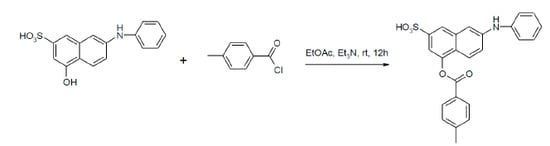
Efficient Synthesis and Reaction Kinetics of Readily Water Soluble Esters Containing Sulfonic Groups
Abstract
:1. Introduction
2. Results and Discussion
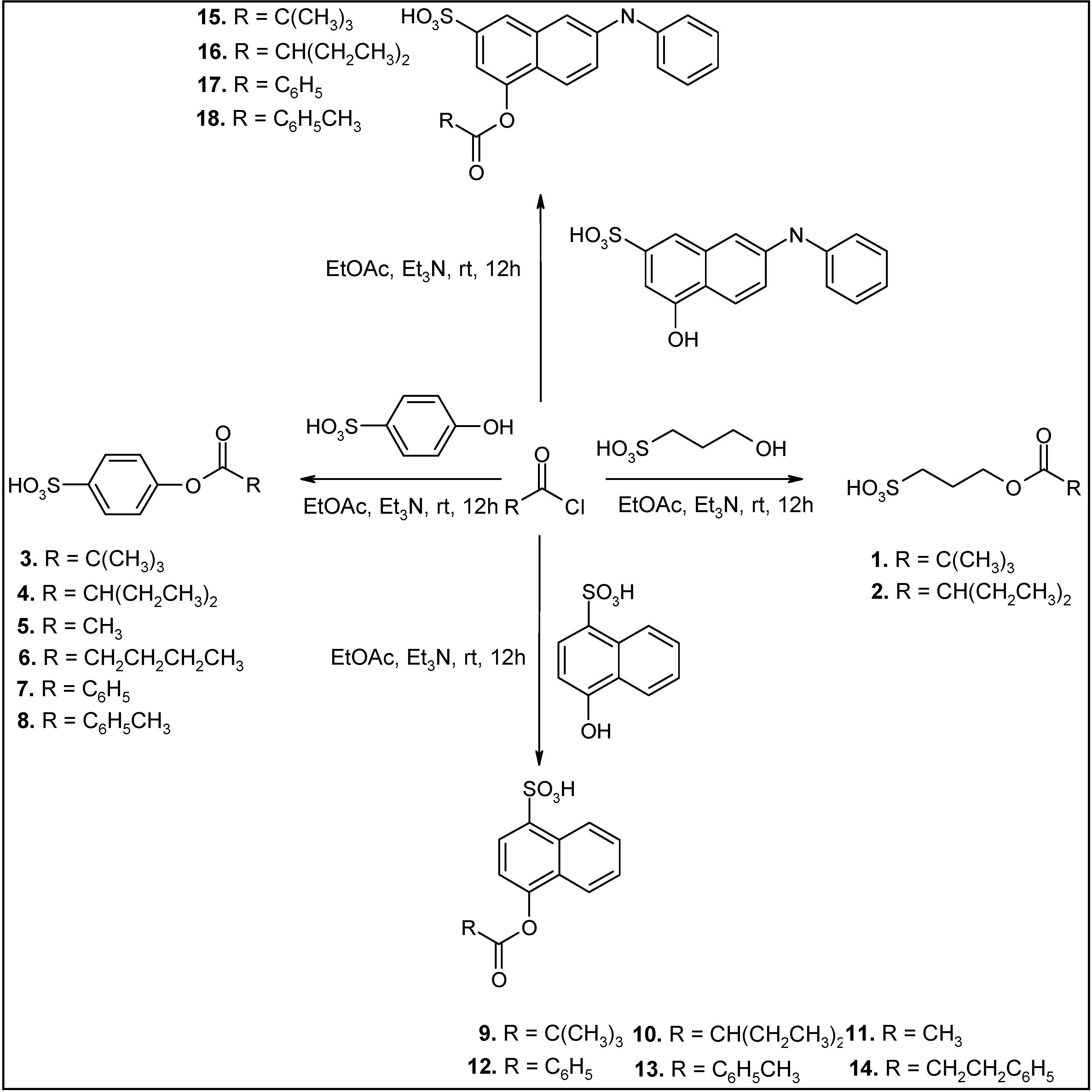
| Entry | Reagent 1 (R1-COCl) | Reagent 2 (R2-OH) | Product (R1-COO-R2) | Yield (%) |
