1. Introduction
The genus
Stemona (Stemonaceae) has been used as a source of insecticides and antitussive remedies in China, Japan and Southeastern Asian countries for a long time [
1,
2]. Till now, about 140 alkaloids have been isolated from the
Stemona genus and structurally identified, most of which share the common pyrrolo[1,2-α]azepine nucleus, while several contain a pyrido[1,2-α]azepine skeleton [
3,
4,
5,
6,
7,
8,
9].
Stemona cochinchinensis Gagnep. is one of the three endemic
Stemona species in Vietnam. We and others have reported twelve alkaloids with a basic pyrrolo[1,2-α]azepine nucleus, and six alkaloids with pyrido[1,2-α]azepine skeletons from this species [
4,
6,
8,
10]. In our preliminary investigation on this plant, we also reported three bisbenzopyrans [
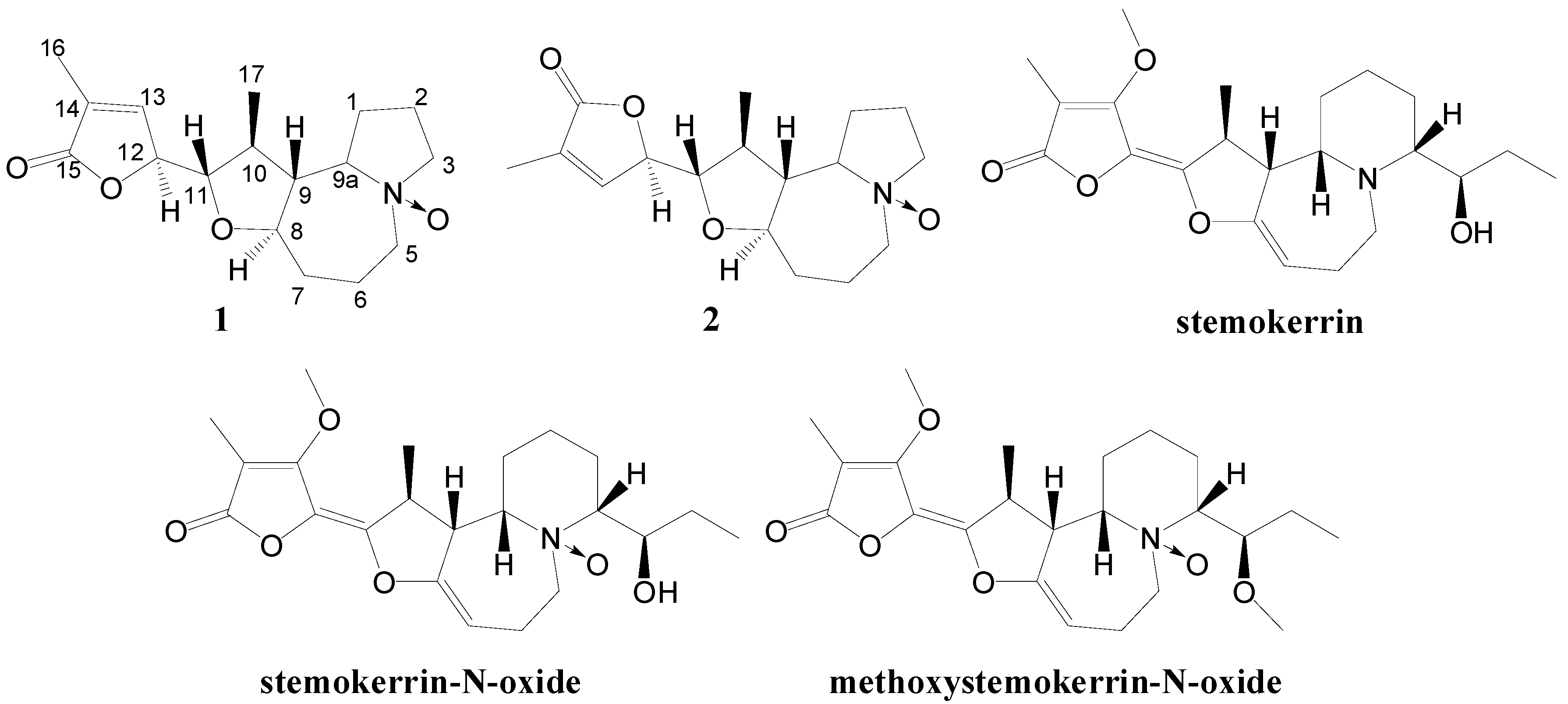
10]. Herein a further investigation of the minor alkaloidal constituents from the title plant was carried out. We describe the isolation and structural elucidation of two new N-oxide alkaloids with pyrrolo[1,2-α]azepine skeletons, namely bisoneostemocochinine-N-oxide (
1) and neostemocochinine-N-oxide (
2) (
Figure 1). Three known alkaloids were also obtained and identified as stemokerrin [
4], stemokerrin-N-oxide [
7] and methoxystemokerrin-N-oxide [
4]. Their structures were elucidated by 1D- and 2D-NMR analysis together with other spectroscopic studies. Furthermore, the N-oxide
Stemona alkaloids were reviewed and their NMR spectral characteristics were discussed. The major alkaloid, stemokerrin, was chosen to test for anti-tussive activity using a citric acid-induced guinea pig model.
