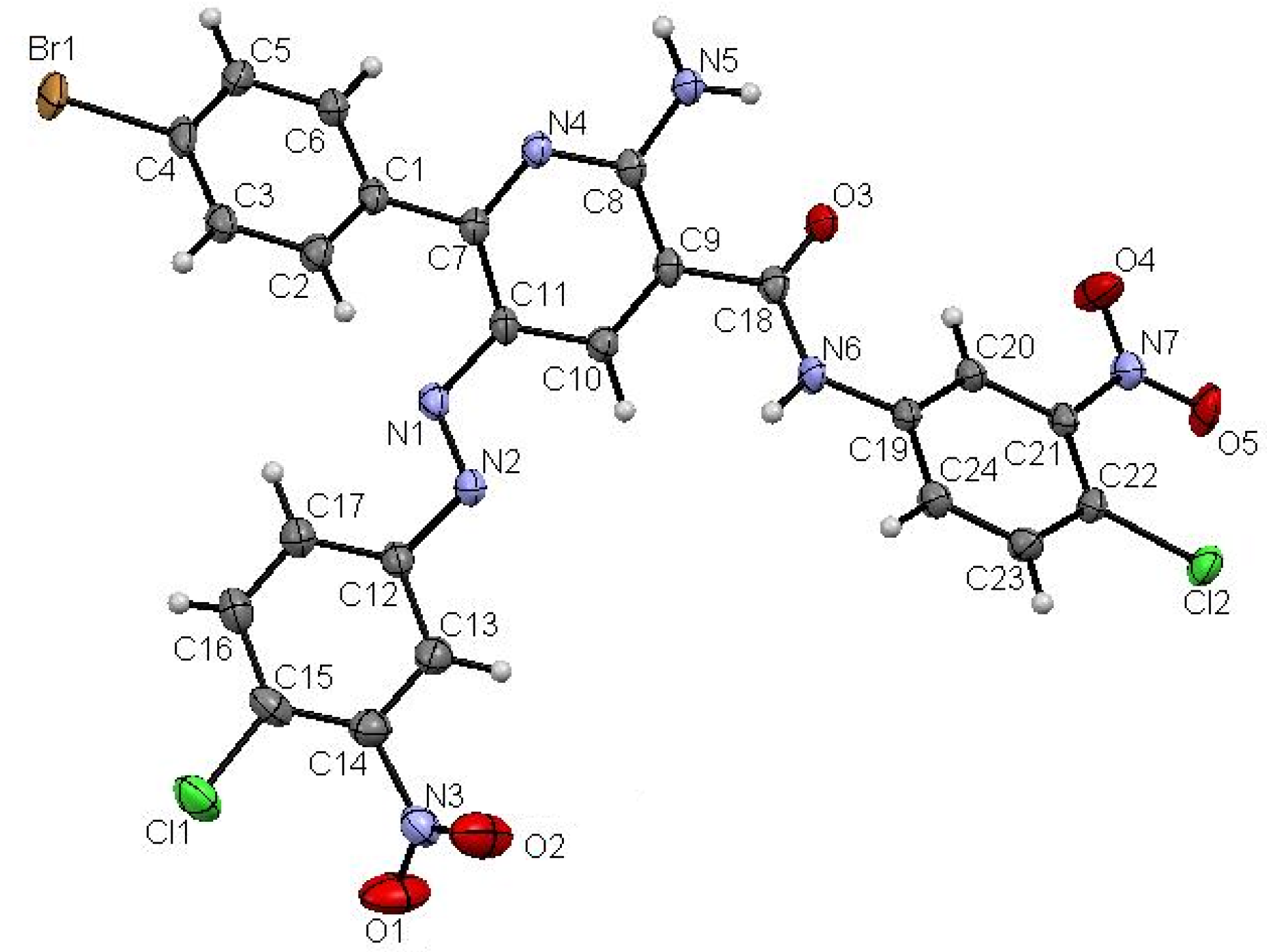
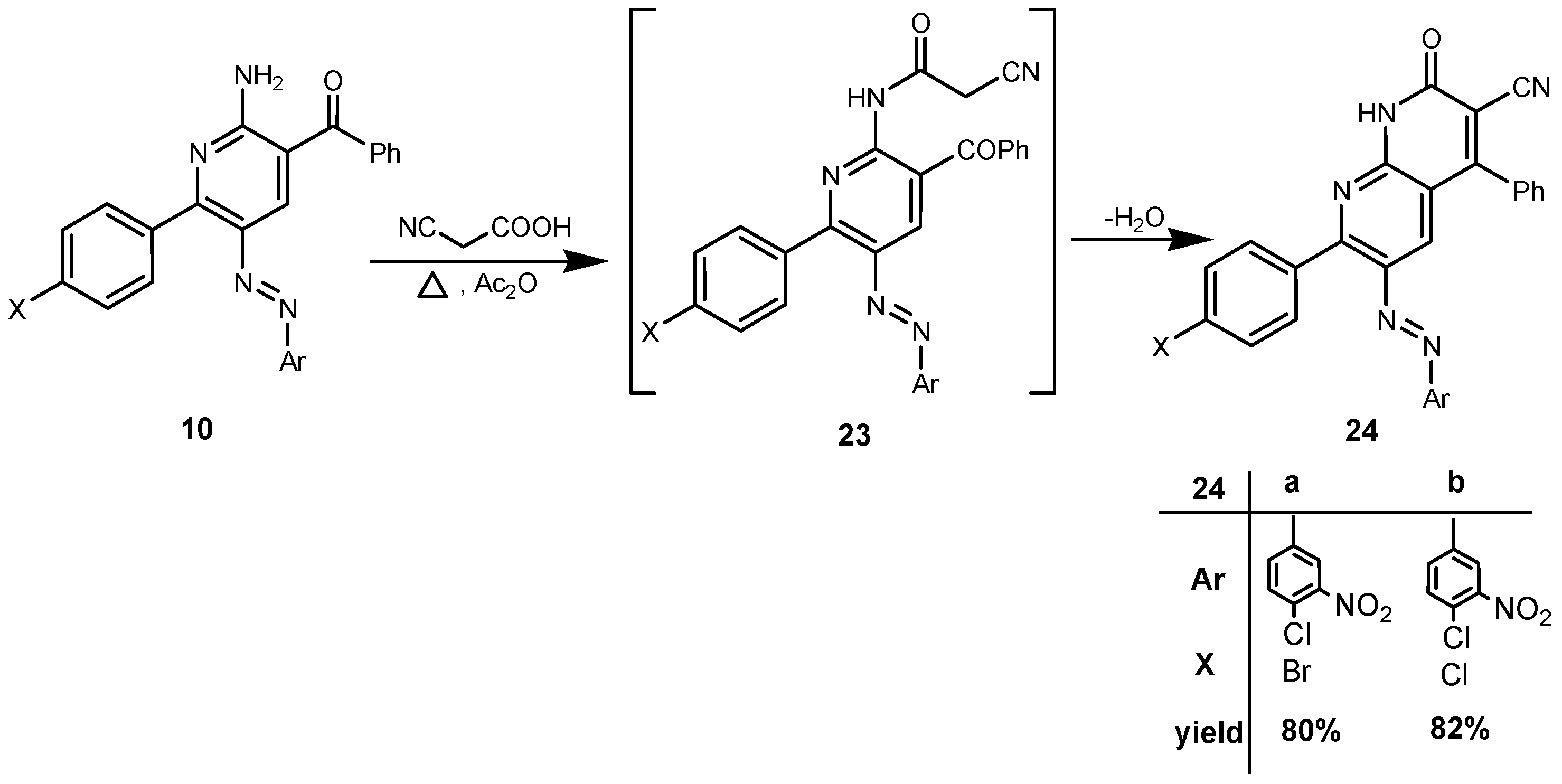
3. Experimental
3.1. General
3.2. Synthesis
3.2.1. General Procedure for the Preparation of Pyridazin-3-one Derivatives 3a–l
3.2.2. General Procedure for the Synthesis of Azolopyrimidines 5–7
3.2.3. General Procedure for the Preparation of Compounds 16–20
3.2.4. General Procedure for the Preparation of Naphthyridine Derivatives 24a,b
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References and Notes
- Zhmurenko, L.A.; Molodavkin, G.M.; Voronina, T.A.; Lezina, V.P. Synthesis and antidepressant and anxiolytic activity of derivatives of pyrazolo[4,3-c]pyridine and 4-phenylhydrazinonicotinic acids. Pharm. Chem. J. 2012, 46, 15–19. [Google Scholar] [CrossRef]
- Smyth, L.A.; Matthews, T.P.; Horton, P.N.; Hursthouse, M.B.; Collins, I. Synthesis and reactivity of 3-amino-1H-pyrazolo[4,3-c]pyridin-4(5H)-ones: Development of a novel kinase-focussed library. Tetrahedron 2010, 66, 2843–2854. [Google Scholar] [CrossRef]
- Suksrichavalit, T.; Prachayasittikul, S.; Nantasenamat, C.; Isarankura-Na-Ayudhya, C.; Prachayasittikul, V. Copper complexes of pyridine derivatives with superoxide scavenging and antimicrobial activities. Eur. J. Med. Chem. 2009, 44, 3259–3265. [Google Scholar] [CrossRef]
- Gaonkar, S.L.; Rai, K.M.L.; Prabhuswamy, B. Synthesis of novel 3-[5-ethyl-2-(2-phenoxy-ethyl)-pyridin]-5-substituted isoxazoline libraries via 1,3-dipolar cycloaddition and evaluation of antimicrobial activities. Med. Chem. Res. 2007, 15, 407–417. [Google Scholar] [CrossRef]
- Butnariu, R.M.; Caprosu, M.D.; Bejan, V.; Mangalagiu, I.I.; Ungureanu, M.; Poiata, A.; Tuchilus, C.; Florescu, M. Pyridazine and phthalazine derivatives with potential antimicrobial activity. J. Heterocycl. Chem. 2007, 44, 1149–1152. [Google Scholar] [CrossRef]
- Kandile, N.G.; Mohamed, M.I.; Zaky, H.; Mohamed, H.M. Novel pyridazine derivatives: Synthesis and antimicrobial activity evaluation. Eur. J. Med. Chem. 2009, 44, 989–1996. [Google Scholar]
