Volatile Profile, Phytochemicals and Antioxidant Activity of Virgin Olive Oils from Croatian Autochthonous Varieties Mašnjača and Krvavica in Comparison with Italian Variety Leccino
Abstract
:1. Introduction
2. Results and Discussion
2.1. Basic Quality Parameters of Investigated Virgin Olive Oils
| No. | Cultivar | Acidity a (%) | Peroxide value b (mEq O2/kg) | K232 c | K270 d |
|---|---|---|---|---|---|
| 1. | Krvavica | 0.22 ± 0.01 | 3.35 ± 0.12 | 1.62 ± 0.1 | 0.12 ± 0.02 |
| 2. | Mašnjača | 0.14 ± 0.02 | 3.32 ± 0.16 | 1.58 ± 0.4 | 0.11 ± 0.01 |
| 3. | Leccino | 0.15 ± 0.01 | 5.37 ± 0.10 | 1.65 ± 0.8 | 0.14 ± 0.02 |
2.2. Volatile Compounds
| No. | Compounds | RI | Mašnjača | Krvavica | Leccino | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Area [%] | Area [%] | Area [%] | |||||||||
| Min | Max | Mean ± SD | Min | Max | Mean ± SD | Min | Max | Mean ± SD | |||
| 1. | Isoprene | <900 | 3.3 | 3.6 | 3.5 ± 0.15 | 1.6 | 1.7 | 1.7 ± 0.05 | 1.1 | 1.2 | 1.2 ± 0.05 |
| 2. | Ethyl acetate | <900 | 1.1 | 1.2 | 1.2 ± 0.50 | 0.8 | 0.8 | 0.8 ± 0.00 | - | - | - |
| 3. | Pent-1-en-3-one | <900 | 5.6 | 6.2 | 5.9 ± 0.30 | 2.0 | 2.0 | 2.0 ± 0.00 | 4.2 | 4.5 | 4.4 ± 0.15 |
| 4. | Pentan-3-one | <900 | 5.2 | 6.1 | 5.7 ± 0.45 | 3.8 | 3.9 | 3.9 ± 0.05 | - | - | - |
| 5. | (E)-Pent-2-enal | <900 | 0.6 | 0.6 | 0.6 ± 0.00 | - | - | - | 0.3 | 0.3 | 0.3 ± 0.00 |
| 6. | (Z)-Hex-3-enal | <900 | 21.9 | 25.0 | 23.5 ± 1.50 | 15.0 | 15.0 | 15.0 ± 0.00 | - | - | - |
| 7. | Hexanal | <900 | - | - | - | 8.8 | 9.4 | 9.1 ± 0.30 | 1.1 | 1.1 | 1.1 ± 0.00 |
| 8. | (E)-Hex-2-enal | <900 | 27.6 | 28.9 | 28.3 ± 0.65 | 41.6 | 44.4 | 43.0 ± 1.40 | 73.4 | 74.0 | 73.7 ± 0.30 |
| 9. | 3-Ethyloct-1,5-diene(isomer I) | 939 | 13.8 | 14.4 | 14.1 ± 0.30 | 3.3 | 4.2 | 3.8 ± 0.45 | 7.1 | 7.5 | 7.3 ± 0.20 |
| 10. | 3-Ethyloct-1,5-diene(isomer II) | 997 | 10.7 | 10.9 | 10.8 ± 0.10 | 3.3 | 4.1 | 3.7 ± 0.40 | 5.1 | 6.0 | 5.6 ± 0.45 |
| 11. | trans-β-Ocimene | 1054 | 0.6 | 0.7 | 0.7 ± 0.05 | 1.9 | 2.5 | 2.2 ± 0.30 | - | - | - |
| 12. | α-Copaene | 1380 | 1.6 | 2.2 | 1.9 ± 0.30 | 0.8 | 1.1 | 1.0 ± 0.15 | 0.0 | 0.1 | 0.1 ± 0.05 |
| (92.0%–99.8%) | (82.9%–89.1%) | (92.3%–94.7%) | |||||||||
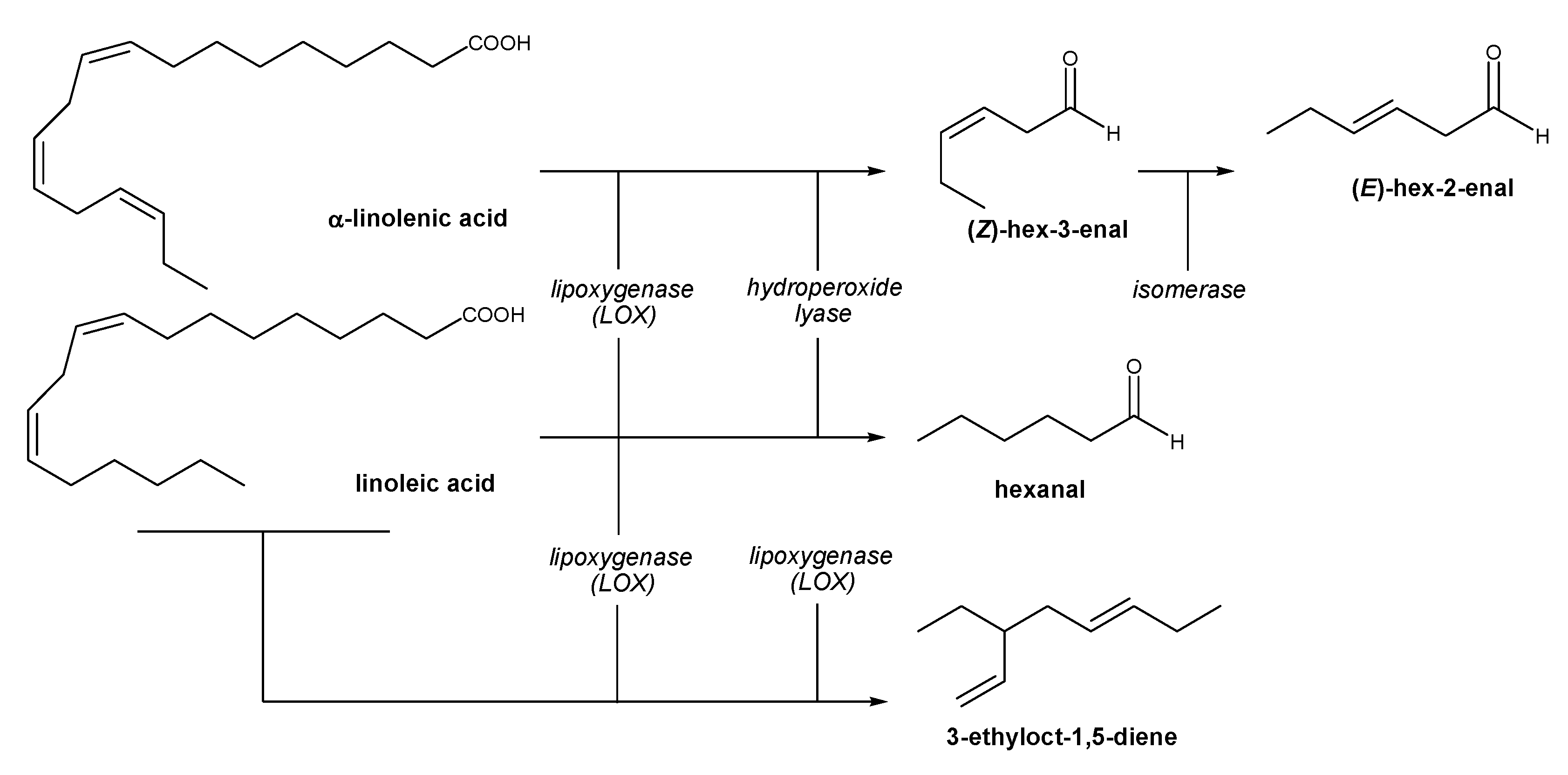
2.3. Tocopherols, Chlorophylls and Carotenoids
