Functional Properties of a Cysteine Proteinase from Pineapple Fruit with Improved Resistance to Fungal Pathogens in Arabidopsis thaliana
Abstract
:1. Introduction
2. Results and Discussion
2.1. Molecular Cloning of the AcCP2 Gene
2.2. Structure Characteristics of the AcCP2 Protein
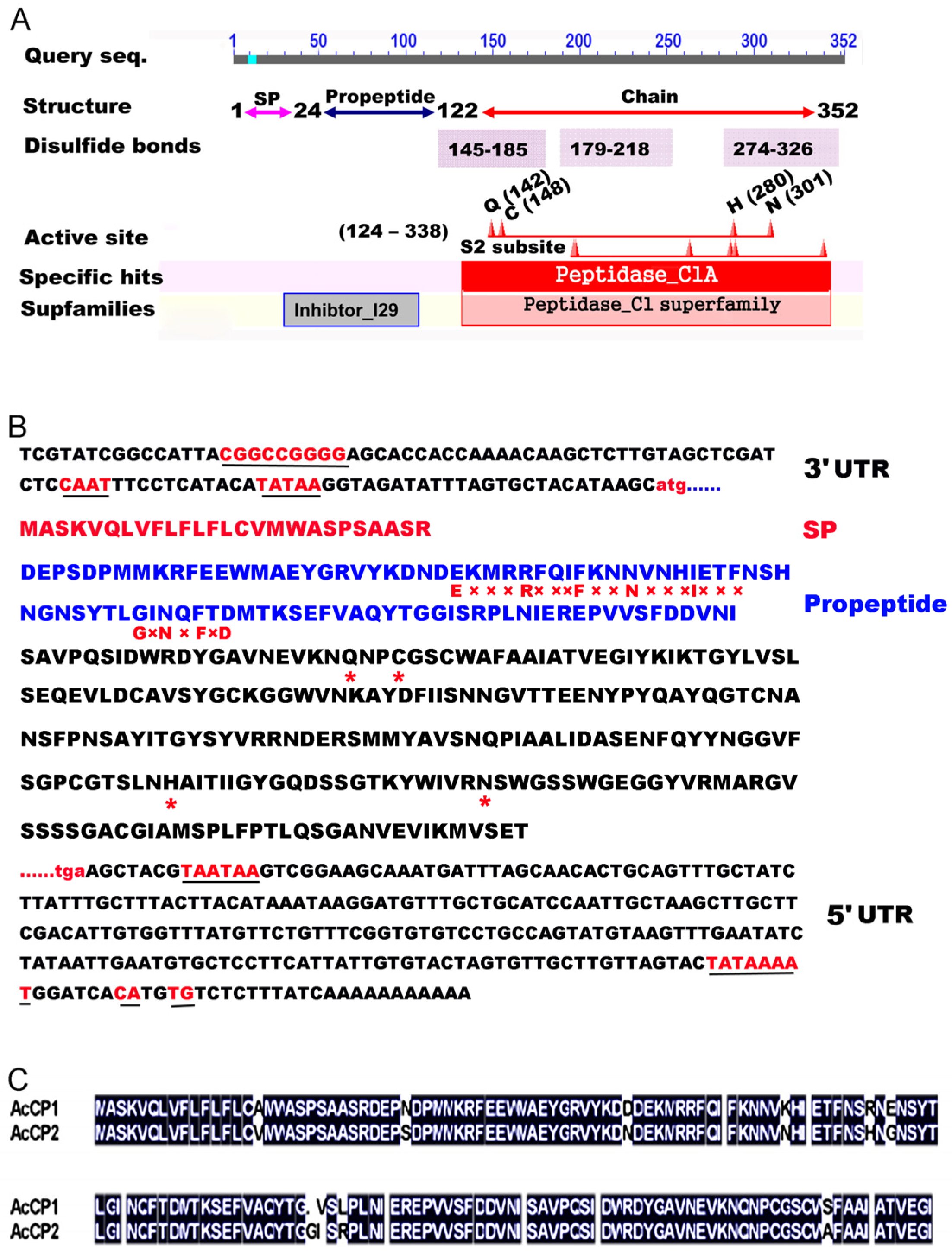
2.3. Expression Patterns of AcCP2 During Pineapple-Fruit Development
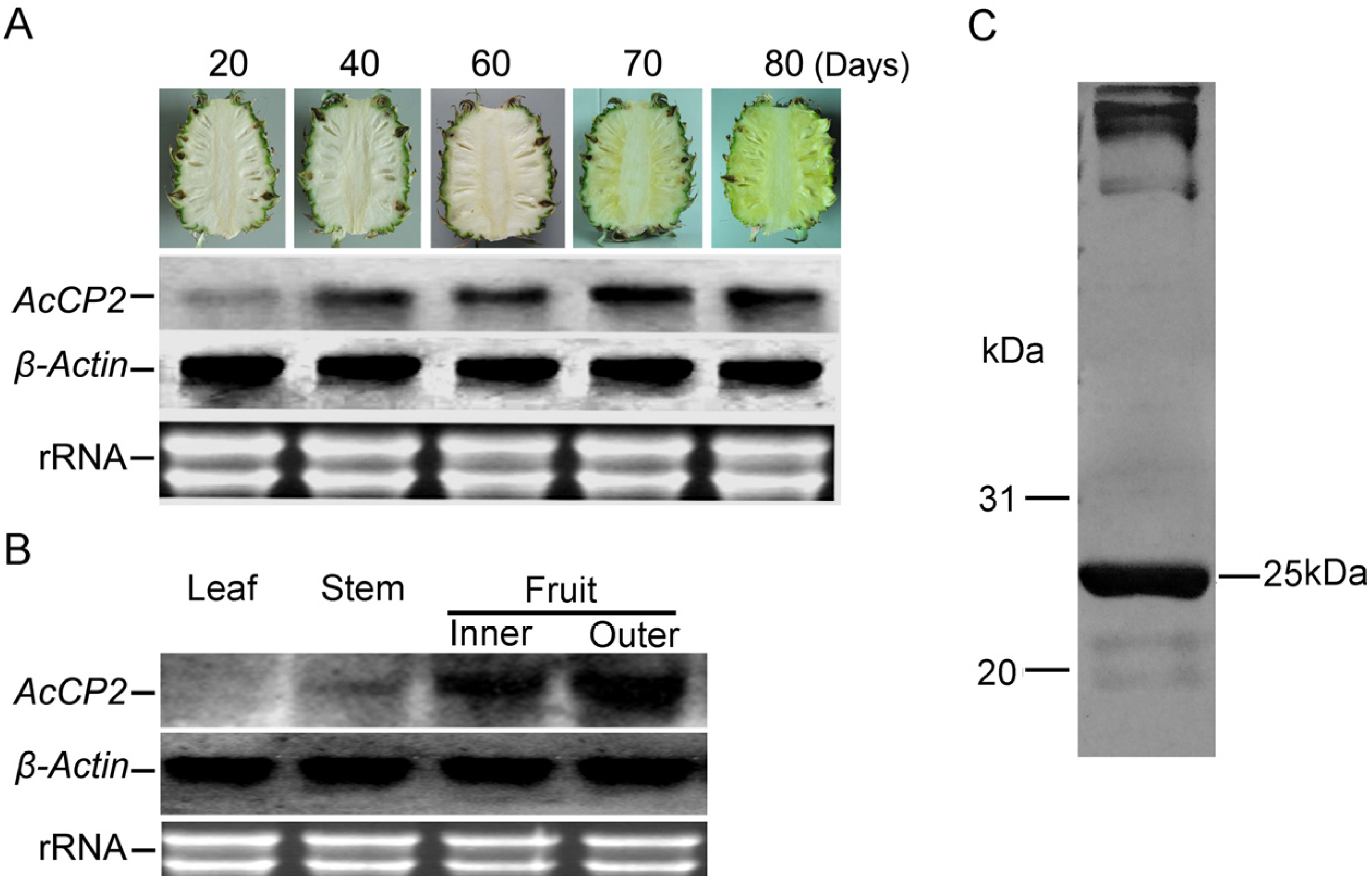
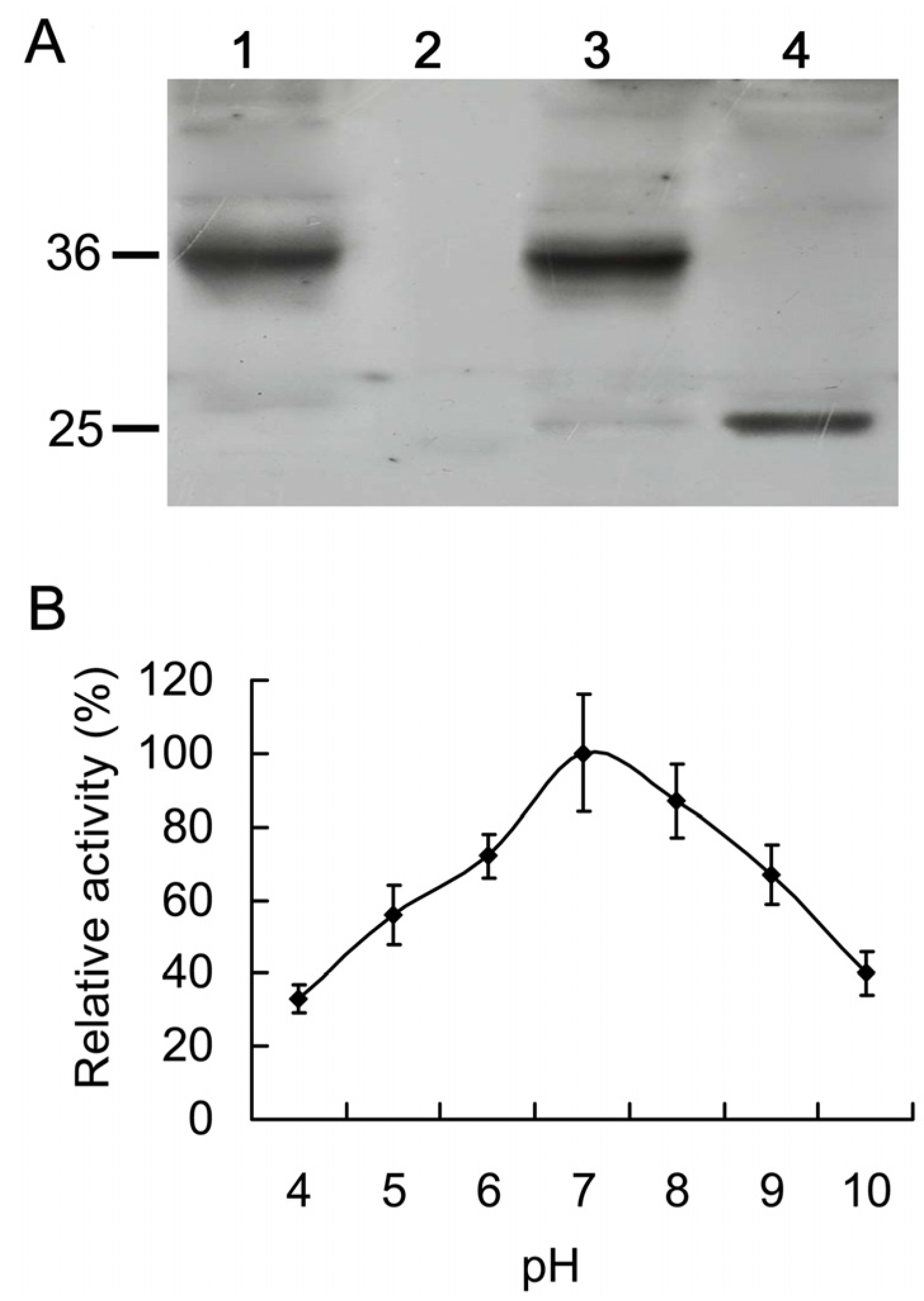
2.4. Expression of His-AcCP2 Recombinant Protein
2.5. Catalytic Characteristics of His-AcCP2 Recombinant Protein Using Synthetic Substrates
| Proteinase | Substrates | Km (μM) | kcat (S−1) | kcat/Km (Mm−1·S−1) |
|---|---|---|---|---|
| AcCP2 a | Z-Arg-Arg-NH-Mec | 72.3 ± 6.4 | 0.004 ± 0.001 | 0.005 |
| Bz-Phe-Val-Arg-NH-Mec | 3.2 ± 1.1 | 15.4 ± 4.8 | 4812.5 | |
| Commercial bromelain b | Z-Arg-Arg-NH-Mec | 82.5 ± 10.2 | 0.003 ± 0.001 | 0.003 |
| Bz-Phe-Val-Arg-NH-Mec | 4.6 ± 1.0 | 14.12 ± 5.2 | 3065 |
2.6. Disease Resistance Analysis in Transgenic Arabidopsis Plants
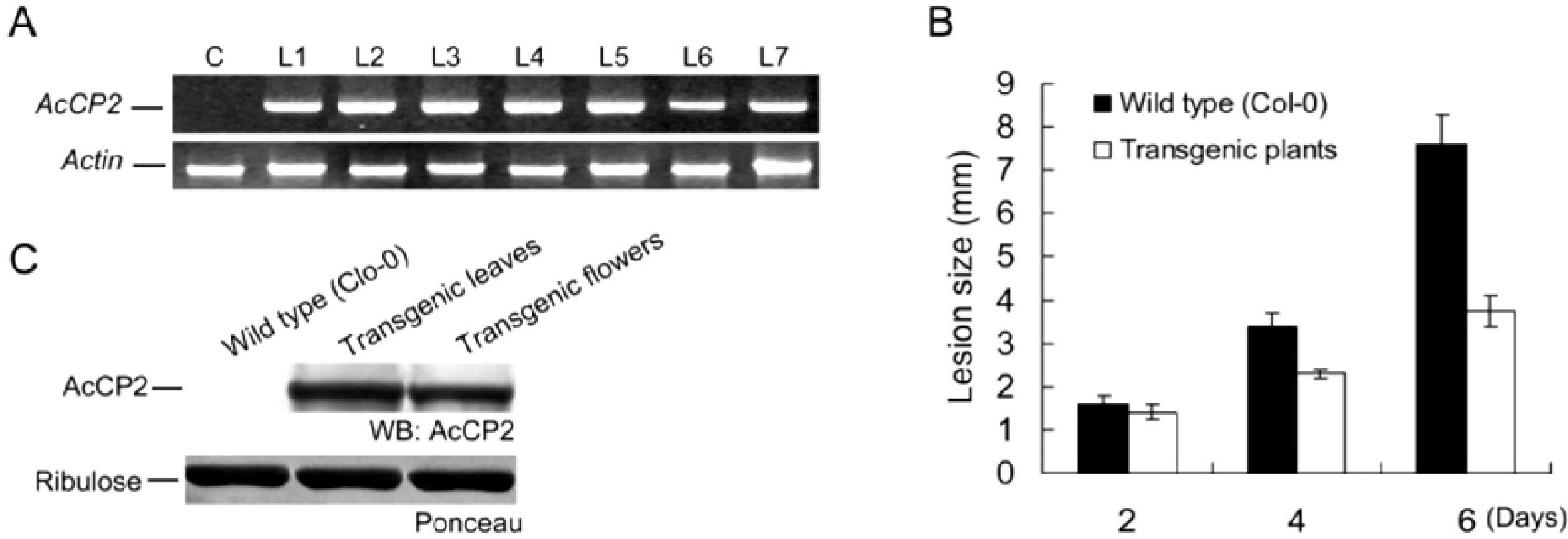
2.7. Discussion
3. Experimental
3.1. Plant Materials
3.2. Isolation of AcCP2 Gene
3.3. Extraction and Purification of Plant Proteins
3.4. RNA Blot Analysis
3.5. Expression and Purification of pPIC9K-AcCP2 in P. pastoris
3.6. Antibodies and Western Blots
3.7. Enzymatic Activity of Recombinant Proteinase
3.8. Measurement of Sugar Contents and Invertase Activity in Pineapple Fruits
3.9. Agrobacterium-Mediated Floral Dip Transformation of Arabidopsis
3.10. Treatment of Arabidopsis Plants
3.11. Bioinformatic Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Solomon, M.; Belenghi, B.; Delledonne, M.; Menachem, E.; Levine, A. The involvement of cysteine proteases and protease inhibitor genes in the regulation of programmed cell death in plants. Plant Cell 1999, 11, 431–444. [Google Scholar]
