Anti-Inflammatory Effect of 3-O-[(6'-O-Palmitoyl)-β-D-glucopyranosyl Sitosterol] from Agave angustifolia on Ear Edema in Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Analysis
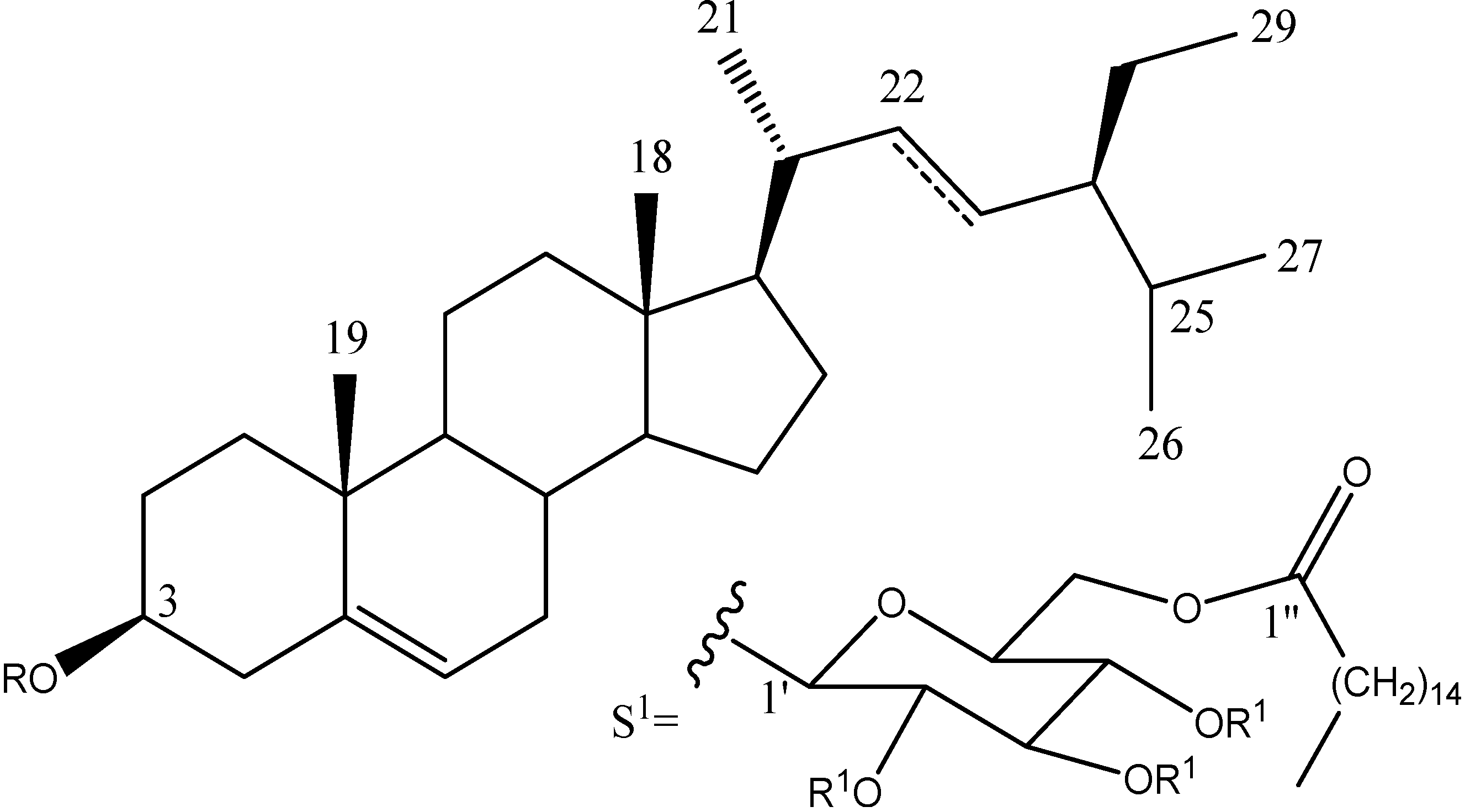
| 1a | --- | S1 | Ac |
| 2 | --- | Glc | --- |
2.2. Effect of A. angustifolia on 12-Ortho-Tetradecanoylphorbol-13-Acetate (TPA)-Induced Mouse Ear Edema Assay
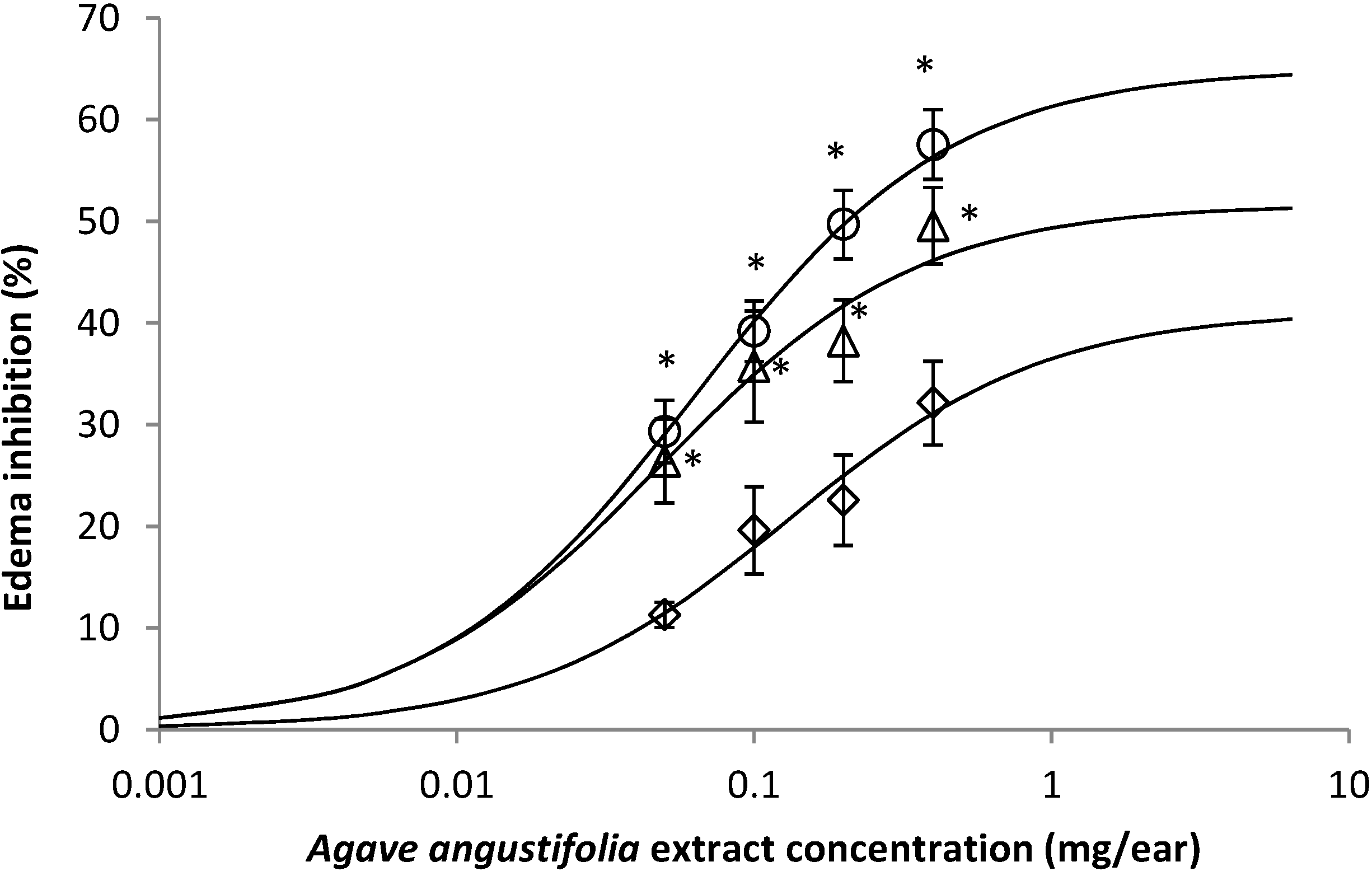
2.3. Effect of A. angustifolia on the Concentration of Cytokines from Mouse Ear
| Treatment | Concentration (mg/ear) | IL-1β | IL-4 | IL-6 | IL-10 | TNF-α |
|---|---|---|---|---|---|---|
| TPA | 0.00025 | 14.4 ± 0.9 | 0.9 ± 0.1 | 7.3 ± 0.6 | 0.7 ± 0.6 | 12.4 ± 0.6 |
| DEX | 0.1 | 5.1 ± 1.1 * | 1.3 ± 0.2 | 1.8 ± 0.2 * | 0.9 ± 0.3 | 2.1 ± 0.4 * |
| AaAc | 0.05 | 10.4 ± 1.2 * | 1.1 ± 0.1 | 7.1 ± 0.6 * | 0.8 ± 0.1 | 10.9 ± 1.9 |
| 0.10 | 9.7 ± 1.5 * | 1.1 ± 0.1 | 6.9 ± 0.6 * | 0.8 ± 0.1 | 10.3 ± 1.8 | |
| 0.20 | 9.4 ± 1.2 * | 1.0 ± 0.2 | 5.8 ± 1.0 * | 0.9 ± 0.1 | 8.8 ± 1.1 * | |
| 0.40 | 8.9 ± 1.4 * | 1.2 ± 0.1 | 5.2 ± 0.9 * | 0.9 ± 0.1 | 8.6 ± 1.5 * | |
| 0.80 | 8.3 ± 1.6 * | 1.3 ± 0.3 | 4.4 ± 0.5 * | 1.1 ± 0.2 | 8.4 ± 1.1 * | |