| VOO Sample | α-Tocopherol (mg/kg) | γ-Tocopherol (mg/kg) | Chlorophylls (mg/kg) | Carotenoids (mg/kg) |
|---|---|---|---|---|
| Krvavica | 176.4 ± 0.9 | 33.7 ± 0.1 | 4.6 ± 0.2 | 1.8 ± 0.1 |
| Mašnjača | 223.9 ± 0.4 | 36.1 ± 0.2 | 4.2 ± 0.2 | 2.1 ± 0.1 |
| Leccino | 312.4 ± 0.3 | 42.5 ± 0.1 | 7.3 ± 0.6 | 4.2 ± 0.1 |
2.4. Total Phenolic Content and Phenolic Compounds Determined by LC-DAD
| VOO sample | Total phenols (mg GAE/kg) | DPPH (mmol TEAC/kg) |
|---|---|---|
| Krvavica | 121.4 ± 1.9 | 0.5 ± 0.0 |
| Mašnjača | 143.4 ± 4.9 | 0.5 ± 0.0 |
| Leccino | 246.6 ± 2.6 | 1.3 ± 0.0 |
| No. | Compounds | RT | LOD | LOQ | Mašnjača | Krvavica | Leccino | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | |||||||||
| Min | Max | Mean ± SD | Min | Max | Mean ± SD | Min | Max | Mean ± SD | |||||
| 1. | Hydroxytyrosol a | 10.9 | 0.34 | 1.02 | 3.9 | 5.0 | 4.4 ± 0.8 | 14.9 | 21.9 | 18.4 ± 5.0 | 4.3 | 6.8 | 5.5 ± 1.8 |
| 2. | Tyrosol a | 13.4 | 0.40 | 1.21 | 3.9 | 4.9 | 4.4 ± 0.7 | 18.9 | 24.7 | 21.8 ± 4.1 | 3.0 | 4.8 | 3.9 ± 1.3 |
| 3. | Vanillic acid a | 15.1 | 0.04 | 0.11 | nd | 0.1 | 0.4 | 0.3 ± 0.2 | nd | ||||
| 4. | p-Coumaric acid a | 19.2 | 0.23 | 0.70 | nd | 1.2 | 1.3 | 1.3 ± 0.1 | nd | ||||
| 5. | 3,4-DHPEA-EDA b | 22.4 | 0.94 | 2.86 | 60.2 | 64.2 | 62.2 ± 2.8 | 20.5 | 29.2 | 24.8 ± 6.2 | 146.5 | 159.7 | 153.1 ± 9.3 |
| 6. | Pinoresinol a | 25.2 | 0.16 | 0.47 | 0.5 | 2.1 | 1.3 ± 1.1 | 2.1 | 2.4 | 2.3 ± 0.2 | 2.7 | 3.2 | 3.0 ± 0.4 |
| 7. | Luteolin a | 25.7 | 0.46 | 1.40 | 4.8 | 5.1 | 4.9 ± 0.2 | 8.6 | 8.7 | 8.6 ± 0.0 | 5.0 | 6.2 | 5.6 ± 0.9 |
| 8. | p-HPEA-EDA b | 26.3 | 0.94 | 2.86 | 45.2 | 46.0 | 45.6 ± 0.6 | 40.2 | 45.6 | 42.9 ± 3.9 | 124.1 | 141.7 | 132.9 ± 12.5 |
| 9. | Apigenin a | 28.2 | 0.36 | 1.10 | 2.1 | 2.5 | 2.3 ± 0.3 | 2.7 | 2.9 | 2.8 ± 0.2 | 2.1 | 2.6 | 2.3 ± 0.3 |
| 10. | 3,4-HPEA-EA b | 28.5 | 0.94 | 2.86 | 60.9 | 85.4 | 73.2 ± 17.3 | 73.0 | 95.5 | 84.2 ± 15.9 | 110.6 | 138.2 | 124.4 ± 19.5 |
2.5. Antioxidant Activity of the Hydrophilic Fraction of the Olive Oils (DPPH Assay)
3. Experimental
3.1. Olive Oil Samples and Preparation of Their Hydrophilic Fractions
3.2. Determination of Oil Quality Parameters
3.3. Headspace Solid-Phase Microextraction (HS-SPME)
3.4. Gas Chromatography and Mass Spectrometry (GC-MS)
3.5. Liquid Chromatography and Diode Array Detector (LC-DAD)
