A Novel Extended N-Methyl Monopyrrolotetrathiafulvalene Based on 2-Methylene-4,5-Bis(Methylthio)-1,3-Dithiole
Abstract
:1. Introduction
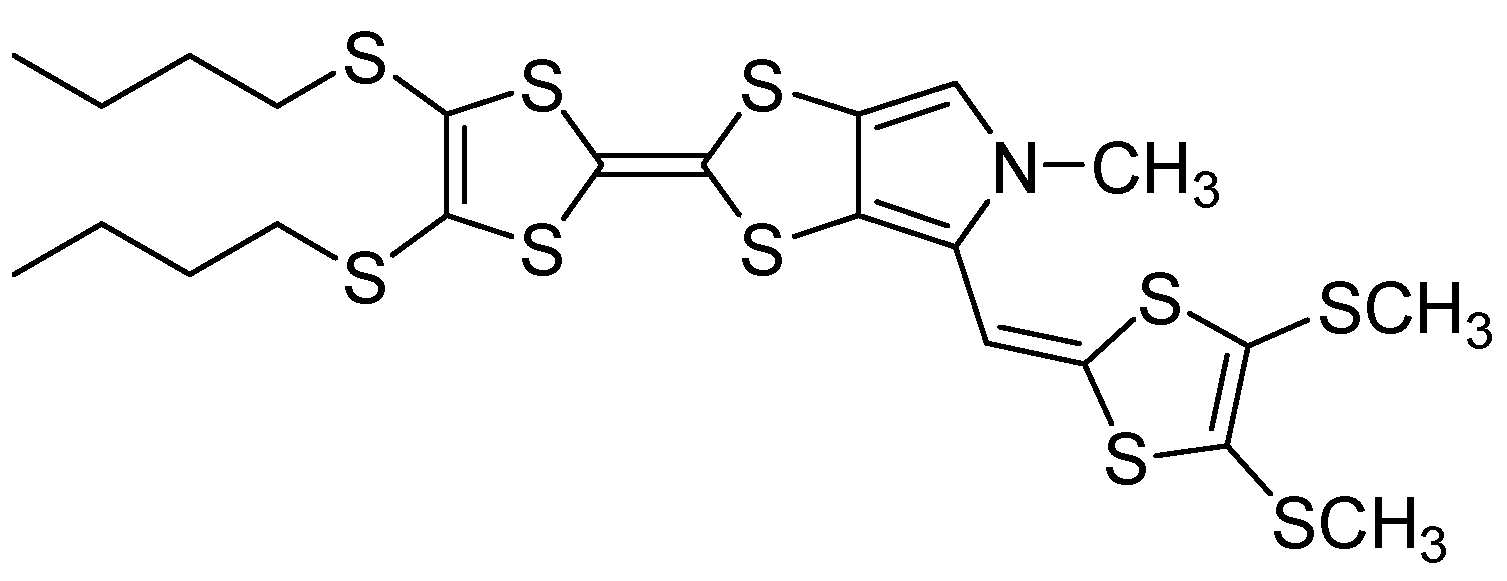
2. Results and Discussion
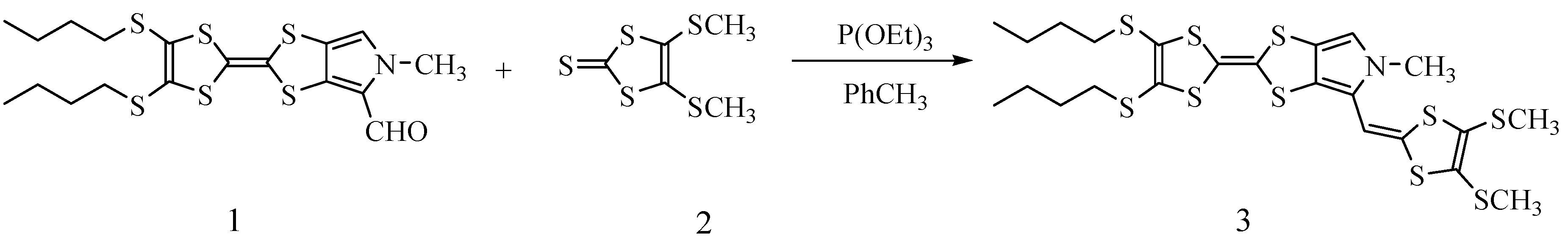
2.1. Synthesis of Target Compound 3
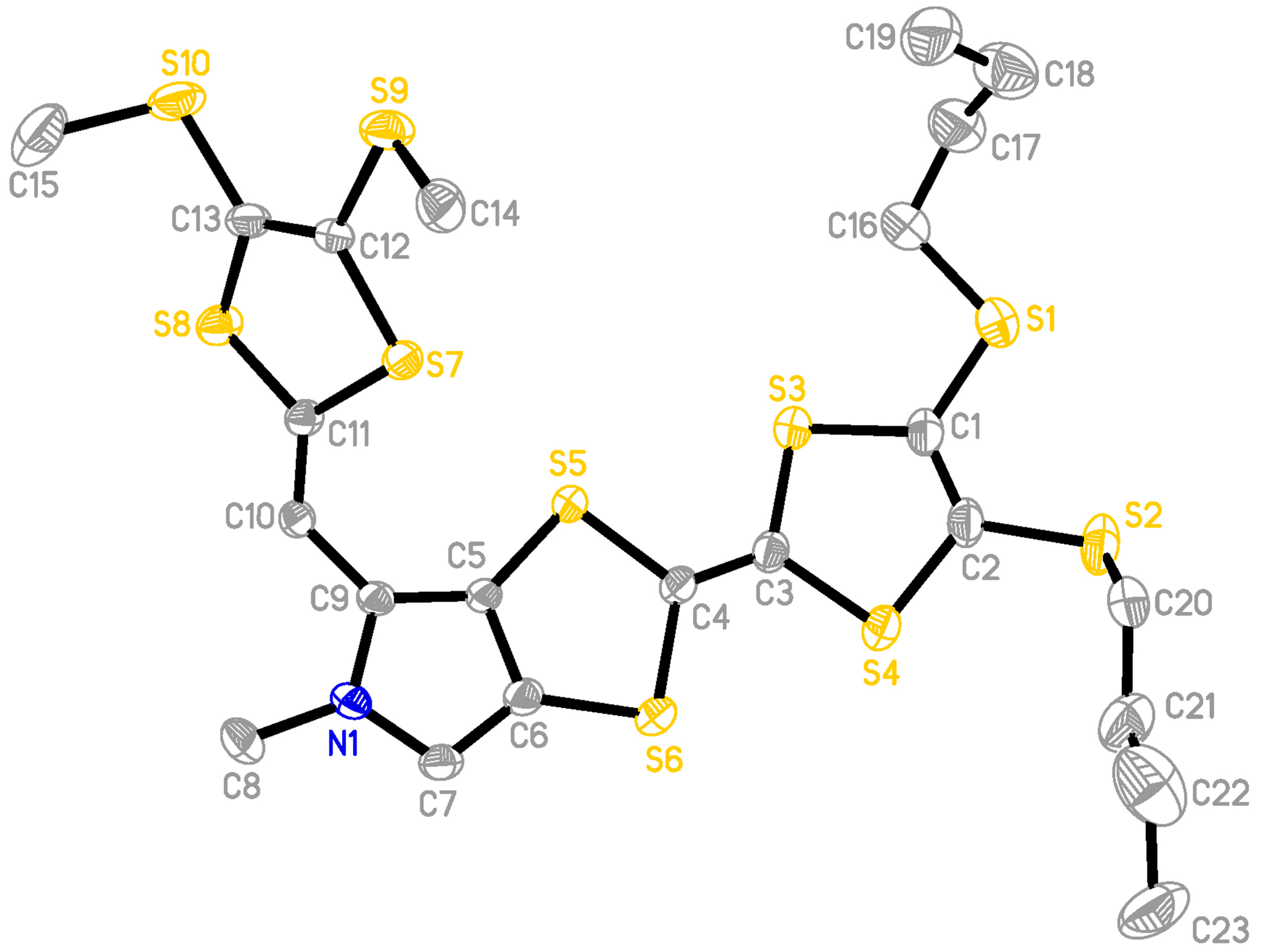
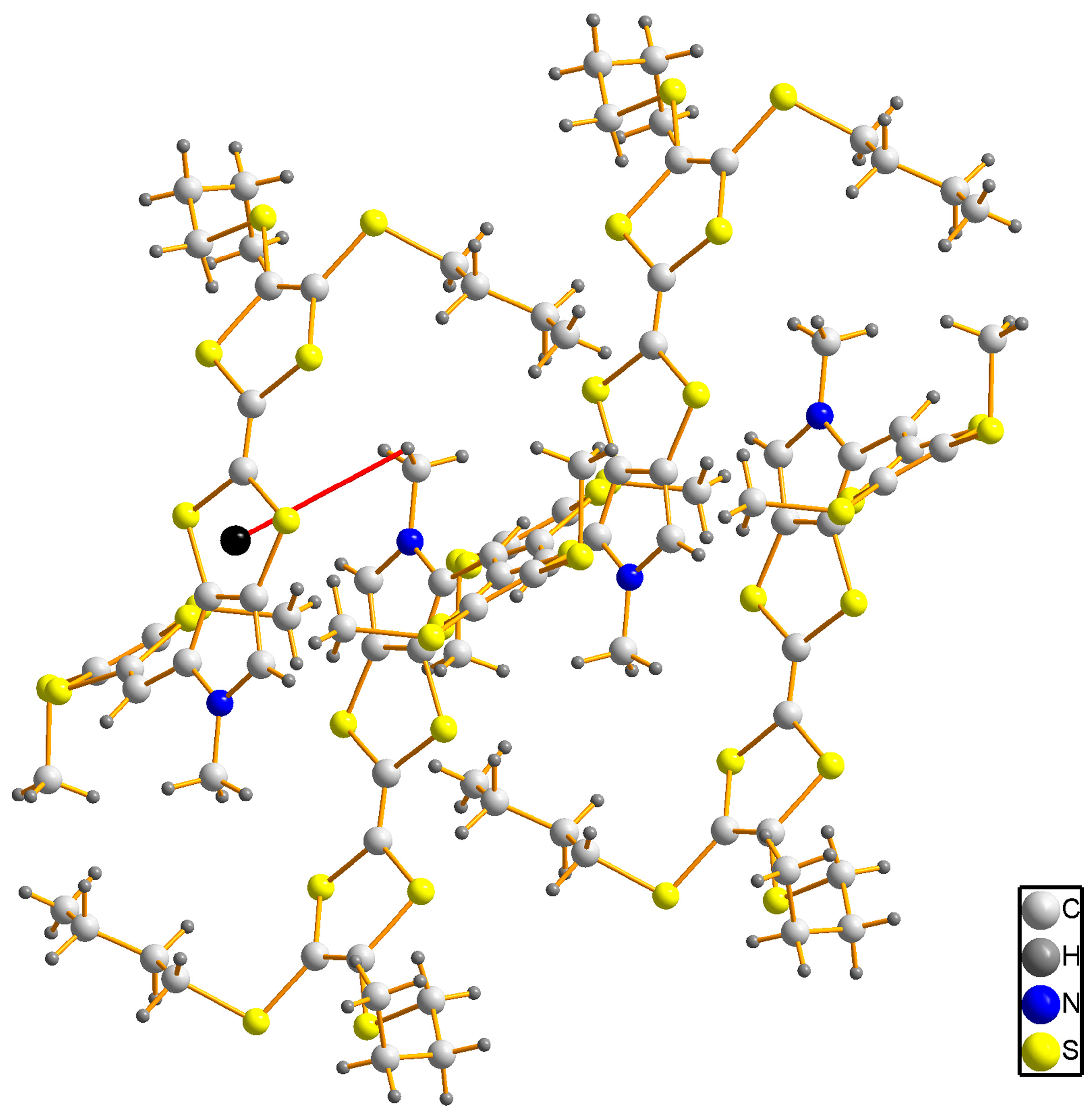
2.2. X-ray Structural Analysis of 3



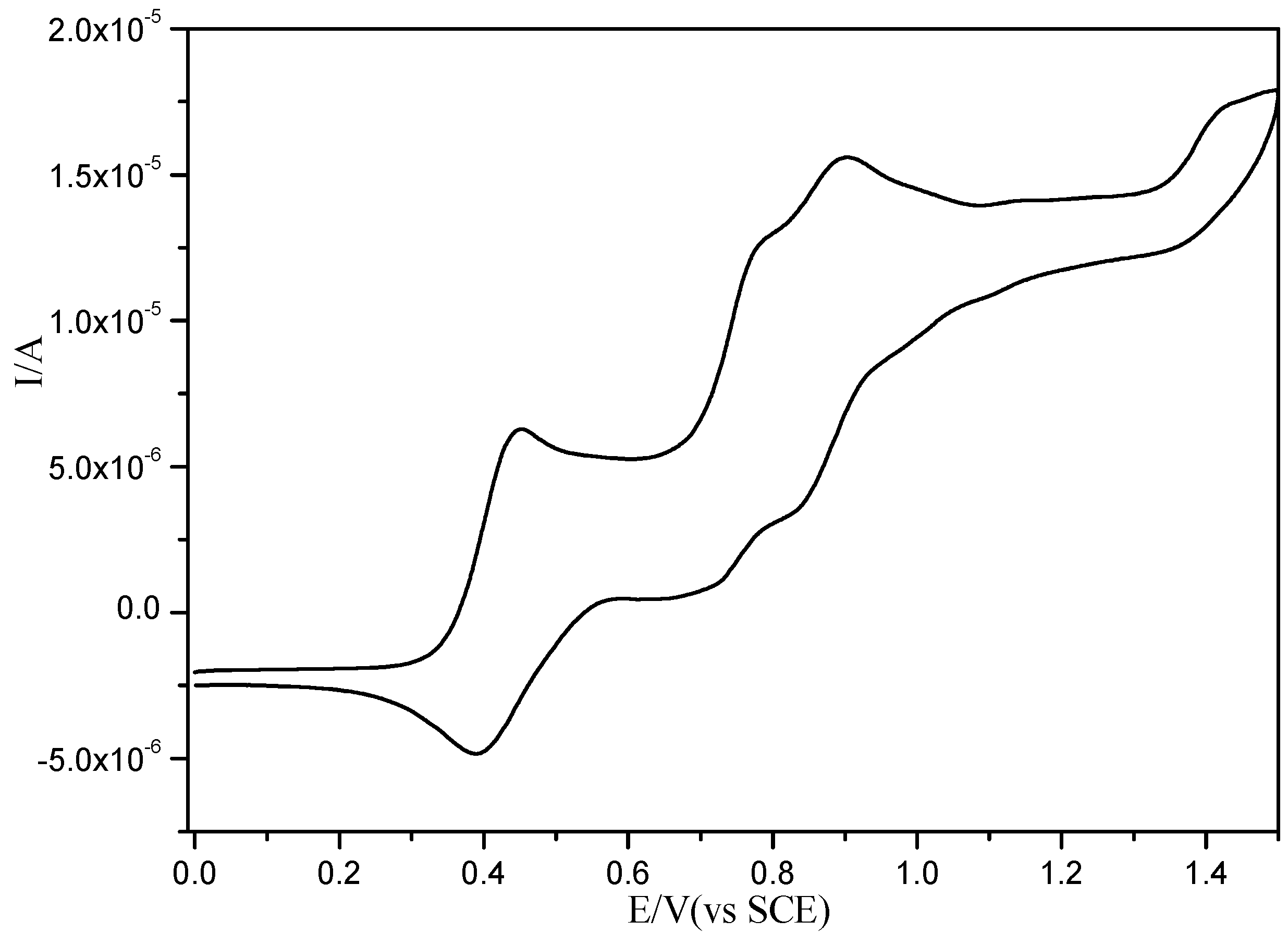
2.3. Electrochemistry
| Compound | E1/2 1 (V) | E1/2 2 (V) | Eox 3 (V) |
|---|---|---|---|
| 1 | 0.470 | 0.773 | - |
| 3 | 0.422 | 0.869 (0.750) | 1.423 |
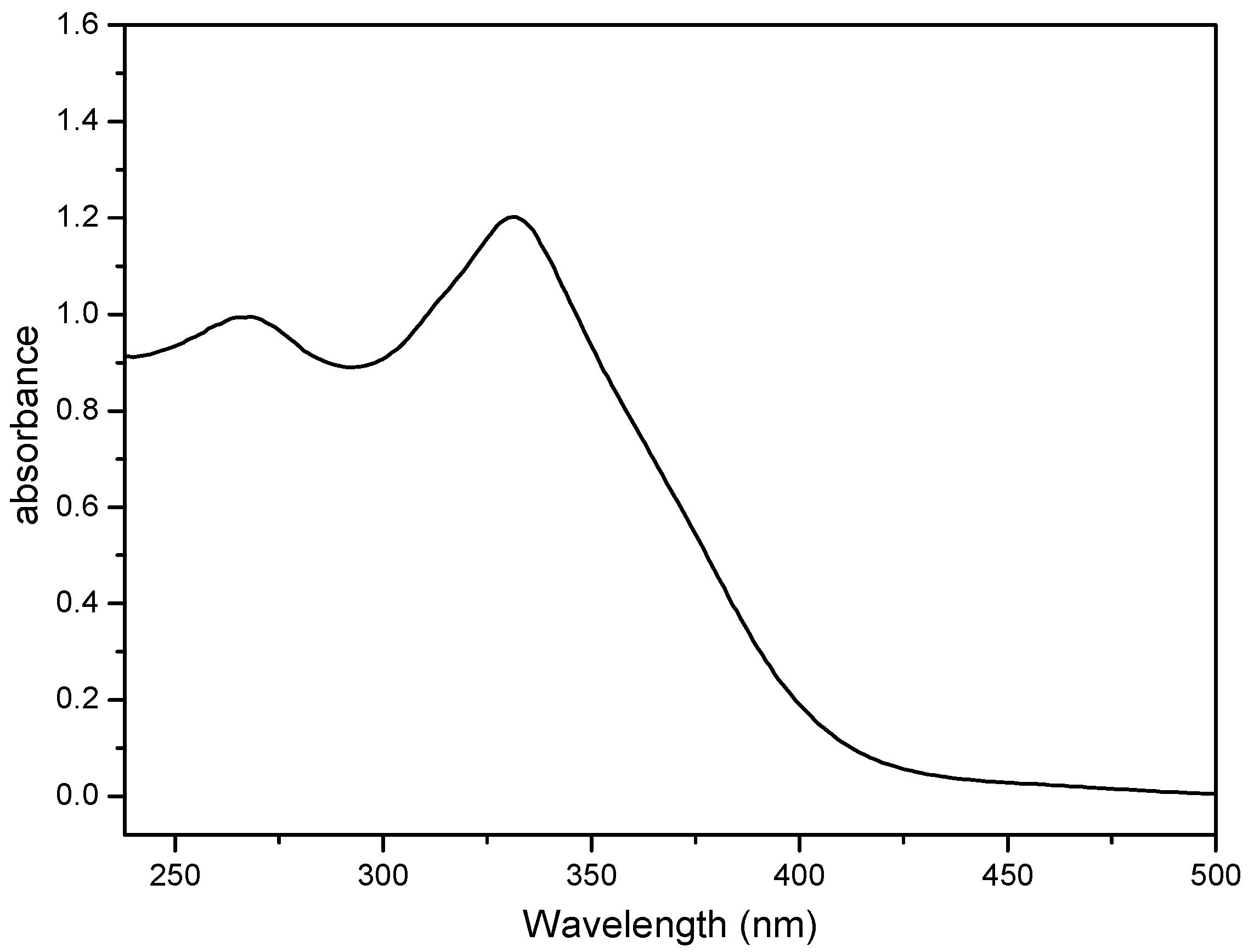
2.4. Electronic Absorption Spectroscopy
3. Experimental Section
3.1. General Information
3.2. Synthesis of N-Methyl-4-[4,5-Bis(Metylthio)-l,3-Dithiol-2-Yliden]-2-[4,5-Bis-(1-Butyithio)-l,3-Dithio1-2-Yli-Den]-(1,3)-Dithiolo[4,5-c]Pyrrole (3)
3.3. X-ray Crystallography
| Chemical Formula | C23 H29 N S10 |
|---|---|
| Formula weight | 640.07 |
| Temperature | 296(2) K |
| Wavelength | 0.71070 Å |
| Crystal system | Triclinic |