|---|---|---|---|---|
| 1 |  |  |  | 85 |
| 2 |  |  |  | 80 |
| 3 |  |  |  | 99 |
| 4 |  |  |  | 96 |
| 5 |  |  |  | 92 |
| 6 |  |  |  | 86 |
| 7 |  |  |  | 98 |
| 8 |  |  |  | 96 |
| 9 |  |  |  | 99 |
| 10 |  |  |  | 95 |
| 11 |  |  |  | 99 |
| 12 |  |  |  | 98 |
| 13 |  |  |  | 96 |
| 14 |  |  |  | 40 |
| 15 |  |  |  | 80 |
| 16 |  |  |  | 78 |
| 17 |  |  |  | 85 |
| 18 |  |  |  | 82 |
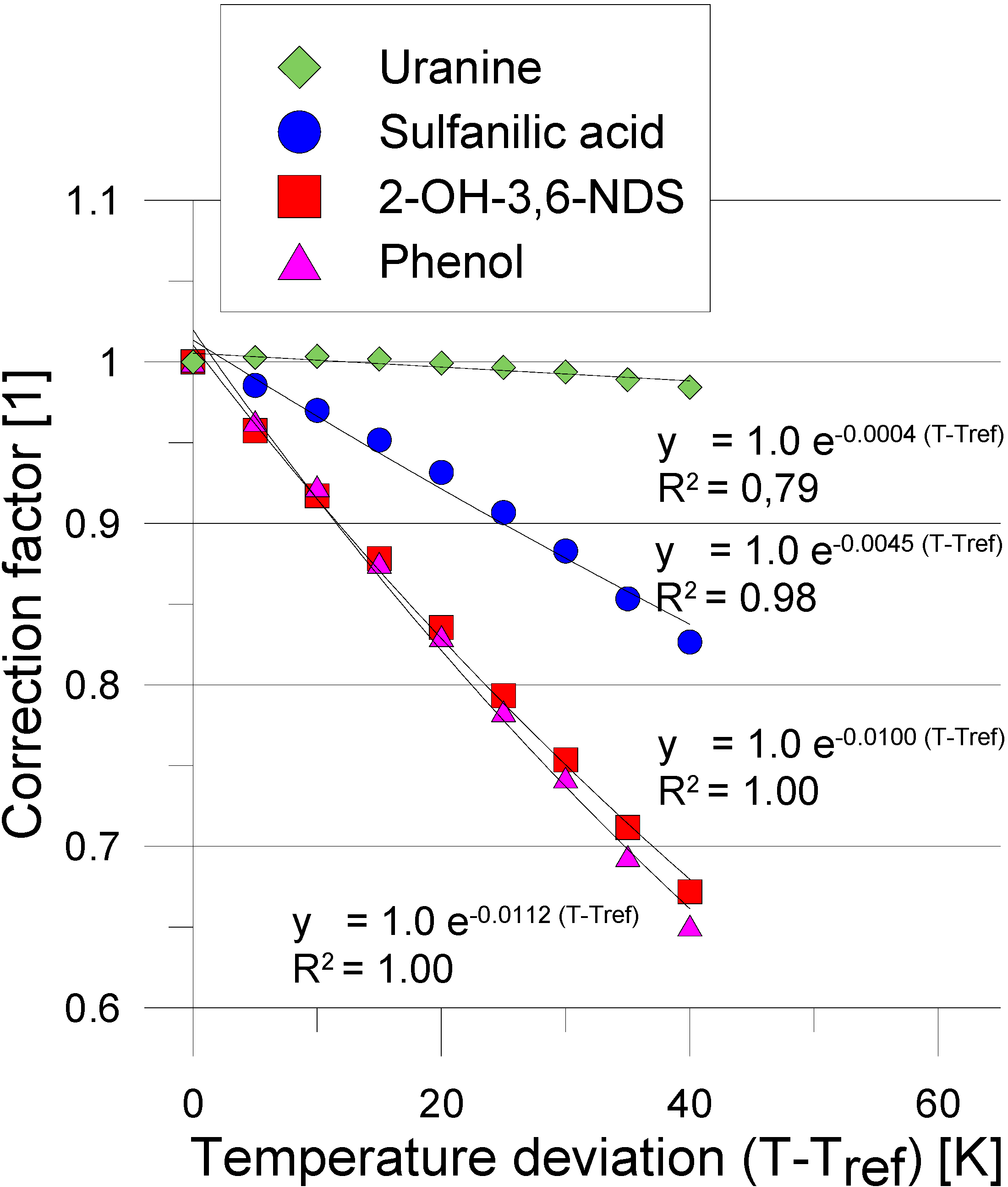
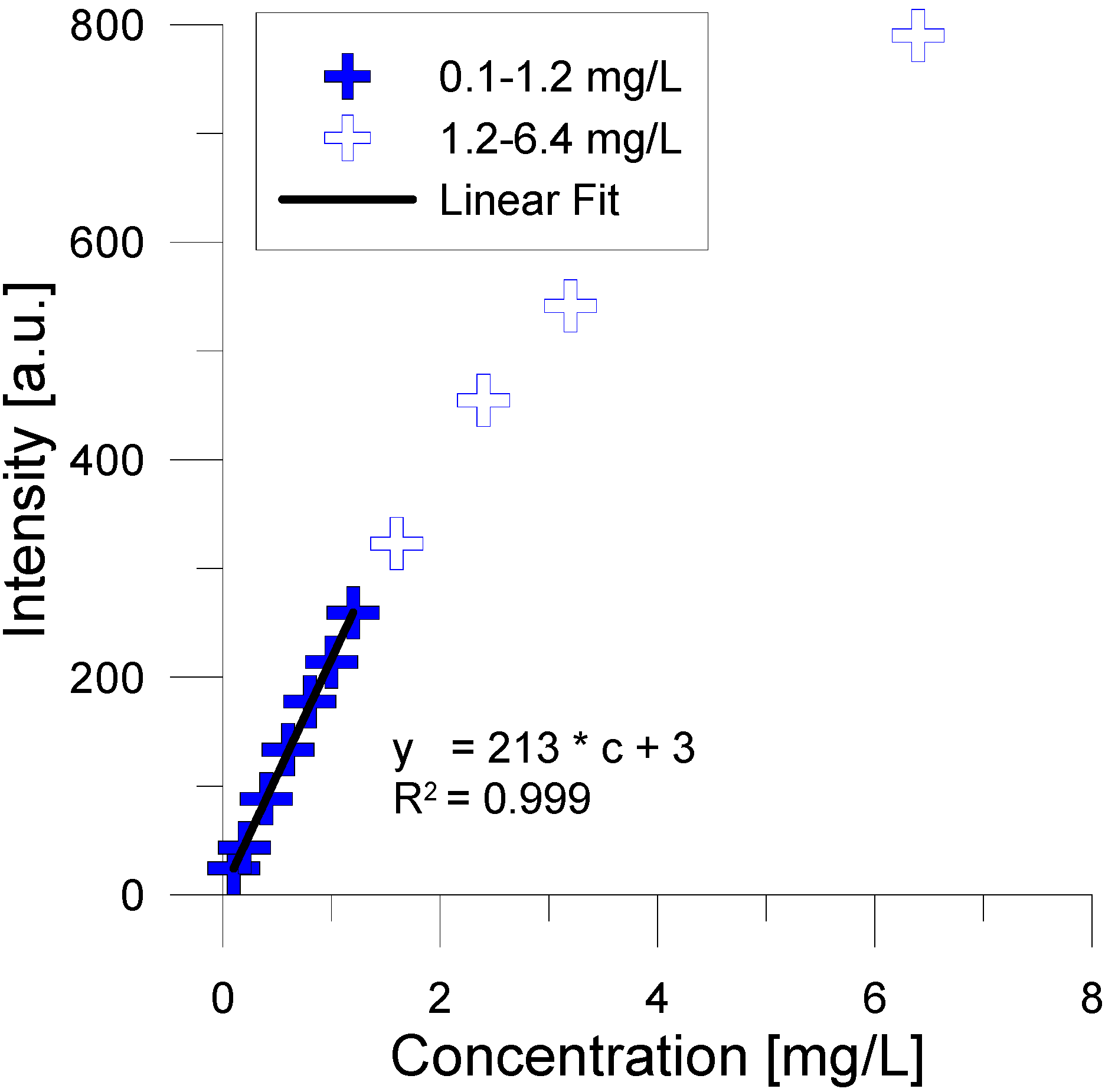
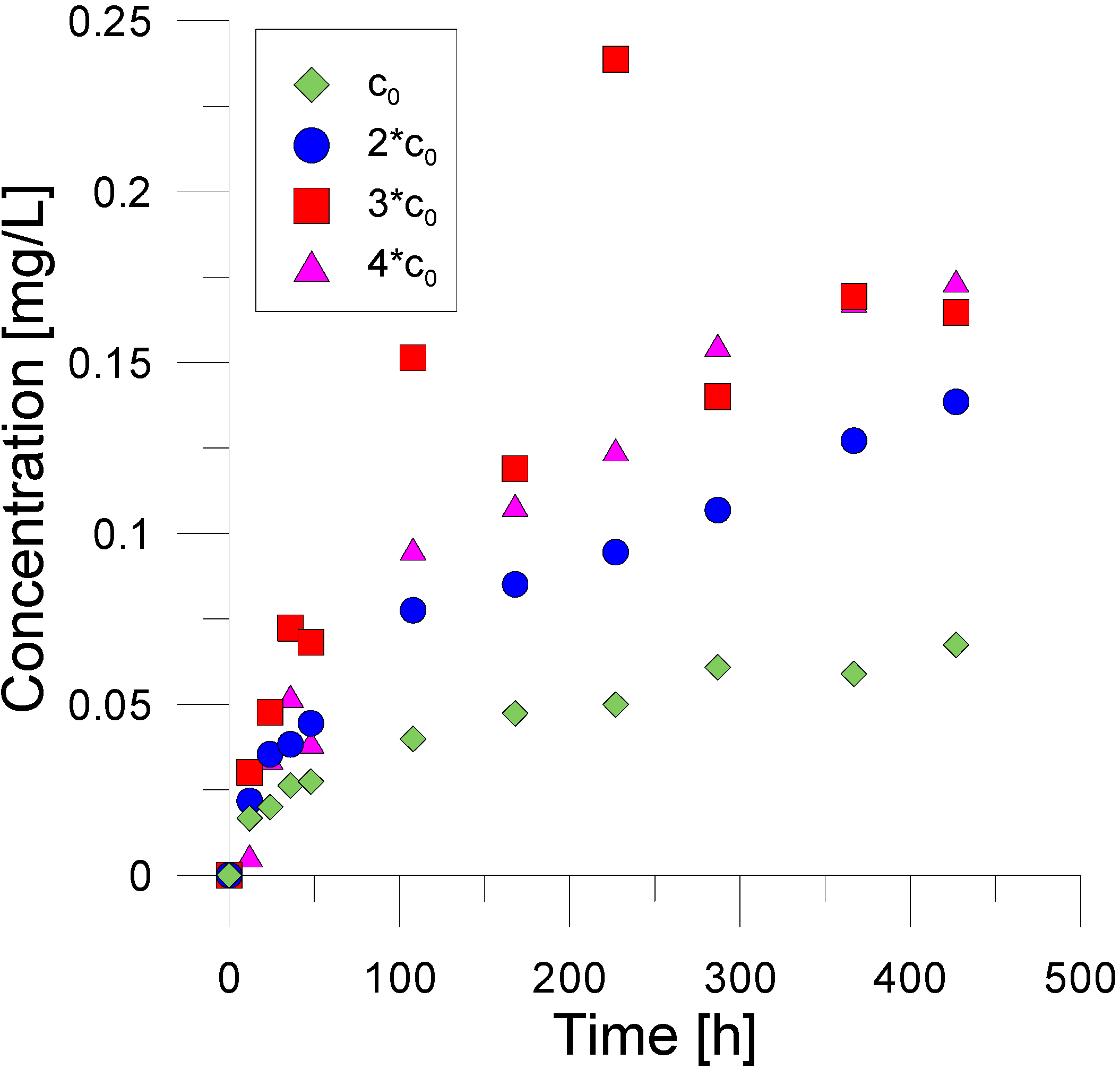
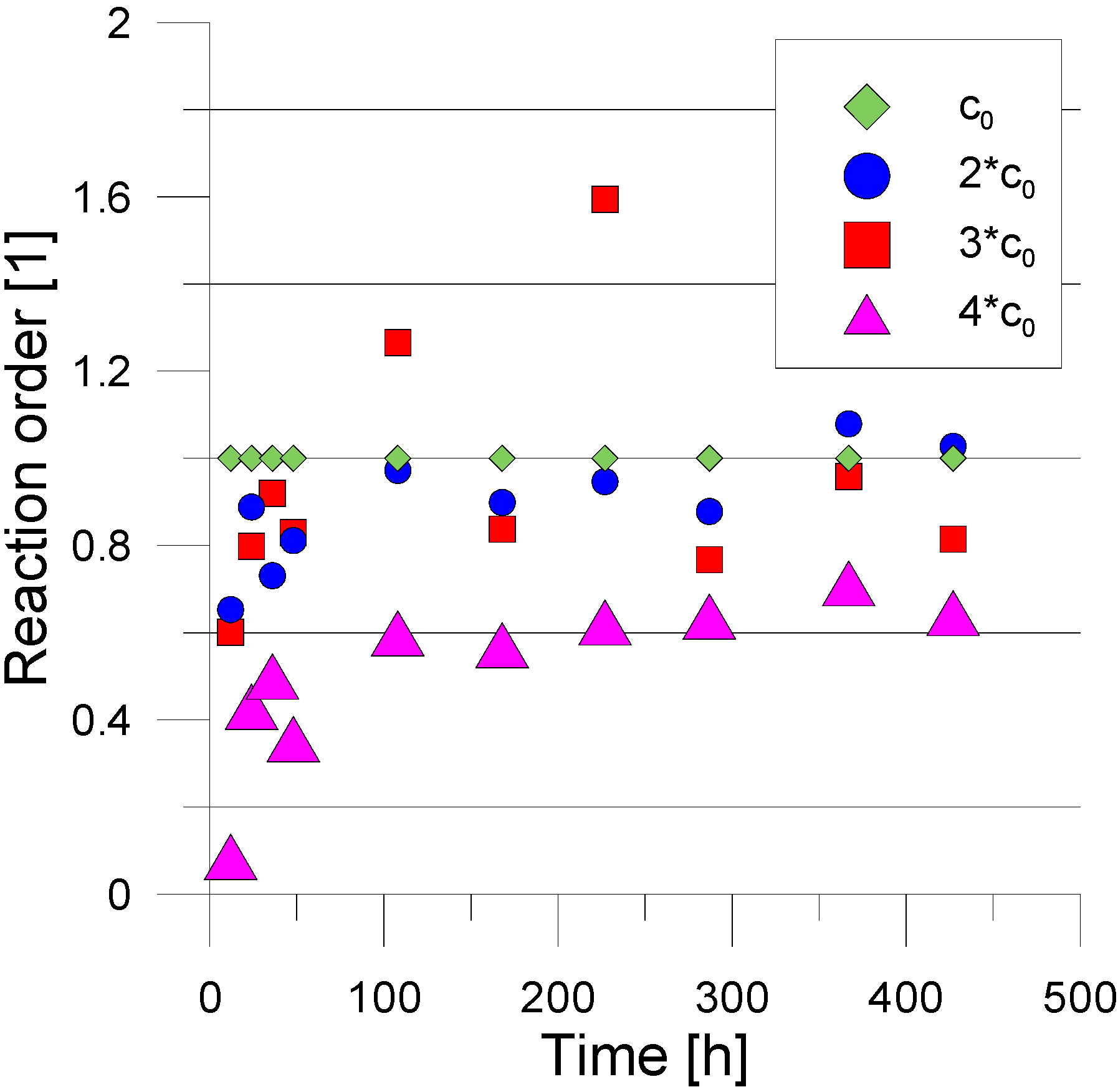
3. Experimental
3.1. General Information
3.2. Synthesis: General Procedure of Preparing Esters Containing Sulfonic Groups 1–18
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nottebohm, M.; Licha, T.; Sauter, M. Tracer design for tracking thermal fronts ingeothermal reservoirs. Geothermics 2012, 43, 37–44. [Google Scholar] [CrossRef]
- Tester, J.W.; Robinson, B.A.; Ferguson, J.H. The theory and selection of chemically reactive tracers for reservoir thermal capacity production. In Proceedings of the Twelfth Workshop on Geothermal Reservoir Engineering, Stanford University, Stanford, CA, USA, 20–22 January 1987. SGP-TR-109.
