Kingianic Acids A–G, Endiandric Acid Analogues from Endiandra kingiana
Abstract
:1. Introduction
2. Results and Discussion

| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δc | δH (J in Hz) | δc | δH (J in Hz) | δc | |
| 1 | 2.71 m | 41.8 | 2.72 dd (1.62, 5.1) | 41.8 | 2.41 m | 39.9 |
| 2 | 2.42 dt (8.5, 5.5) | 39.7 | 2.44 dt (9.3, 3.7) | 39.7 | 2.72 m | 41.8 |
| 3 | 1.73 m | 38.8 | 1.76 m | 38.9 | 1.74 m | 38.9 |
| 4 | 2.00 t (8.5) | 40.6 | 2.07 t (8.0) | 40.2 | 1.85 t (7.5) | 39.0 |
| 5 | 2.34 t (6.5) | 39.8 | 2.37 t (6.0) | 39.9 | 2.38 m | 39.8 |
| 6 | 1.55 d (13.0) 1.90 ddd (13.0, 7.5, 5.5) | 38.5 | 1.54 d (12.8) 1.90 ddd (13.0, 7.4, 5.4) | 38.4 | 1.58 d (12.8) 1.93 dt (7.1, 12.9) | 38.4 |
| 7 | 2.57 t (5.0) | 38.3 | 2.56 t (4.9) | 38.3 | 2.57 t (5.5) | 38.3 |
| 8 | 2.86 d (3.5) | 48.8 | 2.87 d (2.6) | 48.8 | 2.89 d (3.2) | 48.6 |
| 9 | 2.98 dt (7.0, 4.0) | 34.8 | 2.98 m | 34.8 | 3.05 t (3.6) | 34.8 |
| 10 | 6.22 t (4.0) | 131.3 | 6.22 m | 131.3 | 6.24 t (3.7) | 131.9 |
| 11 | 6.22 t (4.0) | 132.0 | 6.22 m | 132.0 | 6.24 t (3.7) | 132.3 |
| 1' | 2.72 m 2.78 m | 41.7 | 2.83 m 2.86 m | 41.9 | 2.35 m | 39.3 |
| 2' | - | 134.7 | - | 140.9 | 6.02 dt (6.9, 15.7) | 126.9 |
| 3' | 6.66 s | 109.1 | 7.15 d (7.1) | 128.7 | 6.29 d (15.7) | 130.5 |
| 4' | - | 147.5 | 7.28 t (7.6) | 128.3 | - | 131.4 |
| 5' | - | 145.7 | 7.19 t (7.3) | 125.8 | 6.90 d (1.2) | 108.4 |