3.6. Determination of Tocopherols (Vitamin E)
3.7. Determination of Total Chlorophylls and Carotenoids
3.8. Determination of Total Phenolic Content (Folin-Ciocalteu Assay)
3.9. Free Radical Scavenging Activity (DPPH Assay)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Angerosa, F.; Mostallino, R.; Basti, C.; Vito, R. Virgin olive oil odor notes: Their relationships with volatile compounds from the lipoxygenase pathway and secoiridoid compounds. Food Chem. 2000, 68, 283–287. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G., Jr.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Angerosa, F. Influence of volatile compounds on virgin olive oil quality evaluated by analytical approaches and sensor panels. Eur. J. Lipid Sci. Technol. 2002, 104, 639–660. [Google Scholar] [CrossRef]
- Angerosa, F.; Basti, C.; Vito, R. Virgin olive oil volatile compounds from lipoxygenase pathway and characterization of some Italian cultivars. J. Agric. Food. Chem. 1999, 47, 836–839. [Google Scholar] [CrossRef]
- Luna, G.; Morales, M.T.; Aparicio, R. Characterization of 39 varietal virgin olive oils by their volatile composition. Food Chem. 2006, 98, 243–252. [Google Scholar] [CrossRef]
- Baccouri, O.; Bendini, A.; Cerretani, L.; Guerfel, M.; Baccouri, B.; Lercker, G.; Zorrouk, M.; Ben Miled, D.D. Comparative study on volatile compounds from Tunisian and Sicilian monovarietal virgin olive oils. Food Chem. 2008, 111, 322–328. [Google Scholar] [CrossRef]
- Aparicio, R.; Morales, M.T. Characterization of olive ripeness by green aroma compounds of virgin olive oil. J. Agric. Food. Chem. 1998, 46, 1116–1122. [Google Scholar] [CrossRef]
- Di Giovacchino, L.; Costantini, N.; Serraiocco, A.; Surricchio, G.; Basti, C. Natural antioxidants and volatile compounds of virgin olive oils obtained by two or three-phases centrifugal decamters. Eur. J. Lipid Sci. Technol. 2001, 103, 279–285. [Google Scholar] [CrossRef]
- Vichi, S.; Guadayol, J.M.; Caixach, J.; López-Tamames, E.; Buxaderas, S. Comparative study of different extraction techniques for the analysis of virgin olive oil aroma. Food Chem. 2007, 105, 1171–1178. [Google Scholar] [CrossRef]
- Ranalli, A.; de Mattia, G.; Patumi, M.; Proietti, P. Quality of virgin olive oil as influenced by origin area. Grasas Aceites 1999, 50, 249–259. [Google Scholar] [CrossRef]