- Krüger, J.; Thomas, C.M.; Golstein, C.; Dixon, M.S.; Smoker, M.; Tang, S.J.; Mulder, L.; Jones, J.D.G. A tomato cysteine protease required for Cf-2-dependent disease resistance and suppression of autonecrosis. Science 2002, 296, 744–747. [Google Scholar] [CrossRef]
- Alonso, J.M.; Granell, A. A putative vacuolar processing protease is regulated by ethylene and also during fruit ripening in citrus fruit. Plant Physiol. 1995, 109, 541–547. [Google Scholar]
- Mohan, S.; Ma, P.W.K.; Williams, W.P.; Luthe, D.S. A naturally occurring plant cysteine protease possesses remarkable toxicity against insect pests and synergizes Bacillus thuringiensis toxin. PLoS One 2008, 3, e1786. [Google Scholar] [CrossRef]
- Bozhkov, P.V.; Suarez, M.F.; Filonova, L.H.; Daniel, G.; Zamyatnin, A.A.; Rodriguez-Nieto, S.; Zhivotovsky, B.; Smertenko, A. Cysteine protease mcII-Pa executes programmed cell death during plant embryogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 14463–14468. [Google Scholar] [CrossRef]
- Toyooka, K.; Okamoto, T.; Minamikawa, T. Mass transport of proform of a KDEL-tailed cysteine proteinase (SH-EP) to protein storage vacuoles by endoplasmic reticulum-derived vesicle is involved in protein mobilization in germinating seeds. J. Cell Boil. 2000, 148, 453–463. [Google Scholar] [CrossRef]
- Souza, D.P.; Freitas, C.D.T.; Pereira, D.A.; Nogueira, F.C.; Silva, F.D.A.; Salas, C.E.; Ramos, M.V. Laticifer proteins play a defensive role against hemibiotrophic and necrotrophic phytopathogens. Planta 2011, 234, 183–193. [Google Scholar] [CrossRef]
- MEROPS the Peptidase Database. Available online: http://merops.sanger.ac.uk/ (accessed on 12 October 2013).