| 6' | 6.72 d (8.0) | 108.1 | 7.28 t (7.6) | 128.4 | - | 148.0 |
| 7' | 6.60 d (8.0) | 121.5 | 7.15 d (7.1) | 128.6 | - | 146.7 |
| 8' | 5.92 s | 100.8 | 6.73 d (8.0) | 108.2 | ||
| 9' | 6.76 d (8.0) | 120.3 | ||||
| 10' | 5.94 s | 101.0 | ||||
| C=O | - | 179.3 | - | 179.4 | - | 178.3 |
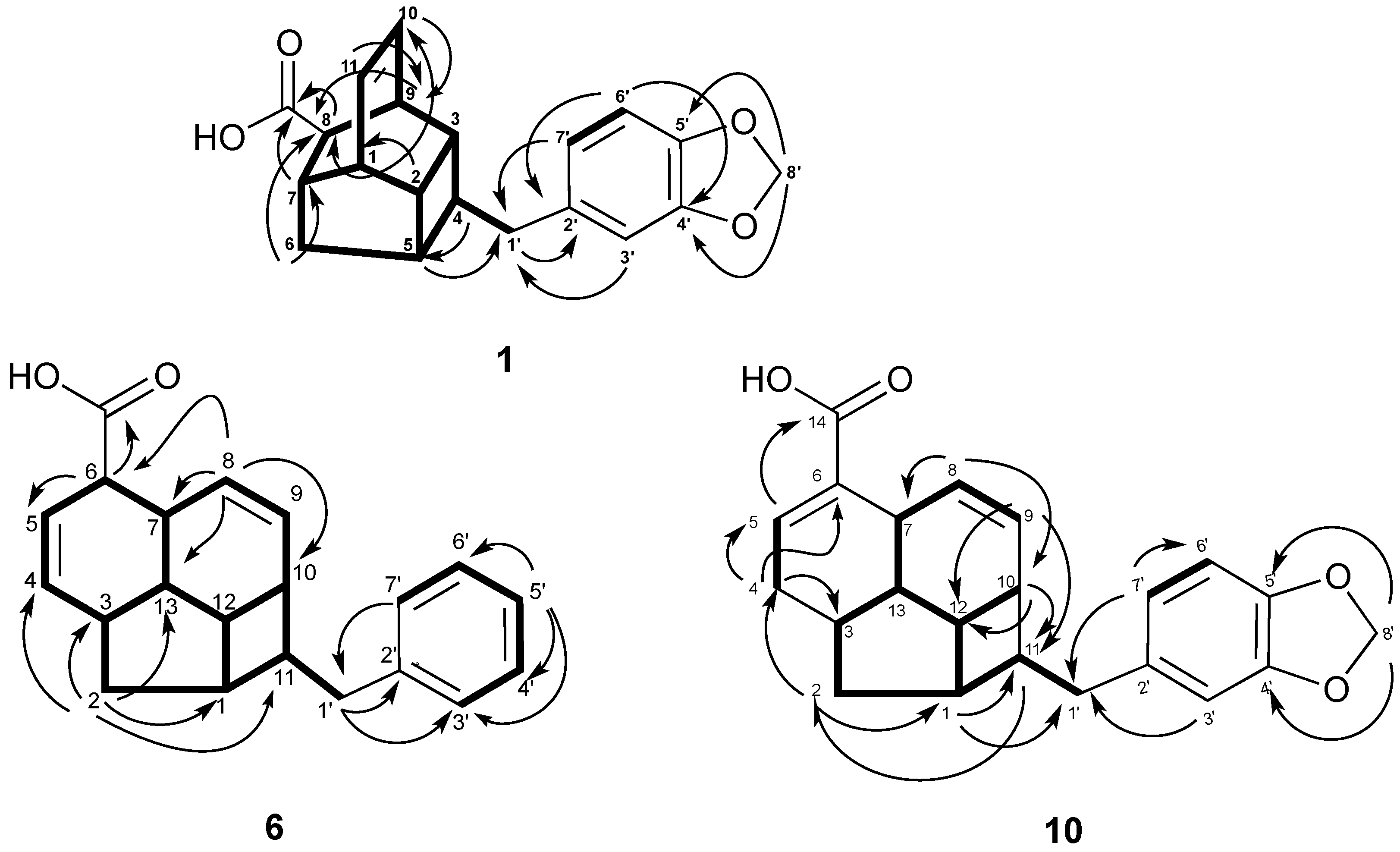
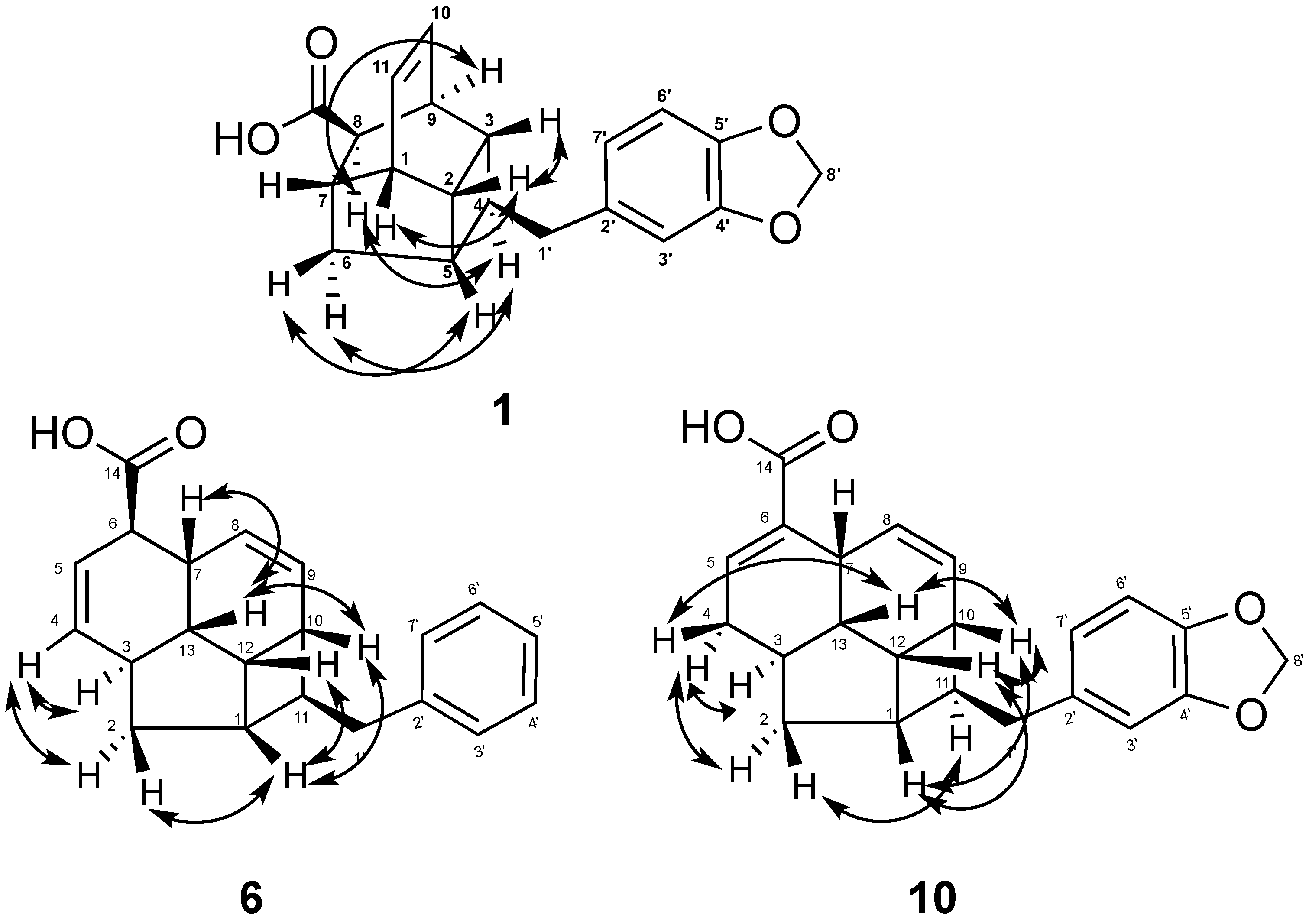
| Position | 4 | 5 | ||
|---|---|---|---|---|
| δH (J in Hz) | δc | δH (J in Hz) | δc | |
| 1 | 2.73 m | 41.9 | 2.76 dd (5.1, 10.8) | 42.4 |
| 2 | 2.40 m | 39.8 | 2.46 m | 39.9 |
| 3 | 1.76 m | 39.0 | 2.62 m | 40.3 |
| 4 | 1.88 t (7.4) | 38.9 | 2.44 m | 35.3 |
| 5 | 2.36 t (7.4) | 39.9 | 2.37 t (6.6) | 40.2 |
| 6 | 1.61 d (12.7) 1.93 m | 38.4 | 1.74 d (12.6) 1.94 m | 39.3 |
| 7 | 2.58 t (5.1) | 38.3 | 2.31 t (4.6) | 43.1 |
| 8 | 2.90 d (3.8) | 48.6 | 3.25 d (2.5) | 47.9 |
| 9 | 3.06 t (3.8) | 34.8 | 2.72 m | 39.6 |
| 10 | 6.23 d (3.1) | 131.4 | 5.93 t (7.0) | 132.3 |
| 11 | 6.24 d (3.0) | 131.9 | 6.29 t (7.3) | 130.5 |
| 12 | 140.2 | |||
| 13 | 6.61 s | 109.5 | ||
| 14 | 146.9 | |||
| 15 | 145.2 | |||
| 16 | 6.67 d (8.0) | 107.4 | ||
| 17 | 6.54 d (8.0) | 121.6 | ||
| 18 | 5.89 s | 100.7 | ||
| 1' | 2.43 m | 39.4 | 2.64 m | 40.4 |
| 2' | 6.19 m | 128.7 | ||
| 3' | 6.38 d (15.8) | 131.0 | ||
| 4' | - | 137.7 | ||
| 5' | 7.35 d (7.2) | 126.0 | ||
| 6' | 7.30 t (7.6) | 128.5 | ||
| 7' | 7.20 t (7.3) | 127.0 | ||