- Vichi, S.; Pizzale, L.; Conte, L.S.; Buxaderas, S.; Lopez-Tamames, E. Solid-phase microextraction in the analysis of virgin olive oil volatile fraction: Characterization of virgin olive oils from two distinct geographical areas of northern Italy. J. Agric. Food. Chem. 2003, 51, 6572–6577. [Google Scholar] [CrossRef]
- Dabbou, S.; Dabbou, S.; Selvaggini, R.; Urbani, S.; Taticchi, A.; Servili, M.; Hammami, M. Comparison of the Chemical composition and the organoleptic profile of virgin olive oil from two wild and two cultivated tunisian olea europaea. Chem. Biodivers. 2011, 8, 189–202. [Google Scholar] [CrossRef]
- Cioffi, G.; Pesca, M.S.; de Caprariis, P.; Braca, A.; Severino, L.; de Tommasi, N. Phenolic compounds in olive oil and olive pomace from Cilento (Campania, Italy) and their antioxidant activity. Food Chem. 2010, 121, 105–111. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura- Carretero, A.; Fernández-Gutiérrez, A. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical method. An overview of the last decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef]
- Giuffrida, D.; Salvo, F.; Salvo, A.; Cossignani, L.; Dugo, G. Pigments profile in monovarietal virgin olive oils from various Italian olive varieties. Food Chem. 2011, 124, 1119–1123. [Google Scholar] [CrossRef]
- Carrasco-Pancorbo, A.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Del Carlo, M.; Gallina-Toschi, T.; Lercker, G.; Compagnone, D.; Fernandez-Gutierrez, A. Evaluation of the antioxidant capacity of individual phenolic compounds in virgin olive oil. J. Agric. Food Chem. 2005, 53, 8918–8925. [Google Scholar] [CrossRef]
- Vinha, A.F.; Ferreres, F.; Silva, B.M.; Valentao, P.; Gonçalves, A.; Pereira, J.A.; Oliveira, M.B.; Seabra, R.M.; Andrade, P.B. Phenolic profiles of Portuguese olive fruits (Olea. europaea L.): Influences of cultivar and geographical origin. Food Chem. 2005, 89, 561–568. [Google Scholar] [CrossRef]
- International Olive Oil Council. Trade standards for Olive Oil and Olive-Pomace Oils, COI/T.15/NC No 3/Rev.7. 2012.
- Commission Regulation (EC) No 1989/2003, amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-pomace oil and on the relevant methods of analysis. Official Journal of the European Union, No L 295/57. 13 November 2003.