- Maier, F.; Schaffer, M.; Licha, T. Temperature Determination Using Thermo-Sensitive Tracers: Experimental Validation in an Isothermal Column Heat Exchanger. Geothermics 2015, 53, 533–539. [Google Scholar] [CrossRef]
- Mabey, W.; Mill, T. Critical review of hydrolysis of organic compounds in water under environmental conditions. J. Phys. Chem. Ref. Data 1978, 7, 383–415. [Google Scholar] [CrossRef]
- Nottebohm, M.; Licha, T.; Ghergut, I.; Nödler, K.; Sauter, M. Development of Thermosensitive Tracers for Push-Pull Experiments in Geothermal Reservoir Characterization. In Proceedings of the World Geothermal Congress, Bali, Indonesia, 25–29 April 2010.
- Zengbin, W.; Lixin, X.; Feng, N.; Jianfang, S.; Qianru, S.; Xiulan, Z. Study of sulfonated polyether ether ketone with pendant lithiated fluorinated sulfonic groups as ion conductive binder in lithium-ion batteries. J. Power Sources 2014, 256, 28–31. [Google Scholar] [CrossRef]
- Grass, M.; Woldt, B.; Gevers, A.; Buchholz, S.; Meier, M.; Montero de Espinosa, L. Ester Having a Sulphonic Group. WO2014095103, 26 June 2014. [Google Scholar]
- Eba, C.; Okano, A.; Nakano, H.; Iwasaki, Y. A chromogenic substrate for solid-phase detection of phospholipase A2. Anal. Biochem. 2014, 447, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Greene, T.W.; Wuts, P.G.M. Protective Groups in Organic Synthesis, 3rd ed.; John Wiley and Sons Ltd.: New York, NY, USA, 1999. [Google Scholar]
- Neises, B.; Steglich, W. Esterification of carboxylic acids with dicyclohexylcarbodiimide/4-dimethylaminopyridine: Tert-butyl ethyl fumarate. Org. Synth. 1985, 63, 183. [Google Scholar] [CrossRef]
- Riemenschneider, W.; Bolt, H.B. “Esters, Organic” Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar] [CrossRef]
- Favorskii, A.E. Isomeric transformations of halides of alcohols, and of sulfovinic acids leading to the regrouping of carbon atoms. J. Russ. Phys. Chem. Soc. 1918, 50, 43–80. [Google Scholar]
- Bayer, A.; Villiger, V. Berichte der deutschen chemischen gesellschaft. Eur. J. Inorg. Chem. 1900, 33, 858. [Google Scholar]
- Crudden, C.M.; Chen, A.C.; Calhoun, L.A. A demonstration of the primary stereoelectronic effect in the Baeyer-Villiger oxidation of alpha-fluorocyclohexanones. Angew. Chem. Int. Ed. 2000, 39, 2851–2855. [Google Scholar] [CrossRef]
- Roger, R.; Neilson, D.G. The Chemistry of Imidates. Chem. Rev. 1961, 61, 179–211. [Google Scholar] [CrossRef]
- Guggenheim, E.A. On the determination of the velocity constant of a unimolecular reaction. Philosophical Magazine Series 7. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1926, 2, 538–543. [Google Scholar] [CrossRef]
- Nödler, K.; Licha, T.; Bester, K.; Sauter, M. Development of a multi-residue analytical method, based on liquid chromatography-tandem mass spectrometry, for the simultaneous determination of 46 micro-contaminants in aqueous samples. J. Chromatogr. A 2010, 1217, 6511–6521. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idzik, K.R.; Nödler, K.; Maier, F.; Licha, T. Efficient Synthesis and Reaction Kinetics of Readily Water Soluble Esters Containing Sulfonic Groups. Molecules 2014, 19, 21022-21033. https://doi.org/10.3390/molecules191221022
Idzik KR, Nödler K, Maier F, Licha T. Efficient Synthesis and Reaction Kinetics of Readily Water Soluble Esters Containing Sulfonic Groups. Molecules. 2014; 19(12):21022-21033. https://doi.org/10.3390/molecules191221022
Chicago/Turabian StyleIdzik, Krzysztof R., Karsten Nödler, Friedrich Maier, and Tobias Licha. 2014. "Efficient Synthesis and Reaction Kinetics of Readily Water Soluble Esters Containing Sulfonic Groups" Molecules 19, no. 12: 21022-21033. https://doi.org/10.3390/molecules191221022
APA StyleIdzik, K. R., Nödler, K., Maier, F., & Licha, T. (2014). Efficient Synthesis and Reaction Kinetics of Readily Water Soluble Esters Containing Sulfonic Groups. Molecules, 19(12), 21022-21033. https://doi.org/10.3390/molecules191221022