| 8' | 7.30 t (7.6) | 128.5 | ||
| 9' | 7.35 d (7.2) | 126.0 | ||
| 10' | ||||
| C=O | - | 177.5 | 176.9 | |
| Position | 6 | 7 | 10 | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δc | δH (J in Hz) | δc | δH (J in Hz) | δc | |
| 1 | 2.45 m | 41.0 | 2.48 m | 40.9 | 2.42 m | 40.9 |
| 2 | 1.30 dt (6.3, 12.1) 1.53 dd (6.4, 11.9) | 34.7 | 1.29 m 1.79 m | 35.0 | 1.20 m 1.53 m | 37.0 |
| 3 | 2.55 m | 36.9 | 2.08 m | 44.1 | 2.04 m | 35.6 |
| 4 | 6.19 d (9.7) | 134.4 | 4.23 d (9.8) | 73.6 | 2.08 m, 2.47 m | 32.2 |
| 5 | 5.72 d (9.6) | 123.9 | 7.03 brs | 145.6 | 7.23 brs | 144.6 |
| 6 | 3.00 m | 49.0 | - | 134.3 | - | 134.6 |
| 7 | 2.84 m | 32.8 | 3.23 brs | 33.2 | 3.26 brs | 33.3 |
| 8 | 5.40 m | 129.8 | 5.43 d (10.5) | 127.7 | 5.39 d (10.0) | 127.1 |
| 9 | 5.42 m | 129.1 | 5.53 d (10.5) | 126.2 | 5.54 d (10.0) | 127.0 |
| 10 | 2.39 m | 34.5 | 2.42 m | 33.7 | 2.40 m | 33.2 |
| 11 | 1.82 m | 46.9 | 1.76 m | 46.8 | 1.74 m | 46.9 |
| 12 | 2.70 dd (7.7, 16.2) | 32.9 | 2.80 m | 33.6 | 2.77 m | 34.0 |
| 13 | 1.73 m | 42.0 | 1.86 m | 40.8 | 1.68 m | 42.2 |
| 1' | 2.81 m | 42.9 | 2.71 d (8.0) | 42.5 | 2.70 d (8.0) | 42.6 |
| 2' | - | 140.7 | - | 134.6 | - | 134.6 |
| 3' | 7.15 d (6.8) | 128.6 | 6.64 s | 108.9 | 6.65 s | 109.0 |
| 4' | 7.24 t (6.7) | 128.4 | - | 147.5 | - | 147.4 |
| 5' | 6.20 dt (2.1, 7.3) | 125.8 | - | 145.6 | - | 145.6 |