| AaF13 | 0.05 | 13.9 ± 1.8 | 1.0 ± 0.1 | 6.0 ± 0.5 * | 0.7 ± 0.1 | 10.8 ± 1.6 * |
| 0.10 | 12.2 ± 1.4 | 1.1 ± 0.0 | 5.1 ± 0.7 * | 0.9 ± 0.1 | 9.4 ± 2.3 * | |
| 0.20 | 11.1 ± 1.0 * | 1.3 ± 0.3 | 4.2 ± 0.9 * | 1.0 ± 0.1 | 8.3 ± 1.7 * | |
| 0.40 | 9.1 ± 1.3 * | 1.6 ± 0.3 * | 3.3 ± 0.6 * | 1.1 ± 0.1 | 6.4 ± 2.9 * | |
| 0.80 | 8.7 ± 2.3 * | 1.8 ± 0.2 * | 2.8 ± 0.3 * | 1.4 ± 0.1 * | 5.8 ± 1.2 * | |
| AaF16 | 0.05 | 13.8 ± 1.6 | 1.3 ± 0.1 | 5.4 ± 1.4 * | 0. 8 ± 0.3 | 7.5 ± 1.4 * |
| 0.10 | 10.4 ± 1.5 * | 1.5 ± 0.4 * | 3.1 ± 0.3 * | 1.2 ± 0.6 | 6.5 ± 1.2 * | |
| 0.20 | 7.6 ± 0.7 * | 2.4 ± 0.4 * | 2.6 ± 0.6 * | 1.6 ± 0.2 * | 4.9 ± 0.9 * | |
| 0.40 | 4.2 ± 0.6 * | 2.7 ± 0.3 * | 1.4 ± 0.2 * | 2.0 ± 0.2 * | 3.5 ± 0.8 * | |
| 0.80 | 2.2 ± 0.3 * | 3.8 ± 0.7 * | 0.8 ± 0.7 * | 3.5 ± 0.7 * | 2.1 ± 0.7 * |
2.4. Effect of A. angustifolia on the Cellularity from Ear Edema Induced by TPA
| Concetration (mg/ear) | Treatment | 0.05 | 0.1 | 0.2 | 0.4 | 0.8 |
|---|---|---|---|---|---|---|
| AaAc |  |  |  |  |  | |
| AaF13 |  |  |  |  |  | |
| AaF16 |  |  |  |  |  | |
| BASAL | DEX | VEHICLE | PANEL 10× | |||