| Space group | P-1 |
| a (Å) | 8.552(2) |
| b (Å) | 11.310(2) |
| c (Å) | 16.150(3) |
| α(°) | 109.55(3)° |
| β(°) | 91.45(3)° |
| γ(°) | 91.28(3)° |
| V (Å3) | 1470.6(5) |
| Z | 2 |
| Calculated density | 1.445 Mg·m−3 |
| Absorption coefficient | 0.77 mm−1 |
| F(000) | 668 |
| Crystal size | 0.39 mm × 0.25 mm × 0.06 mm |
| θ Range for data collection | 3.01–27.55 |
| Index ranges | −11 ≤ h ≤ 11 |
| −14 ≤ k ≤ 14 | |
| −20 ≤ l ≤ 20 | |
| Completeness to θ | 98.5% |
| Reflections collected | 14,464 |
| Independent reflections | 6633 [Rint = 0.067] |
| Absorption correction | None |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restrains/parameters | 6633/6/345 |
| Goodness-of-fit on F2 | 1.024 |
| Final R indices [I > 2σ(I)] | R1 = 0.0642, wR2 = 0.1278 |
| R indices (all data) | R1 = 0.1379, wR2 = 0.1601 |
| Largest diff. peak and hole Å−3 | 0.41 and −0.38 eÅ−3 |
3.4. Theoretical Calculations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Segura, J.L.; Martín, N. New concepts in tetrathiafulvalene chemistry. Angew. Chem. Int. Ed. 2001, 40, 1372–1409. [Google Scholar] [CrossRef]