| 6' | 7.24 t (6.7) | 128.4 | 6.71 d (7.9) | 108.1 | 6.71 d (7.9) | 108.0 |
| 7' | 7.15 d (6.8) | 128.6 | 6.59 d (7.9) | 121.3 | 6.60 d (7.9) | 121.3 |
| 8' | 5.91 s | 100.7 | 5.91 s | 100.7 | ||
| C=O | 179.4 | 170.3 | 178.0 | |||
| Compounds | Bcl-xL/Bak binding affinity (%) | Mcl-1/Bid binding affinity (%) | Cytotoxicity (IC50 in µM,mean ± s.d., n = 3) | ||||
|---|---|---|---|---|---|---|---|
| 20 μM | 100 μM | 20 μM | 100 μM | HT-29 | A549 | PC3 | |
| 1 | 3 ± 1.5 | 21 ± 1.8 | 3 ± 2.0 | 36 ± 2.3 | 35.0 ± 0.2 | 85.4 ± 0.2 | >100 |
| 3 | 9 ± 1.5 | 25 ± 1.7 | 30 ± 2.2 | 75 ± 1.1 | >100 | 85.3 ± 0.2 | >100 |
| 5 | 2 ± 1.4 | 1 ± 0.8 | 3 ± 1.3 | 8 ± 5.5 | 17.1 ± 0.1 | 15.4 ± 0.2 | 77.2 ± 0.2 |
| 6 | 4 ± 1.6 | 22 ± 2.9 | 28 ± 3.7 | 80 ± 0.7 | NT | NT | NT |
| 7 | 5 ± 1.3 | 19 ± 1.6 | 8 ± 1.1 | 47 ± 2.9 | NT | NT | NT |
| 8 | 0 | 10 ± 0.5 | 4 ± 0.8 | 39 ± 0.9 | >100 | >100 | >100 |
| 9 | 6 ± 1.5 | 26 ± 2.5 | 25 ± 2.1 | 81 ± 2.4 | >100 | 38.1 ± 0.1 | >100 |
| U-Bak (Ki) | 12 ± 1 nM | ||||||
| U-Bid (Ki) | 16 ± 2 nM | ||||||
| ABT-737 (Ki) | 57 ± 10 nM | 47 ± 22 nM | |||||
| Cisplatin | 70.3 ± 1.1 | 36.2 ± 1.4 | 44.5 ± 7.7 | ||||
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectral Data
 ± 0 (c 0.20, CHCl3); UV (MeOH) λmax 233, 286 nm; IR (neat) νmax 3431(OH), 1701 (C=O), 1040, 936 (OCH2O) cm−1; 1H-NMR and 13C-NMR, see Table 1; HRESIMS m/z 323.1279 [M−H]− (calcd for C20H19O4, 323.1284).