- Trejo, S.A.; López, L.M.I.; Caffini, N.O.; Natalucci, C.L.; Canals, F.; Avilés, F.X. Sequencing and characterization of asclepain f: The first cysteine peptidase cDNA cloned and expressed from Asclepias. fruticosa latex. Planta 2009, 230, 319–328. [Google Scholar] [CrossRef]
- Drenth, J.; Jansoniu, J.N.; Koekoek, R.; Swen, H.M.; Wolthers, B.G. Structure of papain. Nature 1968, 218, 929–932. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J.; Bateman, A. MEROPS: The database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2012, 40, D343–D350. [Google Scholar]
- Wiederanders, B.; Kaulmann, G.; Schilling, K. Functions of propeptide parts in cysteine proteases. Curr. Protein Pept. Sci. 2003, 4, 309–326. [Google Scholar] [CrossRef]
- Van der Hoorn, R.A.L. Plant proteases: From phenotypes to molecular mechanisms. Annu. Rev. Plant Biol. 2008, 59, 191–223. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Filho, J.X.; Sales, M.P. Cysteine proteinases cystatins. Braz. Arch. Biol. Techn. 2003, 46, 91–104. [Google Scholar] [CrossRef]
- Gautam, S.S.; Mishra, S.K.; Dash, V.; Goyal, A.K.; Rath, G. Comparative study of extraction, purification and estimation of bromelain from stem and fruit of pineapple plant. Thai J. Pharm. Sci. 2010, 34, 67–76. [Google Scholar]
- Larocca, M.; Rossano, R.; Santamaria, M.; Riccio, P. Analysis of pineapple [Ananas comosus (L.) Merr.] fruit proteinases by 2-D zymography and direct identification of the major zymographic spots by mass spectrometry. Food Chem. 2010, 123, 1334–1342. [Google Scholar] [CrossRef]
- Maurer, H.R. Bromelain: Biochemistry, pharmacology and medical use. Cell Mol. Life Sci. 2001, 58, 1234–1245. [Google Scholar] [CrossRef]
- Harrach, T.; Eckert, K.; Schulze-Forster, K.; Nuck, R.; Grunow, D.; Maurer, H.R. Isolation and partial characterization of basic proteinases from stem bromelain. J. Protein Chem. 1995, 14, 41–52. [Google Scholar]