 |  |  | ||||
| AaAc |  |  |  |  |  | |
| AaF13 |  |  |  |  |  | |
| AaF16 |  |  |  |  |  | |
| Controls | BASAL | DEX | VEHICLE | PANEL 40× | ||
 |  |  | ||||
3. Experimental Section
3.1. Plant Material
3.2. Animals
3.3. Extraction and Isolation
3.4. 12-Ortho-Tetradecanoylphorbol-13-Acetate (TPA)-Induced Mouse Ear Edema Assay
3.5. Quantification of Cytokines from Mouse Ear
3.6. Histology
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Colunga-Garciamarin, P.; May-Pat, F. Agave studies in Yucatan, Mexico. I. Past and present germplasm diversity and use. Econ. Bot. 1993, 47, 312–327. [Google Scholar]
- Arellano, R.J.A.; Flores, G.J.S.; Tun, G.J.; Cruz, B.M.M.C. Etnoflora Yucatanense: Nomenclatura, Forma de Vida, Uso, Manejo y Distribución de las Especies Vegetales de la Península de Yucatán; Universidad Autónoma de Yucatán: Merida Yuc, Mexico, 2003; pp. 11–12. [Google Scholar]
- Valenzuela-Zapata, A.G. Las denominaciones de origen Tequila y Mezcal y la biodiversidad en el género Agave sp. Available online: http://www.esac.pt/cernas/comunicacoes_BioDO/3.%20Ana%20Valenzuela_PDF.pdf (accessed on 10 August 2012).