± 0 (c 0.20, CHCl3); UV (MeOH) λmax 233, 286 nm; IR (neat) νmax 3431(OH), 1701 (C=O), 1040, 936 (OCH2O) cm−1; 1H-NMR and 13C-NMR, see Table 1; HRESIMS m/z 323.1279 [M−H]− (calcd for C20H19O4, 323.1284). ± 0 (c 0.12, CHCl3); UV (MeOH) λmax 212, 287 nm; IR (neat) νmax 3432(OH), 1721 (C=O) cm−1; 1H-NMR and 13C-NMR, see Table 1; HRESIMS m/z 279.1398 [M−H]− (calcd for C19H19O2, 279.1385).
± 0 (c 0.12, CHCl3); UV (MeOH) λmax 212, 287 nm; IR (neat) νmax 3432(OH), 1721 (C=O) cm−1; 1H-NMR and 13C-NMR, see Table 1; HRESIMS m/z 279.1398 [M−H]− (calcd for C19H19O2, 279.1385). ± 0 (c 0.20, CHCl3); UV (MeOH) λmax 212, 290 nm; IR (neat) νmax 3437 (OH), 1697 (C=O), 1037, 923 (OCH2O) cm−1; 1H-NMR and 13C-NMR, see Table 1; HRESIMS m/z 349.1439 [M−H]− (calcd for C22H21O4, 349.1440).
± 0 (c 0.20, CHCl3); UV (MeOH) λmax 212, 290 nm; IR (neat) νmax 3437 (OH), 1697 (C=O), 1037, 923 (OCH2O) cm−1; 1H-NMR and 13C-NMR, see Table 1; HRESIMS m/z 349.1439 [M−H]− (calcd for C22H21O4, 349.1440). ± 0 (c 0.10, CHCl3); UV (MeOH) λmax 212, 289 nm; IR (neat) νmax 3440(OH), 1693 (C=O) cm−1; 1H-NMR and 13C-NMR, see Table 2; HRESIMS m/z 305.1539 [M−H]− (calcd for C21H21O2, 305.1542).
± 0 (c 0.10, CHCl3); UV (MeOH) λmax 212, 289 nm; IR (neat) νmax 3440(OH), 1693 (C=O) cm−1; 1H-NMR and 13C-NMR, see Table 2; HRESIMS m/z 305.1539 [M−H]− (calcd for C21H21O2, 305.1542). ± 0 (c 0.14, CHCl3); UV (MeOH) λmax 234, 286 nm; IR (neat) νmax 3444(OH), 1665 (C=O), 1039, 938 (OCH2O) cm−1; 1H-NMR and 13C-NMR, see Table 2; HRESIMS m/z 323.1298 [M−H]− (calcd for C20H19O4, 323.1284).
± 0 (c 0.14, CHCl3); UV (MeOH) λmax 234, 286 nm; IR (neat) νmax 3444(OH), 1665 (C=O), 1039, 938 (OCH2O) cm−1; 1H-NMR and 13C-NMR, see Table 2; HRESIMS m/z 323.1298 [M−H]− (calcd for C20H19O4, 323.1284). ± 0 (c 0.16, CHCl3); UV (MeOH) λmax 232, 288 nm; IR (neat) νmax 3432 (OH), 1696 (C=O) cm−1; 1H-NMR and 13C-NMR, see Table 3; HRESIMS m/z 305.1557 [M−H]− (calcd for C21H21O2, 305.1542).
± 0 (c 0.16, CHCl3); UV (MeOH) λmax 232, 288 nm; IR (neat) νmax 3432 (OH), 1696 (C=O) cm−1; 1H-NMR and 13C-NMR, see Table 3; HRESIMS m/z 305.1557 [M−H]− (calcd for C21H21O2, 305.1542). ± 0 (c 0.14, CHCl3). UV (MeOH) λmax 234, 286 nm. IR (neat) νmax 2600–3300 (OH), 1687 (C=O), 1632 (C=C) and 1040, 937 (OCH2O) cm−1. 1H-NMR and 13C-NMR, see Table 3. HREIMS: m/z 365.1401 [M−H]− (calcd for C22H21O5, 365.1389).