- Hosni, H.M.; Abdulla, M.M. Anti-inflammatory and analgesic activities of some newly synthesized pyridinedicarbonitrile and benzopyranopyridine derivatives. Acta Pharm. 2008, 58, 175–186. [Google Scholar]
- Dogruer, D.S.; Unlu, S.; Kupeli, E.; Banoglu, E.; Sahin, M.F. Synthesis of 2-[5,6-diphenyl-3(2H)-pyridazinone-2-yl]acetamide and 3-[5,6-diphenyl-3(2H)-pyridazinone-2-yl]propanamide derivatives as analgesic and anti inflamematory agents. Turk. J. Pharm. Sci. 2007, 4, 57–70. [Google Scholar]
- Gökçe, M.; Colak, M.S.; Küpeli, E.; Sahin, M.F. Synthesis and analgesic and anti-inflammatory activity of 6-phenyl/(4-methylphenyl)-3(2H)-pyridazinon-2-propionamide derivatives. Arzneim. Forsch. 2009, 59, 357–363. [Google Scholar]
- Ali, M.A.; Yar, M.S.; Siddiqui, A.A.; Sriram, D.; Yogeeswari, P.; de Clercq, E. Synthesis and anti-HIV activity of N'-nicotinoyl-3-(4'-hydroxy-3'-methylphenyl)-5-[substituted phenyl]-2-pyrazo- lines. Acta Pol. Pharm. 2007, 64, 423–428. [Google Scholar]
- Kumar, S.; Das, S.K.; Dey, S.; Maity, P.; Guha, M.; Choubey, V.; Panda, G.; Bandyopadhyay, U. Antiplasmodial activity of [(aryl)arylsulfanylmethyl]pyridine. Antimicrob. Agents Chemother. 2008, 52, 705–715. [Google Scholar] [CrossRef]
- Lourenço, M.C.S.; de Souza, M.V.N.; Pinheiro, A.C.; de Lima Ferreira, M.; Goncalves, R.S.B.; Nogueira, T.C.M.; Peralta, M.A. Evaluation of anti-tubercular activity of nicotinic and isoniazid analogues. ARKIVOC 2007, xv, 181–191. [Google Scholar]
- Sharma, P.C.; Jain, S. Synthesis and in vitro antibacterial activity of some novel N-nicotinoyl-1-ethyl-6-fluoro-1,4-dihydro-7-piperazin-1-yl-4-oxoquinoline-3-carboxylates. Acta Pol. Pharm. 2008, 65, 551–556. [Google Scholar]
- Shafiee, A.; Rastkari, N.; Sharifzadeh, M. Anticonvulsant activities of new 1,4-dihydropyridine derivatives containing 4-nitroimidazolyl substituents. Daru 2004, 12, 81–86. [Google Scholar]
- Rubat, C.; Coudert, P.; Refouvelet, B.; Tronche, P.; Bastide, P.; Bastide, J. Anticonvulsant activity of 3-oxo-5-substituted benzylidene-6-methyl-(4H)-2-pyridazinylacetamides and 2-pyridazinylacetyl-hydrazides. Chem. Pharm. Bull. 1990, 38, 3009–3013. [Google Scholar] [CrossRef]