- Rowan, A.D.; Buttle, D.J.; Barrett, A.J. The cysteine proteases of the pineapple plant. Biochem. J. 1990, 266, 869–875. [Google Scholar]
- Hale, L.P.; Greer, P.K.; Trinh, C.T.; James, C.L. Proteinase activity and stability of natural bromelain preparations. Int. Immunopharmacol. 2005, 5, 783–793. [Google Scholar] [CrossRef]
- Yamada, F.; Takahashi, N.; Murachi, T. Purification and characterization of a protease from pineapple fruit, fruit bromelain FA2. J. Biochem. 1976, 79, 1223–1234. [Google Scholar]
- Corzo, C.A.; Waliszewski, K.N.; Welti-Chanes, J. Pineapple fruit bromelain affinity to different protein substrates. Food Chem. 2012, 133, 631–635. [Google Scholar] [CrossRef]
- Ritonja, A.; Rowan, A.D.; Buttle, D.J.; Rawlings, N.D.; Turk, V.; Barrett, A.J. Stem bromelain: Amino acid sequence and implications for weak binding of cystatin. Fed. Eur. Biochem. Soc. Lett. 1989, 247, 419–424. [Google Scholar] [CrossRef]
- Amid, A.; Ismail, N.A.; Yusof, F.; Salleh, H.M. Expression, purification, and characterization of a recombinant stem bromelain from Ananas comosus. Process Biochem. 2011, 46, 2232–2239. [Google Scholar] [CrossRef]
- Aoki, H.; Nazmul Ahsan, M.; Watabe, S. Heterologous expression in Pichia pastoris and single-step purification of a cysteine proteinase from northern shrimp. Protein Expres. Purif. 2003, 31, 213–221. [Google Scholar] [CrossRef]
- Beers, E.P.; Jones, A.M.; Dickerman, A.W. The S8 serine, C1A cysteine and A1 aspartic protease families in Arabidopsis. Photochemistry 2004, 65, 43–58. [Google Scholar] [CrossRef]
- Neuteboom, L.W.; Matsumoto, K.O.; Christopher, D.A. An extended AE-rich N-terminal trunk in secreted pineapple cystatin enhances inhibition of fruit bromelain and is posttranslationally removed during ripening. Plant Physiol. 2009, 151, 515–527. [Google Scholar] [CrossRef]