± 0 (c 0.14, CHCl3). UV (MeOH) λmax 234, 286 nm. IR (neat) νmax 2600–3300 (OH), 1687 (C=O), 1632 (C=C) and 1040, 937 (OCH2O) cm−1. 1H-NMR and 13C-NMR, see Table 3. HREIMS: m/z 365.1401 [M−H]− (calcd for C22H21O5, 365.1389).  ± 0 (c 0.12, CHCl3). UV (MeOH) λmax 234, 286 nm. IR (neat) νmax 1685 (C=O), 1630 (C=C) and 1039, 935 (OCH2O) cm−1. 1H-NMR and 13C-NMR, see Table 3. HREIMS: m/z 349.1431[M−H]− (calcd for C22H21O4, 349.1440). A colourless crystal was obtained from MeOH, crystallized in the monoclinic crystal system with P21/c space group. Cell parameters: a = 6.141(2)Å; b = 23.448(8) Å; c = 12.366(4) Å; β = 104.38˚; V = 1834.58(8) Å3, T 100 K. For the X-ray crystallographic data of compound 10 see Supporting Information. Supplementary crystallographic data have been deposited with the CCDC as CCDC-918161.
± 0 (c 0.12, CHCl3). UV (MeOH) λmax 234, 286 nm. IR (neat) νmax 1685 (C=O), 1630 (C=C) and 1039, 935 (OCH2O) cm−1. 1H-NMR and 13C-NMR, see Table 3. HREIMS: m/z 349.1431[M−H]− (calcd for C22H21O4, 349.1440). A colourless crystal was obtained from MeOH, crystallized in the monoclinic crystal system with P21/c space group. Cell parameters: a = 6.141(2)Å; b = 23.448(8) Å; c = 12.366(4) Å; β = 104.38˚; V = 1834.58(8) Å3, T 100 K. For the X-ray crystallographic data of compound 10 see Supporting Information. Supplementary crystallographic data have been deposited with the CCDC as CCDC-918161. 3.5. Bcl-xL and Mcl-1 Binding Affinity Assays
3.6. Cell Viability Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Litaudon, M.; Bousserouel, H.; Awang, K.; Nosjean, O.; Martin, M.-T.; Tran Huu Dau, M.-E.; Hadi, H.A.; Boutin, J.A.; Sévenet, T.; Guéritte, F. A dimeric sesquiterpenoid from a Malaysian Meiogyne as a new inhibitor of Bcl-xL/BakBH3 domain peptide interaction. J. Nat. Prod. 2009, 72, 480–483. [Google Scholar] [CrossRef]
- Leverrier, A.; Dau, M.E.T.H.; Retailleau, P.; Awang, K.; Guéritte, F.; Litaudon, M. Kingianin A: A new natural pentacyclic compound from Endiandra kingiana. Org. Lett. 2010, 12, 3638–3641. [Google Scholar] [CrossRef]
- Leverrier, A.; Martin, M.-T.; Servy, C.; Ouazzani, J.; Retailleau, P.; Awang, K.; Mukhtar, M.R.; Guéritte, F.; Litaudon, M. Rearranged diterpenoids from the biotransformation of ent trachyloban-18-oic acid by Rhizopus arrhizus. J. Nat. Prod. 2010, 76, 1121–1125. [Google Scholar]
- Leverrier, A.; Awang, K.; Guéritte, F.; Litaudon, M. Pentacyclic polyketides from Endiandra kingiana as inhibitors of the Bcl-xL/Bak interaction. Phytochemistry 2011, 72, 1443–1452. [Google Scholar] [CrossRef]
- Ng, F.S.P. Tree Flora of Malaya, a Manual for the Forester; Whitmore, T.C., Ed.; Longman: Kuala Lumpur, Malaysia, 1989; Volume 4, p. 141. [Google Scholar]
- Burkill, I.H. A Dictionary of the Economic Products of the Malay Peninsular, 2nd ed.; Government of Malaya and Singapore: Kuala Lumpur, Malaysia, 1966; p. 1773. [Google Scholar]
- Maberley, D.J. Mabberley’s Plant-Book: A Portable Dictionary of Plants, Their Classification and Uses, 3rd ed.; Cambridge University Press: Cambridge, UK, 2008; p. 1021. [Google Scholar]
- Bandaranayake, W.M.; Banfield, J.E.; Black, D.S.C.; Fallon, G.D.; Gatehouse, B.M. Endiandric acid, a novel carboxylic acid from Endiandra introrsa (Lauraceae): X-Ray structure determination. J. Chem. Soc. Chem. Commun. 1980, 162–163. [Google Scholar] [CrossRef]