- Chintakunta, V.K.; Akella, V.; Vedula, M.S.; Mamnoor, P.K.; Mishra, P.; Casturi, S.R.; Vangoori, A.; Rajagopalan, R. 3-O-Substituted benzyl pyridazinone derivatives as COX inhibitors. Eur. J. Med. Chem. 2002, 37, 339–347. [Google Scholar]
- Rathish, I.G.; Javed, K.; Bano, S.; Ahmad, S.; Alam, M.S.; Pillai, K.K. Synthesis and blood glucose lowering effect of novel pyridazinone substituted benzenesulfonylurea derivatives. Eur. J. Med. Chem. 2009, 44, 2673–2678. [Google Scholar] [CrossRef]
- Barbaro, R.; Betti, L.; Botta, M.; Corelli, F.; Giannaccini, G.; Maccari, L.; Manetti, F.; Strappaghetti, G.; Corsano, S. Synthesis, biological evaluation, and pharmacophore generation of new pyridazinone derivatives with affinity toward α1- and α2-adrenoceptors. J. Med. Chem. 2001, 44, 2118–2132. [Google Scholar] [CrossRef]
- Joule, J.A.; Mills, K. Heterocyclic Chemistry, 5th ed.; Wiley Blackwell: Oxford, UK, 2010; pp. 645–664. [Google Scholar]
- Atanasova, M.; Ilieva, S.; Galabov, B. QSAR analysis of 1,4-dihydro-4-oxo-1-(2-thiazolyl)-1,8-naphthyridines with anticancer activity. Eur. J. Med. Chem. 2007, 42, 1184–1192. [Google Scholar] [CrossRef]
- Miguel, F.B.; Monica, C.M.L.G.; Elena, P.M.; Berta, L.; de Beatriz, P.T.; Ana, R.; Nuria, A.; Francisco, L.; Dolores, M.M.; Olivier, L.; et al. Pyrazolo[3,4-c]pyridazines as novel and selective inhibitors of cyclin-dependent kinases. J. Med. Chem. 2005, 48, 6843–6854. [Google Scholar] [CrossRef]
- Malinka, W.; Redzicka, A.; Lozach, O. New derivatives of pyrrolo[3,4-d]pyridazinone and their anticancer effects. Farmaco 2004, 59, 457–462. [Google Scholar] [CrossRef]
- Thapa, P.; Karki, R.; Thapa, U.; Jahng, Y.; Jung, M.-J.; Nam, J.M.; Na, Y.; Kwon, Y.; Lee, E.-S. 2-Thienyl-4-furyl-6-aryl pyridine derivatives: Synthesis, topoisomerase I and II inhibitory activity, cytotoxicity, and structure–activity relationship study. Bioorg. Med. Chem. 2010, 18, 377–386. [Google Scholar] [CrossRef]