- Bryce, M.R. Recent progress on conducting organic charge-transfer salts. Chem. Soc. Rev. 1991, 20, 355–390. [Google Scholar] [CrossRef]
- Bryce, M.R. Current trends in tetrathiafulvalene chemistry: Towards increased dimensionality. J. Mater. Chem. 1995, 5, 1481–1496. [Google Scholar] [CrossRef]
- Siquot, Y.; Frère, P.; Nozdryn, T.; Cousseau, J.; Sallé, M.; Jubault, M.; Orduna, J.; Garıín, J.; Gorgues, A. Synthesis and electrochemical properties of fused [3,4]Furano-tetrathiafulvalenes. Tetrahedron Lett. 1997, 38, 1919–1922. [Google Scholar] [CrossRef]
- Terkia-Derdra, N.; Andreu, R.; Sallé, M.; Levillain, E.; Orduna, J.; Garín, J.; Ortí, E.; Viruela, R.; Pou-Amérigo, R.; Sahraoui, B.; et al. π Conjugation across the tetrathiafulvalene core: Synthesis of extended tetrathiafulvalene derivatives and theoretical analysis of their unusual electrochemical properties. Chem. Eur. J. 2000, 6, 1199–1213. [Google Scholar]
- Shu, P.; Chiang, L.; Emge, T.; Holt, D.; Kistenmacher, T.; Lee, M.; Stokes, J.; Poehler, T.; Bloch, A.; Cowan, D. Synthesis of Δ2,2'-bithieno[3,4-d]-1,3-dithiole (DTTTF) and some of its charge-transfer salts. J. Chem. Soc. Chem. Commun. 1981, 920–921. [Google Scholar] [CrossRef]
- Ketcham, R.; Hornfeldt, A.B.; Gronowitch, S. Synthesis of tetrathiafulvalene doubly fused to the 3,4-position of selenophene. J. Org. Chem. 1984, 9, 1117–1119. [Google Scholar] [CrossRef]
- Danila, I.; Pop, F.; Escudero, C.; Feldborg, L.N.; Puigmartí-Luis, J.; Riobé, F.; Avarvari, N.; Amabilino, D.B. Twists and turns in the hierarchical self-assembly pathways of a non-amphiphilic chiral supramolecular material. Chem. Commun. 2012, 48, 4552–4554. [Google Scholar] [CrossRef] [Green Version]
- Cao, D.; Wang, C.; Giesener, M.A.; Liu, Z.; Stoddart, J.F. A rigid donor–acceptor daisy chain dimer. Chem. Commun. 2012, 48, 6791–6793. [Google Scholar] [CrossRef]
- Kim, D.; Lynch, V.M.; Park, J.S.; Sessler, J.L. Three distinct equilibrium states via self-assembly: Simple access to a supramolecular ion-controlled NAND logic gate. J. Am. Chem. Soc. 2013, 135, 14889–14894. [Google Scholar] [CrossRef]