- Bandaranayake, W.M.; Banfield, J.E.; Black, D.S.C.; Fallon, G.D.; Gatehouse, B.M. Constituents of Endiandra species. I. Endiandric acid, a novel carboxylic acid from Endiandra introrsa (Lauraceae) and a derived lactone. Aust. J. Chem. 1981, 34, 1655–1667. [Google Scholar] [CrossRef]
- Bandaranayake, W.M.; Banfield, J.E.; Black, D.S.C.; Fallon, G.D.; Gatehouse, B.M. Constituents of Endiandra species. III. 4-[(E,E)-5'-Phenylpenta-2',4'-dien-1'-yl]tetra-cyclo[5,4,0,02,5,03,9]undec-10-ene-8-carboxylic acid from Endiandra introrsa (Lauraceae). Aust. J. Chem. 1982, 35, 567–579. [Google Scholar] [CrossRef]
- Bandaranayake, W.M.; Banfield, J.E.; Black, D.S.C. Constituents of Endiandra species. II. (E)-4-(6'-phenyltetracyclo[5.4.2.03,13.010,12]trideca-4',8'-dien-11'yl)but-2-enoic acid from Endiandra introrsa (Lauraceae) and a derived lactone. Aust. J. Chem. 1982, 35, 557–565. [Google Scholar] [CrossRef]
- Bandaranayake, W.M.; Banfield, J.E.; Black, D.S.C. Postulated electrocyclic reactions leading to endiandric acid and related natural products. J. Chem. Soc. Chem. Commun. 1980, 902–903. [Google Scholar] [CrossRef]
- Banfield, J.E.; Black, D.S.C.; Fallon, G.D.; Gatehouse, B.M. Constituents of Endiandra species. V. 2-[3',5'-Dioxo-4'-phenyl-10'-{(E,E)-5''-phenyl-penta-2'',4''-dien-1''-yl}-2',4',6'-triazatetracyclo [5,4,2,02,6,08,11] tridec12'-en-9'-yl]-acetic acid derived from Endiandra introrsa (Lauraceae). Aust. J. Chem. 1983, 36, 627–632. [Google Scholar] [CrossRef]
- Banfield, J.E.; Black, D.S.C.; Johns, S.R.; Willing, R.I. Constituents of Endiandra species. IV. 2-(8'-[(E,E)-5"-phenylpenta-2",4"-dien-1"-yl]bicyclo[4.2.0]octa-2',4'-dien-7'-yl)acetic acid, a biogenetically predicted metabolite of Endiandra introrsa (Lauraceae) and its structure determination by means of 1D and 2D NMR spectroscopy. Aust. J. Chem. 1982, 35, 2247–2256. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Petasis, N.A.; Zipkin, R.E. The endiandric acid cascade. Electrocyclizations in organic synthesis. 4. Biomimetic approach to endiandric acids A-G. Total synthesis and thermal studies. J. Am. Chem. Soc. 1982, 104, 5560–5562. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Zipkin, R.E.; Petasis, N.A. The endiandric acid cascade. Electrocyclizations in organic synthesis. 3. “Biomimetic” approach to endiandric acids A-G. Synthesis of precursors. J. Am. Chem. Soc. 1982, 104, 5558–5560. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Petasis, N.A. Strategies and Tactics in Organic Synthesis; Lindberg, T., Ed.; Academic Press: San Diego, CA, USA, 1984; Volume 1, pp. 155–173. [Google Scholar]
- Chouna, J.R.; Nkeng-Efouet, P.A.; Lenta, B.N.; Devkota, K.P.; Neumann, B.; Stammler, H.-G.; Kimbu, S.F.; Sewald, N. Antibacterial endiandric acid derivatives from Beilschmiedia anacardioides. Phytochemistry 2009, 70, 684–688. [Google Scholar] [CrossRef]
- Chouna, J.R.; Nkeng-Efouet, P.A.; Lenta, B.N.; Wansi, J.D.; Kimbu, S.F.; Sewald, N. Endiandric acid derivatives from the stem bark of Beilschmiedia anacardioides. Phytochem. Lett. 2010, 3, 13–16. [Google Scholar] [CrossRef]
- Talontsi, F.M.; Lamshöft, M.; Bauer, J.O.; Razakarivony, A.A.; Andriamihaja, B.; Strohmann, C.; Spiteller, M. Antibacterial and antiplasmodial constituents of Beilschmiedia cryptocaryoides. J. Nat. Prod. 2013, 76, 97–102. [Google Scholar] [CrossRef]