- Thapa, P.; Karki, R.; Choi, H.; Choi, J.H.; Yun, M.; Jeong, B.-S.; Jung, M.-J.; Nam, J.M.; Na, Y.; Cho, W.-J.; et al. Synthesis of 2-(thienyl-2-yl or -3-yl)-4-furyl-6-aryl pyridine derivatives and evaluation of their topoisomerase I and II inhibitory activity, cytotoxicity, and structure activity relationship. Bioorg. Med. Chem. 2010, 18, 2245–2254. [Google Scholar] [CrossRef]
- Sotelo, E.; Fraiz, N.; Yanez, M.; Terrades, V.; Laguna, R.; Cano, E.; Ravina, E. Pyridazines. Part XXIX: Synthesis and platelet aggregation inhibition activity of 5-substituted-6-phenyl-3(2H)-pyridazinones novel aspects of their biological action. Bioorg. Med. Chem. 2002, 10, 2873–2882. [Google Scholar] [CrossRef]
- Griebel, G.; Perrault, G.; Sanger, D.J. Differences in anxiolytic-like profile of two novel nonbenzodiazepine BZ (omega) receptor agonists on defensive behaviors of mice. Pharmacol. Biochem. Behav. 1999, 62, 689–694. [Google Scholar] [CrossRef]
- Wermuth, C.G.; Schlewer, G.; Bourguignon, J.J.; Maghioros, G.; Bouchet, M.J.; Moire, C.; Kan, J.P.; Worms, P.; Biziere, K. 3-Aminopyridazine derivatives with atypical antidepressant, serotonergic, and dopaminergic activities. J. Med. Chem. 1989, 32, 528–537. [Google Scholar] [CrossRef]
- Caliskan, E.B.; Sukuroglu, M.; Coban, T.; Banoglu, E.; Suzen, S. Screening and evaluation of antioxidant activity of some pyridazine derivatives. J. Enz. Inhib. Med. Chem. 2008, 23, 225–229. [Google Scholar] [CrossRef]
- Chen, K.; Kuo, S.-C.; Hsieh, M.-C.; Mauger, A.; Lin, C.M.; Hamel, E.; Lee, K.-H. Antitumor agents. 178. Synthesis and biological evaluation of substituted 2-aryl-1,8-naphthyridin-4(1H)-ones as antitumor agents that inhibit tubulin polymerization. J. Med. Chem. 1997, 40, 3049–3056. [Google Scholar] [CrossRef]
- Zhang, S.-X.; Bastow, K.F.; Tachibana, Y.; Kuo, S.-C.; Hamel, E.; Mauger, A.; Narayanan, V.L.; Lee, K.-H. Antitumor agents. 196. Substituted 2-thienyl-1,8-naphthyridin-4-ones: Their synthesis, cytotoxicity, and inhibition of tubulin polymerization. J. Med. Chem. 1999, 42, 4081–4087. [Google Scholar] [CrossRef]