- Mulla, K.; Shaik, H.; Thompson, D.W.; Zhao, Y. TTFV-based molecular tweezers and macrocycles as receptors for fullerenes. Org. Lett. 2013, 15, 4532–4535. [Google Scholar] [CrossRef]
- Avellini, T.; Li, H.; Coskun, A.; Barin, G.; Trabolsi, A.; Basuray, A.N.; Dey, S.K.; Credi, A.; Silvi, S.; Stoddart, J.F.; et al. Photoinduced Memory Effect in a Redox Controllable Bistable Mechanical Molecular Switch. Angew. Chem. Int. Ed. 2012, 51, 1611–1615. [Google Scholar] [CrossRef]
- Jeppesen, J.O.; Takimiya, K.; Jensen, F.; Brimert, T.; Nielsen, K.; Thorup, N.; Becher, J. Pyrrolo-annelated tetrathiafulvalenes: The parent systems. J. Org. Chem. 2000, 65, 5794–5805. [Google Scholar] [CrossRef]
- Becher, J.; Brimert, T.; Jeppesen, J.O.; Pedersen, J.; Zubarev, R.; Bjørnholm, T.; Reitzel, N.; Jensen, T.; Kjaer, K.; Levillain, E. Tetrathiafulvaleno-annelated porphyrins. Angew. Chem. Int. Ed. 2001, 40, 2497–2500. [Google Scholar] [CrossRef]
- Hansen, J.A.; Becher, J.; Jeppesen, J.O.; Levillain, E.; Nielsen, M.B.; Petersen, B.M.; Petersenc, J.C.; Şahin, Y. Synthesis and non-linear optical properties of mono-pyrrolotetrathiafulvalene derived donor-π-acceptor dyads. J. Mater. Chem. 2004, 14, 179–184. [Google Scholar] [CrossRef]