- Brömme, D.; Nallaseth, F.S.; Turk, B. Production and activation of recombinant papain-like cysteine proteases. Methods 2004, 32, 199–206. [Google Scholar] [CrossRef]
- Karrer, K.M.; Peiffer, S.L.; DiTomas, M.E. Two distinct gene subfamilies within the family of cysteine protease genes. Proc. Natl. Acad. Sci. USA 1993, 90, 3063–3067. [Google Scholar] [CrossRef]
- Vernet, T.; Berti, P.J.; de Montigny, C.; Musil, R.; Tessier, D.C.; Menard, R.; Magny, M.C.; Storer, A.C.; Thomas, D.Y. Processing of the papain precursor. The ionization state of a conserved amino acid motif within the proregion participates in the regulation of intramolecular processing. J. Biol. Chem. 1995, 270, 10838–10846. [Google Scholar] [CrossRef]
- Gomes, M.T.; Teixeira, R.D.; Lopes, M.T.; Nagem, R.A.; Salas, C.E. X-ray crystal structure of CMS1MS2: A high proteolytic activity cysteine proteinase from Carica candamarcensis. Amino Acids 2012, 43, 2381–2391. [Google Scholar] [CrossRef]
- Barrett, A.J.; Rawlings, N.D. Introduction: The Clans and Families of Cysteine Peptidases. In Handbook of Proteolytic Enzymes, 2nd ed.; Barrett, A.J., Rawlings, N.D., Woessner, J.F., Eds.; Elsevier: London, UK, 2004; pp. 1051–1071. [Google Scholar]
- Smooker, P.M.; Whisstock, J.C.; Irving, J.A.; Siyaguna, S.; Spithill, T.W.; Pike, R.N. For the record: A single amino acid substitution affects substrate specificity in cysteine proteinases from Fasciola hepatica. Protein Sci. 2000, 9, 2567–2572. [Google Scholar] [CrossRef]
- Yamada, T.; Ohta, H.; Shinohara, A.; Iwamatsu, A.; Shimada, H.; Tsuchiya, T.; Masuda, T.; Takamiya, K.I. A cysteine protease from maize isolated in a complex with cystatin. Plant Cell. Physiol. 2000, 41, 185–191. [Google Scholar]