Figure 1.
Structures of the isolated alkaloids.
Figure 1.
Structures of the isolated alkaloids.
2. Results and Discussion
Isoneostemocochinine-N-oxide (
1) was obtained as a yellow amorphous powder. The HR-ESI-MS suggested the molecular formula C
17H
25NO
4 (
m/
z 330.1672 [M+Na]
+; calc. 330.1681). The strong and sharp IR band at 1,775 cm
−1 indicated the existence of a
γ-lactone. The
1H-NMR spectrum (
Table 1) displayed resonances of two methyl groups [δ
H 1.15 (3H, d,
J = 6.1 Hz, CH
3-17) and 1.92 (3H, d,
J = 1.7 Hz, CH
3-16)], an olefinic proton [δ
H 6.98 (q,
J = 1.7 Hz, H-13)], and three low-field protons [δ
H 3.72 (m, H-11), 3.94 (m, H-8) and 4.88 (m, H-12)]. The
13C-NMR spectrum of compound
1 showed 17 resonances, which were classified into one
sp2 carbonyl carbon, two
sp2 olefinic carbons, six
sp3 tertiary carbons, six
sp3 secondary carbons and two
sp3 methyl carbons (
Table 1). The
1H- and
13C-NMR spectra of compound
1 strongly resembled those of isoneostemocochinine [
8]. The molecular weight of compound
1 was 16 Da more than that of isoneostemocochinine, which suggested the possible presence of an N-oxide moiety in the molecule of
1. This was further supported by the down-field shifts of H-3 (3.48, m), H-5α (3.95, m), H-5β (3.60, m), H-9a (3.89, m), C-3 (70.2), C-5 (66.0) and C-9a (80.6) in comparison with those corresponding NMR data of isoneostemocochinine (
Table 2). Therefore the planar structure of compound
1 was determined. The relative configuration of
1 was inferred by the ROESY experiment (broken arrows in
Figure 2). In all the stemoamide-group alkaloids, H-9 is β-oriented [
3]. The correlations of H-11/H-9, CH
3-17/H-9 and CH
3-17/H-11 indicated that H-11 and CH
3-17 were both β-oriented. The relative configuration of C-12 was determined to be rel-S by the ROESY correlations of H-12/H-10 and H-13/CH
3-17. Thus the full structure of
1 was established and the detailed assignments of the
1H- and
13C-NMR resonances were shown in
Table 1.
Table 1.
1H-NMR and 13C-NMR data for compounds 1 and 2 (CDCl3).
Table 1.
1H-NMR and 13C-NMR data for compounds 1 and 2 (CDCl3).
| | 1 | 2 |
|---|
| δH,
J (Hz) | δC | δH,
J (Hz) | δC |
|---|
| 1 | 1.82 m | 20.5 | 1.81 m | 20.6 |
| 1 | 1.57 m | 1.57 m |
| 2 | 1.91 m | 24.6 | 1.91 m | 24.8 |
| 2 | 1.37 m | 1.37 m |
| 3 | 3.48 m (2H) | 70.2 | 3.48 m (2H) | 70.4 |
| 5α | 3.95 m | 66.0 | 3.95 m | 66.0 |
| 5β | 3.60 m | 3.62 m |
| 6 | 1.54 m | 18.8 | 1.54 m | 18.9 |
| 6 | 1.37 m | 1.37 m |
| 7 | 2.06 m | 32.4 | 2.06 m | 32.1 |
| 7 | 1.27 m | 1.27 m |
| 8 | 3.94 m | 83.0 | 4.05 m | 82.8 |
| 9 | 2.11 m | 48.7 | 2.17 m | 49.0 |
| 9a | 3.89 m | 80.6 | 3.89 m | 80.5 |
| 10 | 2.22 m | 40.2 | 2.20 m | 39.6 |
| 11 | 3.72 m | 84.6 | 3.82 m | 84.6 |
| 12 | 4.88 m | 80.5 | 4.93 m | 80.2 |
| 13 | 6.98 q (1.7) | 145.8 | 7.07 q (1.7) | 145.8 |
| 14 | | 131.1 | | 131.3 |
| 15 | | 174.2 | | 173.9 |
| 16 | 1.92 d (1.7) | 10.8 | 1.92 d (1.7) | 10.7 |
| 17 | 1.15 d (6.1) | 14.9 | 1.09 d (6.1) | 16.3 |
Figure 2.