- Hou, R.; Li, B.; Chen, T.; Yin, B.; Wu, L. 2,3-[(3,6-Dioxaoctane-1,8-diyl)bis(sulfanediyl-methylene)]-6,7-bis(methylsulfanyl)-1,4,5,8-tetrathiafulvalene. Acta Crystallogr. 2009, E65, o2783. [Google Scholar]
- Zheng, N.; Li, B.; Ma, C.; Chen, T.; Kan, Y.; Yin, B. The pyridazineetetrathiafulvalene conjugates: Synthesis, photophysical, and electrochemical properties. Tetrahedron 2012, 68, 1782–1789. [Google Scholar] [CrossRef]
- Jeppesen, J.O.; Takimiya, K.; Jensen, F.; Becher, J. Pyrrolo Annelated Tetrathiafulvalenes: The Parent Systems. Org. Lett. 1999, 1, 1291–1294. [Google Scholar] [CrossRef]
- Guerro, M.; Dam, T.U.; Bakhta, S.; Kolli, B.; Roisnel, T.; Lorcy, D. Tetrathiafulvalene hydrazone an efficient precursor of various chelating electroactive ligands. Tetrahedron 2011, 67, 3427–3433. [Google Scholar] [CrossRef]
- CCDC CIF Depository Request Form. Available online: http://www.ccdc.cam.ac.uk/data_request/cif (accessed on 27 November 2014).