- Dahl, S.W.; Halkier, T.; Lauritzen, C.; Dolenc, I.; Pedersen, J.; Turk, V.; Turk, B. Human recombinant pro-dipeptidyl peptidase I (cathepsin C) can be activated by cathepsins L and S but not by autocatalytic processing. Biochemistry 2001, 40, 1671–1678. [Google Scholar] [CrossRef]
- Koia, J.H.; Moyle, R.L.; Botella, J.R. Microarray analysis of gene expression profiles in ripening pineapple fruits. BMC Plant Biol. 2012, 12, 240. [Google Scholar] [CrossRef]
- Ariizumi, T.; Higuchi, K.; Arakaki, S.; Sano, T.; Asamizu, E.; Ezura, H. Genetic suppression analysis in novel vacuolar processing enzymes reveals their roles in controlling sugar accumulation in tomato fruits. J. Exp. Bot. 2011, 62, 2773–2786. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.M.; Wang, W.; Du, L.Q.; Xie, J.H.; Yao, Y.L.; Sun, G.M. Expression patterns, activities and carbohydrate-metabolizing regulation of sucrose phosphate synthase, sucrose synthase and neutral invertase in pineapple fruit during development and ripening. Int. J. Mol. Sci. 2012, 13, 9460–9477. [Google Scholar] [CrossRef]
- Matarasso, N.; Schuster, S.; Avni, A. A novel plant cysteine protease has a dual function as a regulator of 1-aminocyclopropane-1-carboxylic acid synthase gene expression. Plant Cell 2005, 17, 1205–1216. [Google Scholar] [CrossRef]
- Yamada, K.; Shimada, T.; Nishimura, M.; Hara-Nishimura, I. A VPE family supporting various vacuolar functions in plants. Physiol. Plant 2005, 123, 369–375. [Google Scholar] [CrossRef]
- Konno, K.; Hirayama, C.; Nakamura, M.; Tateishi, K.; Tamura, Y.; Hattori, M.; Kohno, K. Papain protects papaya trees from herbivorous insects: Role of cysteine proteases in latex. Plant J. 2004, 37, 370–378. [Google Scholar] [CrossRef]