Key NOE correlations of compounds 1 and 2.
Figure 2.
Key NOE correlations of compounds 1 and 2.
Table 2.
Comparison of NMR data of N-oxide Stemona alkaloids and their precursors.
Table 2.
Comparison of NMR data of N-oxide Stemona alkaloids and their precursors.
| Compound | δH | δC | Ref |
|---|
| H-3 | Δ | H-5 | Δ | H-9a | Δ | C-3 | Δ | C-5 | Δ | C-9a | Δ |
|---|
| isoneostemocochinine N-oxide | 3.48 (2H) | 0.20 | 3.95/3.60 | 0.47/0.64 | 3.89 | 0.25 | 70.2 | 18.2 | 66.0 | 16.2 | 80.6 | 19.9 | [8] |
| isoneostemocochinine | 3.28 (2H) | 3.48/2.96 | 3.64 | 52.0 | 49.8 | 60.7 |
| neostemocochinine N-oxide | 3.48 (2H) | 0.20 | 3.95/3.62 | 0.46/0.64 | 3.89 | 0.25 | 70.4 | 18.3 | 66.0 | 16.1 | 80.5 | 19.8 | [8] |
| neostemocochinine | 3.28 (2H) | 3.49/2.98 | 3.64 | 52.1 | 49.9 | 60.7 |
| stemaphylline N-oxide | 3.57 (2H) | 0.56/1.06 | 3.33 (2H) | 0.39/0.82 | 4.65 | 1.72 | 71.0 | 16.7 | 67.2 | 14.9 | 81.6 | 16.8 | [11] |
| stemaphylline | 3.01/2.51 | 2.94/2.51 | 2.93 | 54.3 | 52.3 | 64.8 | [11] |
| stenine A | 3.79/3.69 | 0.14/0.22 | 3.87/3.54 | 0.74/0.58 | 3.69 | 1.62 | 72.2 | 19.5 | 67.7 | 15.4 | 84.3 | 19.0 | [12] |
| stenine B | 3.65/3.47 | 3.13/2.96 | 2.07 | 52.7 | 52.3 | 65.3 | [12] |
| N-oxytuberostemonine | 3.67 | 0.24 | 3.78/3.52 | 0.31/0.85 | 4.01 | 0.94 | 77.5 | 12.5 | 69.7 | 21.6 | 88.9 | 25.3 | [13] |
| tuberostemonine | 3.43 | 3.47/2.67 | 3.07 | 65.0 | 48.1 | 63.6 | [14] |
| | H-4 | Δ | H-6 | Δ | H-10a | Δ | C-4 | Δ | C-6 | Δ | C-10a | Δ | |
| stemocurtisine N-oxide | 3.44/3.29 | 0.42/0.42 | 4.12/3.01 | 0.74/0.05 | 3.66 | 0.22 | 69.6 | 16.0 | 66.4 | 13.4 | 82.3 | 20.3 | [9] |
| stemocurtisine | 3.02/2.87 | 3.38/2.96 | 3.44 | 53.6 | 53.0 | 62.0 | [15] |
| oxystemokerrine N-oxide | 3.31 | 0.72 | 4.04/3.55 | 0.68/0.49 | 3.93 | 0.43 | 79.4 | 13.6 | 61.9 | 17.7 | 85.4 | 19.0 | [4] |
| oxystemokerrine | 2.59 | 3.36/3.06 | 3.50 | 65.8 | 44.2 | 66.4 | [4] |
| stemokerrine N-oxide | 3.22 | 0.62 | 3.46/3.42 | 0/76/0.86 | 3.22 | 0.39 | 81.9 | 12.0 | 54.1 | 14.4 | 78.3 | 15.9 | [7] |
| methoxystemokerrine N-oxide | 3.26 | 0.66 | 3.36/2/63 | 0.66/0.07 | 3.21 | 0.38 | 84.3 | 14.4 | 56.2 | 16.5 | 78.5 | 16.1 | [4] |
| stemokerrine | 2.60 | | 2.70/2.56 | | 2.83 | | 69.9 | | 39.7 | | 62.4 | | [4] |
Neostemocochinine-N-oxide (
2) was obtained as a yellow amorphous powder. The molecular formula was determined by the HR-ESI-MS to be C
17H
25NO
4 (
m/
z 330.1689 [M+Na]
+; calc. 330.1681), the same as that of
1. The presence of a
γ-lactone was indicated by the strong and sharp absorption band at 1770 cm
−1 in the IR spectrum. The
1H- and
13C-NMR data of
2 (
Table 1) were similar to those of neostemocochinine [
8]. The molecular weight difference and down-field shifts of H-3, H-5, H-9a, C-3, C-5 and C-9a in
1H- and
13C-NMR (
Table 2) suggested that compound
2 was an N-oxide of neostemocochinine. A careful analysis of the spectroscopic data resulted in the conclusion that compounds