- Wu, J.; Kang, S.; Luo, L.; Shi, Q.; Ma, J.; Yin, J.; Song, B.; Hu, D.; Yang, S. Synthesis and antifungal activities of novel nicotinamide derivatives containing 1,3,4-oxadiazole. Chem. Cent. J. 2013, 7, 64. [Google Scholar] [CrossRef]
- Behbehani, H.; Ibrahim, H.M.; Elnagdi, M.H. Non-concerted nucleophilic [4+1] cycloaddition of (dimethylamino)methoxycarbene to arylazonicotinates in the synthesis of pyrazolo[3,4-c]pyridines and pyrazolo[4',3':4,5]pyrido[2,3-d]pyrimidines. Tetrahedron 2013, 69, 6176–6184. [Google Scholar] [CrossRef]
- Behbehani, H.; Ibrahim, H.M.; Makhseed, S.; Mahmoud, H. Applications of 2-arylhydrazononitriles in synthesis: Preparation of new indole containing 1,2,3-triazole, pyrazole and pyrazolo[1,5-a]pyrimidine derivatives and evaluation of their antimicrobial activities. Eur. J. Med. Chem. 2011, 46, 1813–1820. [Google Scholar] [CrossRef]
- Behbehani, H.; Ibrahim, H.M.; Makhseed, S.; Elnagdi, M.H.; Mahmoud, H. 2-Aminothiophenes as building blocks in heterocyclic synthesis: Synthesis and antimicrobial evaluation of a new class of pyrido[1,2-a]thieno[3,2-e]pyrimidine, quinoline and pyridin-2-one derivatives. Eur. J. Med. Chem. 2012, 52, 51–65. [Google Scholar]
- Behbehani, H.; Ibrahim, H.M. 4-Thiazolidinones in heterocyclic synthesis: Synthesis of novel enaminones, azolopyrimidines and 2-arylimino-5-arylidene-4-thiazolidinones. Molecules 2012, 17, 6362–6385. [Google Scholar] [CrossRef]
- Behbehani, H.; Ibrahim, H.M. Organocatalysis in heterocyclic synthesis: DABCO as a mild and efficient catalytic system for the synthesis of a novel class of quinazoline, thiazolo[3,2-a]quinazoline and thiazolo[2,3-b]quinazoline derivatives. Chem. Cent. J. 2013, 7, 82. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Behbehani, H.; Elnagdi, M.H. Approaches towards the synthesis of a novel class of 2-amino-5-arylazonicotinate, pyridazinone and pyrido[2,3-d]pyrimidine derivatives as potent antimicrobial agents. Chem. Cent. J. 2013, 7, 123. [Google Scholar] [CrossRef]