- López, L.; Camas, A.; Shivaji, R.; Ankala, A.; Williams, W.P.; Luthe, D.S. Mir1-CP, a novel defense cysteine protease accumulates in maize vascular tissues in response to herbivory. Planta 2007, 226, 517–527. [Google Scholar] [CrossRef]
- Raimbault, A.K.; Zuily-Fodil, Y.; Soler, A.; Mora, P.; de Carvalho, C.M.H. The expression patterns of bromelain and AcCYS1 correlate with blackheart resistance in pineapple fruits submitted to postharvest chilling stress. J. Plant Physiol. 2013, 170, 1442–1446. [Google Scholar] [CrossRef]
- Jung, Y.J.; Choi, C.S.; Park, J.H.; Kang, H.W.; Choi, J.E.; Nou, I.S.; Lee, S.Y.; Kang, K.K. Overexpression of the pineapple fruit bromelain gene (BAA) in transgenic Chinese cabbage (Brassica rapa) results in enhanced resistance to bacterial soft rot. Electron J. Biotech. 2008, 11, 1–9. [Google Scholar]
- López-García, B.; Hernandez, M.; Segundo, B.S. Bromelain, a cysteine protease from pineapple (Ananas comosus) stem, is an inhibitor of fungal plant pathogens. Lett. Appl. Microbial. 2012, 55, 62–67. [Google Scholar] [CrossRef]
- Nieuwenhuizen, N.J.; Maddumage, R.; Tsang, G.K.; Fraser, L.G.; Cooney, J.M.; de Silva, H.N.; Green, S.; Richardson, K.A.; Atkinson, R.G. Mapping, complementation, and targets of the cysteine protease actinidin in kiwifruit. Plant Physiol. 2012, 158, 376–388. [Google Scholar] [CrossRef]
- Zhang, X.; Henriques, R.; Lin, S.S.; Niu, Q.W.; Chua, N.H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef]
- Candiano, G.; Bruschi, M.; Musante, L.; Santucci, L.; Ghiggeri, G.M.; Carnemolla, B.; Orecchia, P.; Zardi, L.; Righetti, P.G. Blue silver: A very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 2004, 25, 1327–1333. [Google Scholar] [CrossRef]
- Nagarathnam, R.; Rengasamy, A.; Balasubramanian, R. Purification and properties of cysteine protease from rhizomes of Curcuma longa (Linn.). J. Sci. Food Agric. 2010, 90, 97–105. [Google Scholar] [CrossRef]