- Argueta, A.; Cano, L.; Rodarte, M. Atlas de las Plantas de la Medicina Tradicional Mexicana, 1st ed.; Instituto Nacional Indigenista: Mexico D.F., Mexico, 1994; pp. 930–933. [Google Scholar]
- Barraza-Morales, A.; Sánchez-Teyer, F.L.; Robert, M.; Esqueda, M.; Gardea, A. Variabilidad genética en Agave angustifolia Haw. de la sierra Sonorense, Mexico, determinada con marcadores AFLP. Rev. Fitotec. Mex. 2006, 29, 1–8. [Google Scholar]
- Cano, L. Flora Medicinal de Veracruz Inventario Etnobotánico, 1st ed.; Universidad Veracruzana: Xalapa Ver, Mexico, 1998; pp. 49–50. [Google Scholar]
- Monroy-Ortriz, C.; Castillo-España, P. Plantas Medicinales Utilizadas en el Estado de Morelos; Universidad Autónoma del Estado de Morelos: Cuernavaca Mor, Mexico, 2007; pp. 25–26. [Google Scholar]
- Abdel, K.S.; Melek, F.; Miyase, T.; Gaber, N.; Mina, S. New steroidal saponin from Agave angustifolia. Planta Med. 2010, 76, 446. [Google Scholar]
- Zeng, X.; Li, C.Y.; Wang, H.; Qiu, Q.; Qiu, G.; He, X. Unusual lipids and acylglucosylsterols from the roos of Livistona chinencis. Phytochem. Lett. 2013, 6, 36–40. [Google Scholar]
- Peana, A.T.; Moretti, M.D.; Manconi, V.; Desole, G.; Pippia, P. Anti-inflammatory activity of aqueous extracts and steroidal sapogenins of Agave americana. Planta Med. 1997, 63, 199–202. [Google Scholar] [PubMed]
- García, M.D.; Quílez, A.M.; Sáenz, M.T.; Martínez-Domínguez, M.E.; de la Puerta, R. Anti-inflammatory activity of Agave intermixta Trel. and Cissus sicyoides L., species used in the Caribbean tradicional medicine. J. Ethnopharmacol. 2000, 71, 395–400. [Google Scholar] [PubMed]
- Stanley, P.L.; Steiner, S.; Havens, M.; Tramposch, K.M. Mouse skin inflammation induced by multiple topical applications of 12-O-tetradecanoylphorbol-13-acetate. Skin Pharmacol. 1991, 4, 262–271. [Google Scholar] [PubMed]
- Gábor, M. Models of acute inflammation in the ear. Methods Mol. Biol. 2003, 225, 129–137. [Google Scholar] [PubMed]
- Chung, W.Y.; Park, J.H.; Kim, M.J.; Kim, H.O.; Hwang, J.K.; Lee, S.K.; Park, K.K. Xanthorrhizol inhibits 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced acute inflammation and two-stage mouse skin carcinogenesis by blocking the expression of ornithine decarboxylase, cyclooxygenase-2 and inducible nitric oxide synthase through mitogen-activated protein kinases and/or the nuclear factor-kB. Carcinogenesis 2007, 28, 1224–1231. [Google Scholar] [PubMed]
- Ho, Y.S.; Lai, C.S.; Liu, H.I.; Ho, S.Y.; Tai, C.; Pan, M.H.; Wang, Y.J. Dihydrolipoic acid inhibits skin tumor promotion through anti-inflammation and anti-oxidation. Biochem. Pharmacol. 2007, 73, 1786–1795. [Google Scholar] [PubMed]