- Crystallographic data for 3d (ref. CCDC 982246) can be obtained on request from the director, Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EW, UK.
- Crystallographic data for 3l (ref. CCDC 982247) can be obtained on request from the director, Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EW, UK.
- Behbehani, H.; Ibrahim, H.M. A strategy for the synthesis of 2-aryl-3-dimethylaminopyrazolo-[3,4-c]pyridines that utilizes [4+1] cycloaddition reactions of 5-arylazo-2,3,6-trisubstituted pyridines. Tetrahedron. 2013, 69, 10535–10543. [Google Scholar] [CrossRef]
- Al-Mousawi, S.M.; Moustafa, M.S.; Abdelshafy, I.A.; Elnagdi, M.H. Reassignment of the structures of condensation products of α-keto α'-formylarylhydrazones with ethyl cyanoacetate: A novel route to ethyl 5-arylazo-2-hydroxynicotinates. Tetrahedron Lett. 2011, 52, 202–204. [Google Scholar] [CrossRef]
- Crystallographic data for 18 (ref. CCDC 943164) can be obtained on request from the director, Cambridge Crystallographic Data Center, 12 Union Road, Cambridge CB2 1EW, UK.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Sample Availability: Samples of all compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ibrahim, H.M.; Behbehani, H. Synthesis of A New Class of Pyridazin-3-one and 2-Amino-5-arylazopyridine Derivatives and Their Utility in the Synthesis of Fused Azines. Molecules 2014, 19, 2637-2654. https://doi.org/10.3390/molecules19022637
Ibrahim HM, Behbehani H. Synthesis of A New Class of Pyridazin-3-one and 2-Amino-5-arylazopyridine Derivatives and Their Utility in the Synthesis of Fused Azines. Molecules. 2014; 19(2):2637-2654. https://doi.org/10.3390/molecules19022637
Chicago/Turabian StyleIbrahim, Hamada Mohamed, and Haider Behbehani. 2014. "Synthesis of A New Class of Pyridazin-3-one and 2-Amino-5-arylazopyridine Derivatives and Their Utility in the Synthesis of Fused Azines" Molecules 19, no. 2: 2637-2654. https://doi.org/10.3390/molecules19022637