2 and
1 shared the same planar structure. The major differences of their NMR data involved the chemical shifts of H-11, H-12, H-13 and C-10 (
Table 1), suggesting
2 was a stereoisomer of
1. The relative configuration of
2 was disclosed by the ROESY spectrum (broken arrows in
Figure 2). The correlations of H-11/H-9 and H-11/CH
3-17 revealed that H-9, H-11 and CH
3-17 were β-oriented. The correlations of H-12/H-10, H-12/H-8 and H-13/H-8 suggested an rel-
R-configuration of C-12. The stereochemistry of C-11 and C-12 was consistent with that of neostemocochinine [
8].
The natural occurring N-oxide
Stemona alkaloids are rare, and till now only nine were isolated and identified including compounds
1 and
2 (
Figure 1 and
Figure 3 ).
Figure 3.
N-oxide Stemona alkaloids.
Figure 3.
N-oxide Stemona alkaloids.
Five of them, including isoneostemocochinine N-oxide (
1), neostemocochinine N-oxide (
2), stemaphylline N-oxide [
11], stenine A [
12] and N-oxy-tuberostemonine [
13], contain pyrrolo[1,2-α]azepine skeletons. The other four including stemocurtisine N-oxide [
9], oxystemokerrine N-oxide [
4], stemokerrine N-oxide [
7] and methoxystemokerrine N-oxide [
4] contain pyrido[1,2-α]azepine skeletons. Most of the N-oxide
Stemona alkaloids were identified from
Stemona species collected from Southeast Asia, including
S. cochinchinensis,
S. saxorum,
S. curtisii,
S. kerrii and
S. aphylla. It might be due to warm and moist environment in this area.
As the electronegativity of oxygen atom is stronger than that of nitrogen, the electron cloud in nitrogen oxides is attracted toward the oxygen end. The deshielding effect results in the down-field shifts of hydrogens and carbons near the nitrogen atom in NMR spectra. Regarding N-oxide
Stemona alkaloids, the chemical shifts of H-3 (H-4), H-5 (H-6) and H-9a (H-10a) are 0.14 to 1.72 ppm more than their corresponding signals in precursors (
Table 2). Additionally, the signals of C-3 (C-4), C-5 (C-6) and C-9a (C-10a) in N-oxide
Stemona alkaloids are down-field shift about 12.0 to 25.3 ppm, compared with their corresponding signals in precursor alkaloids (
Table 2). These data could help to elucidate the structures of N-oxide
Stemona alkaloids.
The major alkaloid, stemokerrin, was tested for anti-tussive activity in the citric acid-induced guinea pig cough model. Stemokerrin showed significant anti-tussive activity, and about 62% cough inhibition was achieved at a single intraperitoneal (ip) dose of 70 mg/kg. Comparing the results from our previous study on stemoninine (about 43% cough inhibition at a single ip dose of 75 mg/kg) [
16], stemokerrin exhibited markedly higher cough suppression potency. The current study thus has disclosed the first pyrido[1,2-α]azepine skeleton alkaloid with potent anti-tussive activity, which might contribute to the development of a new cough inhibition therapy.