- Hariharan, P.C.; Pople, J.A. Accuracy of AHn equilibrium geometries by single determinant molecular orbital theory. Mol. Phys. 1974, 27, 209–214. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Sample Availability: Samples of the compounds 1–3 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, R.; Shang, X.; Xia, Y.; Li, B.; Li, D. A Novel Extended N-Methyl Monopyrrolotetrathiafulvalene Based on 2-Methylene-4,5-Bis(Methylthio)-1,3-Dithiole. Molecules 2014, 19, 20314-20324. https://doi.org/10.3390/molecules191220314
Hou R, Shang X, Xia Y, Li B, Li D. A Novel Extended N-Methyl Monopyrrolotetrathiafulvalene Based on 2-Methylene-4,5-Bis(Methylthio)-1,3-Dithiole. Molecules. 2014; 19(12):20314-20324. https://doi.org/10.3390/molecules191220314
Chicago/Turabian StyleHou, Ruibin, Xiaohong Shang, Yan Xia, Bao Li, and Dongfeng Li. 2014. "A Novel Extended N-Methyl Monopyrrolotetrathiafulvalene Based on 2-Methylene-4,5-Bis(Methylthio)-1,3-Dithiole" Molecules 19, no. 12: 20314-20324. https://doi.org/10.3390/molecules191220314