- Yang, P.-S.; Cheng, M.-J.; Peng, C.-F.; Chen, J.-J.; Chen, I.-S. Endiandric acid analogues from the roots of Beilschmiedia erythrophloia. J. Nat. Prod. 2009, 72, 53–58. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Chang, H.-S.; Wang, G.-J.; Cheng, M.-J.; Chen, C.-H.; Yang, Y.-J.; Chen, I.-S. Anti-inflammatory endiandric acid analogues from the roots of Beilschmiedia tsangii. J. Nat. Prod. 2011, 74, 1875–1880. [Google Scholar] [CrossRef]
- Williams, R.B.; Martin, S.M.; Hu, J.-F.; Norman, V.L.; Goering, M.G.; Loss, S.; O’Neil-Johnson, M.; Eldridge, G.R.; Starks, C.M. Cytotoxic and antibacterial beilschmiedic acids from a gabonese species of Beilschmiedia. J. Nat. Prod. 2012, 75, 1319–1325. [Google Scholar] [CrossRef]
- Gravel, E.; Poupon, E. Biogenesis and biomimetic chemistry: Can complex natural products be assembled spontaneously? Eur. J. Org. Chem. 2008, 1, 27–42. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Chang, H.-S.; Wang, G.-J.; Lin, C.-H.; Chen, I.-S. Secondary metabolites from the roots of Beilschmiedia tsangii and their anti-inflammatory activities. Int. J. Mol. Sci. 2012, 13, 16430–16443. [Google Scholar] [CrossRef]
- Qian, J.; Voorbach, M.J.; Huth, J.R.; Coen, M.L.; Zhang, H.; Ng, S.-C.; Comess, K.M.; Petros, A.M.; Rosenberg, S.H.; Warrior, U.; et al. Discovery of novel inhibitors of Bcl-xL using multiple high-throughput screening platforms. Anal. Biochem. 2004, 328, 131–138. [Google Scholar] [CrossRef]
- Bruker (2000). SAINT and SMART; Bruker AXS Inc.: Madison, WI, USA, 2000. [Google Scholar]
- Sheldrick, G.M. SHELXTL. Version 5.1 Bruker AXS; University of Göttingen: Göttingen, Germany, 2008. [Google Scholar]
- Nikolovska-Coleska, Z.; Wang, R.; Fang, X.; Pan, H.; Tomita, Y.; Li, P.; Roller, P.P.; Krajewski, K.; Saito, N.G.; Stuckey, J.A.; et al. Development and optimization of a binding assay for the XIAP BIR3 domain using fluorescence polarization. Anal. Biochem. 2004, 332, 261–273. [Google Scholar] [CrossRef]
- Cheng, Y.; Prusoff, W.H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [CrossRef]
- Zhang, J.H.; Chung, T.D.; Oldenburg, K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A. Comparison of MTT, XTT, and a novel tetrazolium compound MTS for in vitro proliferation and chemosensitivity assay. Mol. Biol. Cell 1992, 3, 184a–188a. [Google Scholar]
- Sample Availability: Samples of compounds 1–10 are not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Azmi, M.N.; Gény, C.; Leverrier, A.; Litaudon, M.; Dumontet, V.; Birlirakis, N.; Guéritte, F.; Leong, K.H.; Halim, S.N.A.; Mohamad, K.; et al. Kingianic Acids A–G, Endiandric Acid Analogues from Endiandra kingiana. Molecules 2014, 19, 1732-1747. https://doi.org/10.3390/molecules19021732
Azmi MN, Gény C, Leverrier A, Litaudon M, Dumontet V, Birlirakis N, Guéritte F, Leong KH, Halim SNA, Mohamad K, et al. Kingianic Acids A–G, Endiandric Acid Analogues from Endiandra kingiana. Molecules. 2014; 19(2):1732-1747. https://doi.org/10.3390/molecules19021732
Chicago/Turabian StyleAzmi, Mohamad Nurul, Charlotte Gény, Aurélie Leverrier, Marc Litaudon, Vincent Dumontet, Nicolas Birlirakis, Françoise Guéritte, Kok Hoong Leong, Siti Nadiah Abd. Halim, Khalit Mohamad, and et al. 2014. "Kingianic Acids A–G, Endiandric Acid Analogues from Endiandra kingiana" Molecules 19, no. 2: 1732-1747. https://doi.org/10.3390/molecules19021732
APA StyleAzmi, M. N., Gény, C., Leverrier, A., Litaudon, M., Dumontet, V., Birlirakis, N., Guéritte, F., Leong, K. H., Halim, S. N. A., Mohamad, K., & Awang, K. (2014). Kingianic Acids A–G, Endiandric Acid Analogues from Endiandra kingiana. Molecules, 19(2), 1732-1747. https://doi.org/10.3390/molecules19021732