3. Experimental Section
3.2. HPLC Conditions
Analytical HPLC was performed on a Waters 2690 instrument with a 996 Photodiode Array Detector (PAD) and an Alltech ELSD 2000 detector. Chromatographic separation was carried out on an XTerra RP18 column (4.6 × 250 mm, 5 μm, Waters, Milford, MA, USA), using a gradient solvent system comprised of H2O (A) and CH3CN (B) containing 0.1% ammonia, at a flow rate of 1.0 mL/min. Temperature for the ELSD drift tube was set at 105 °C, and the air flow was 3.2 L/min. Preparative HPLC was performed on a Varian SD1 instrument with 320 single wave detector. Chromatographic separation was carried out on a C18 column (220 × 25 mm, 10 μm, Merck, Darmstadt, Germany), using a gradient solvent system comprised of H2O (A) and CH3CN (B) containing 0.1% ammonia, at a flow rate of 15 mL/min.
3.3. Plant Material
The roots of S. cochinchinensis (1.52 kg) were collected in Sonla province of Northern Vietnam in April 2002 by Nguyen Duc Thinh, Director of Sonla State Farm and identified by Vu Ngoc Chuyen, Hanoi University of Pharmacy.
3.4. Extraction and Isolation
The crude alkaloids were extracted as described previously [
8]. The crude alkaloids (15 g) were subjected to column chromatography over silica gel and eluted with petroleum ether–acetone gradients from 9:1 to 1:1, acetone and then methanol, to yield 12 fractions. Fraction 4 (2.115 g) was subjected to column chromatography over silica gel eluting with petroleum ether–acetone (9:1) to obtain stemokerrin (201 mg). Fraction 7 (1.546 g) was separated by column chromatography over silica gel with petroleum ether–acetone (4:1) and then purified with preparative HPLC (CH
3CN–H
2O from 40% to 55% in 0–60 min and then from 55% to 70% in 60–180 min), affording stemokerrin-N-oxide (34 mg) and methoxystemokerrin-N-oxide (13 mg). Fraction 8 (860 mg) was subjected to column chromatography over silica gel (petroleum ether–acetone 4:1) and Sephadex LH-20 (chloroform–methanol (1:1) repeatedly to give five subfractions (1–5). Subfraction 4 was further separated by preparative HPLC (CH
3CN–H
2O 30:70) to yield isoneostemocochinine N-oxide (
1, 8 mg) and neostemocochinine N-oxide (
2, 6 mg).
Isoneostemocochinine N-oxide (
1). Yellow amorphous powder;
−54 (
c = 0.10, CHCl
3); UV (MeOH) λ
max 239.2 (2.35); IR (KBr) ν
max 1775, 1456, 1213, 1094, 1045, 752;
1H- and
13C-NMR see
Table 1; positive ESI-MS
m/
z 308.1 [M+1]
+, 615.1 [2M+1]
+; EIMS
m/
z 291, 289, 271, 256, 194, 192, 177, 164, 162, 134, 120, 111, 96, 84, 70; HR-ESI-MS
m/
z [M+Na]
+ 330.1672 (calcd. for C
17H
25NO
4Na, 330.1681).
Neostemocochinine N-oxide (
2). Yellow amorphous powder;
−12 (
c = 0.10, CHCl
3); UV (MeOH) λ
max 239.1 (2.35); IR (KBr) ν
max 1770, 1439, 1161, 1082, 1051, 754;
1H- and
13C-NMR see
Table 1; positive ESI-MS
m/
z 308.1 [M+1]
+, 615.2 [2M+1]
+; HR-ESI-MS
m/
z [M+Na]
+ 330.1689 (cacld. for C
17H
25NO
4Na, 330.1681).
3.5. Anti-Tussive Activity of Stemokerrin
The citric acid-induced guinea pig cough model was used in this study, as described in our previous study [
16]. Briefly, unrestrained, conscious Dunkin-Hartley guinea pigs of both sexes (300–350 g) were randomly divided into groups with at least five animals in each group. Stemokerrin (dose 70 mg/kg) was given to the guinea pigs via a single ip injection. The treated animal was individually placed into a transparent Perspex airtight chamber. At 30 min after treatment, each animal was exposed to 0.5 M citric acid aerosols for 8 min with a flow rate of 0.5 mL/min. During the aerosol exposure, the animal was continuously monitored, and cough sounds were recorded via a microphone connected to a personal computer and analyzed by Cool Edit 2000 software (Syntrillium, Phoenix, AZ, USA). Cough episodes were determined. The antitussive activity of codeine phosphate as the positive control and response of the vehicle control (Tween 80 in saline (5:95,
v/
v)) were also tested in parallel studies. Antitussive activity was evaluated and expressed as the percentage of cough inhibition based on the comparison of numbers of cough episodes recorded in the alkaloid-treated group with the corresponding vehicle control group.