- Stewart, C.N.; Via, L.E. A rapid CTAB DNA isolation technique useful for RAPD fingerprinting and other PCR applications. Biotechniques 1993, 14, 748–750. [Google Scholar]
- Blast. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 20 March 2012).
- NCBI. Available online: http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi (accessed on 20 March 2012).
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Wessig, H.; Shindyalov, N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Biological Macromolecular Resource. Available online: http://www.rcsb.org/pdb/ (accessed on 10 December 2012).
- PROCHECK. Available online: http://www.ebi.ac.uk/thornton-srv/software/PROCHECK/download.html (accessed on 10 December 2012).
- Sample Availability: Samples of the compounds Fruit proteinases, Z-Arg-Arg-NH-Mec and Bz-Phe-Val-Arg-NH-Mec are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, W.; Zhang, L.; Guo, N.; Zhang, X.; Zhang, C.; Sun, G.; Xie, J. Functional Properties of a Cysteine Proteinase from Pineapple Fruit with Improved Resistance to Fungal Pathogens in Arabidopsis thaliana. Molecules 2014, 19, 2374-2389. https://doi.org/10.3390/molecules19022374
Wang W, Zhang L, Guo N, Zhang X, Zhang C, Sun G, Xie J. Functional Properties of a Cysteine Proteinase from Pineapple Fruit with Improved Resistance to Fungal Pathogens in Arabidopsis thaliana. Molecules. 2014; 19(2):2374-2389. https://doi.org/10.3390/molecules19022374
Chicago/Turabian StyleWang, Wei, Lu Zhang, Ning Guo, Xiumei Zhang, Chen Zhang, Guangming Sun, and Jianghui Xie. 2014. "Functional Properties of a Cysteine Proteinase from Pineapple Fruit with Improved Resistance to Fungal Pathogens in Arabidopsis thaliana" Molecules 19, no. 2: 2374-2389. https://doi.org/10.3390/molecules19022374