- Song, H.Y.; Lee, J.A.; Ju, S.M.; Yoo, K.Y.; Won, M.H.; Kwon, H.J.; Eum, W.S.; Jang, S.H.; Choi, S.Y.; Park, J. Topical transduction of superoxide dismutase mediated by HIV-1 Tat protein transduction domain ameliorates 12-O-tetra-decanoylphprbol-13-acetate (TPA)-induced inflammation in mice. Biochem. Pharmacol. 2008, 75, 1346–1357. [Google Scholar]
- Xian, Y.F.; Lin, Z.X.; Xu, X.Y.; Su, Z.R.; Chen, J.N.; Lai, X.P.; Ip, S.P. Effect of Rhizoma polygonati on 12-O-tetradecanoylphorbol-acetate-induced ear edema in mice. J. Ethnopharmacol. 2012, 142, 851–856. [Google Scholar] [PubMed]
- Dayer, J.M. The process of identifying and understanding cytokines: From basic studies to treating rheumatic diseases. Best Pract. Res. Clin. Rheumatol. 2004, 18, 31–45. [Google Scholar] [PubMed]
- Bazzoni, F.; Tamassia, N.; Rossato, M.; Cassatella, M.A. Understanding the molecular mechanisms of the multifaceted IL-10-mediated anti-inflammatory response: Lessons from neutrophils. Eur. J. Immunol. 2010, 40, 2360–2368. [Google Scholar] [PubMed]
- Bouic, P.J.; Etsebeth, S.; Liebenberg, R.W.; Albrecht, C.F.; Pegel, K.; van Jaarsveld, P.P. Beta-sitosterol and beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: Implications for their use as an immunomodulatory vitamin combination. Int. J. Immunopharmacol. 1996, 18, 693–700. [Google Scholar] [PubMed]
- Lee, J.H.; Lee, J.Y.; Park, J.H.; Jung, H.S.; Kim, J.S.; Kang, S.S.; Kim, Y.S.; Han, Y. Immunoregulatory activity by daucosterol, a beta-sitosterol glycoside, induces protective Th1 immune response against disseminated Candidiasis in mice. Vaccine 2007, 25, 3834–3840. [Google Scholar] [PubMed]
- SPSS Statistical Product and Service Solutions, 11.0 version; SPSS Inc.: Chicago, IL, USA, 2001.
- Sample Availability: Samples of the compounds 3-O-(6'-O-Palmitoyl)-β-d-glucopyranosyl sitosterol are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Valle, E.; Herrera-Ruiz, M.; Salgado, G.R.; Zamilpa, A.; Ocampo, M.L.A.; Aparicio, A.J.; Tortoriello, J.; Jiménez-Ferrer, E. Anti-Inflammatory Effect of 3-O-[(6'-O-Palmitoyl)-β-D-glucopyranosyl Sitosterol] from Agave angustifolia on Ear Edema in Mice. Molecules 2014, 19, 15624-15637. https://doi.org/10.3390/molecules191015624
Hernández-Valle E, Herrera-Ruiz M, Salgado GR, Zamilpa A, Ocampo MLA, Aparicio AJ, Tortoriello J, Jiménez-Ferrer E. Anti-Inflammatory Effect of 3-O-[(6'-O-Palmitoyl)-β-D-glucopyranosyl Sitosterol] from Agave angustifolia on Ear Edema in Mice. Molecules. 2014; 19(10):15624-15637. https://doi.org/10.3390/molecules191015624
Chicago/Turabian StyleHernández-Valle, Elizabeth, Maribel Herrera-Ruiz, Gabriela Rosas Salgado, Alejandro Zamilpa, Martha Lucia Arenas Ocampo, Antonio Jiménez Aparicio, Jaime Tortoriello, and Enrique Jiménez-Ferrer. 2014. "Anti-Inflammatory Effect of 3-O-[(6'-O-Palmitoyl)-β-D-glucopyranosyl Sitosterol] from Agave angustifolia on Ear Edema in Mice" Molecules 19, no. 10: 15624-15637. https://doi.org/10.3390/molecules191015624