- Baccouri, B.; Ben Temime, S.; Campeol, E.; Cioni, P.L.; Daoud, D.; Zarrouk, M. Application of solid-phase microextraction to the analysis of volatile compounds in virgin olive oils from five new cultivars. Food Chem. 2007, 102, 850–856. [Google Scholar] [CrossRef]
- Angerosa, F.; Mostallino, R.; Basti, C.; Vito, R. Influence of malaxation temperature and time on the quality of virgin olive oils. Food Chem. 2001, 72, 19–28. [Google Scholar] [CrossRef]
- Koprivnjak, O.; Conte, L.S.; Bencic, D.; Totis, N. Application of solid-phase microextraction of virgin olive oil volatiles on varieties characterization. Riv. Ital. Sost. Grasse 2003, 80, 35–40. [Google Scholar]
- Brkić Bubola, K.; Koprivnjak, O.; Sladonja, B.; Lukić, I. Volatile compounds and sensory profiles of monovarietal virgin olive oil from Buža, Črna and Rosinjola cultivars in Istria (Croatia). Food Technol. Biotechnol. 2012, 50, 192–198. [Google Scholar]
- Boskou, D. Olive Oil - Chemistry and Technology, 2nd ed.; AOCS Press: Champaign, IL, USA, 2006. [Google Scholar]
- Dabbou, S.; Rjiba, I.; Nakbi, A.; Gazzah, N.; Issaoui, M.; Hammami, M. Compositional quality of virgin olive oils from cultivars introduced in Tunisian arid zones in comparison to Chemlali cultivars. Sci. Hort. 2010, 124, 122–127. [Google Scholar] [CrossRef]
- Ranalli, A.; Angerosa, F. Integral centrifuges for olive oil extraction. The qualitative characteristics of Products. J. Am. Oil Chem. Soc. 1996, 73, 417–422. [Google Scholar] [CrossRef]
- Manzi, P.; Panfili, G.; Esti, M.; Pizzoferrato, L. Natural antioxidants in the unsaponifiable fraction of virgin olive oils from different cultivars. J. Sci. Food Agric. 1998, 77, 115–120. [Google Scholar] [CrossRef]
- Salvador, M.; Aranda, F.; Fregapane, G. Chemical composition of commercial cornicabra virginolive oils from 1995/96 and 1996/97 crops. J. Am. Oil Chem. Soc. 1998, 75, 1305–1311. [Google Scholar] [CrossRef]
- Psomiadou, E.; Tsimidou, M.; Boskou, D. α-Tocopherol content of greek virgin olive oils. J. Agric. Food Chem. 2000, 48, 1770–1775. [Google Scholar] [CrossRef]
- Koprivnjak, O.; Conte, L. Specific components of virgin olive oil as active participants in oxidative processes. Food Technol. Biotech. 1998, 36, 229–234. [Google Scholar]
- Dağdelen, A.; Tümen, G.; Özcan, M.M.; Dündar, E. Phenolics profiles of olive fruits (Olea. europaea L.) and oils from Ayvalık, Domat and Gemlik varieties at different ripening stages. Food Chem. 2013, 136, 41–45. [Google Scholar] [CrossRef]
- Vichi, S.; Romero, A.; Gallardo-Chacón, J.; Tous, J.; López-Tamames, E.; Buxaderas, S. Volatile phenols in virgin olive oils: Influence of olive variety on their formation during fruits storage. Food Chem. 2009, 116, 651–656. [Google Scholar] [CrossRef]
- Guerfel, M.; Ben Mansour, M.; Ouni, Y.; Boujnah, D.; Zarrouk, M. Compositional quality of virgin olive oils from cultivars introduced in two Tunisian locations. Afr. J. Agric. Res. 2012, 7, 2469–2474. [Google Scholar]
- Tsimidou, M. Polyphenols and quality of virgin olive oil in retrospect. Ital. J. Food Sci. 1998, 10, 99–116. [Google Scholar]
- Fogliano, V.; Ritieni, A.; Monti, S.M.; Gallo, M.; Medaglia, D.D.; Ambrosino, M.L. Antioxidant activity of virgin olive oil phenolic compounds in a micellar system. J. Sci. Food Agric. 1999, 79, 1803–1808. [Google Scholar] [CrossRef]
- Visioli, F.; Gallì, C. The effect of minor constituents of olive oil on cardiovascular disease: New findings. Nutr. Rev. 1998, 56, 142–147. [Google Scholar] [CrossRef]
- Uceda, M.; Frias, L. Trend of the quality and quantitative composition of olive fruit oil during ripening. In Proceedings of the International Meeting on Olive Oil, Cordoba, Spain, 1975; pp. 25–46.
- Tuberoso, C.I.G.; Kowalczyk, A.; Sarritzu, E.; Cabras, P. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem. 2007, 103, 1494–1501. [Google Scholar] [CrossRef]
- García-Villalba, R.; Carrasco-Pancorbo, A.; Oliveras-Ferraros, C.; Vázquez-Martín, A.; Menéndez, J.A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Characterization and quantification of phenolic compounds of extra-virgin olive oils with anticancer properties by a rapid and resolutive LC-ESI-TOF MS method. J. Pharm. Biomed. Anal. 2010, 51, 416–429. [Google Scholar] [CrossRef]
- Dierkes, G.; Krieger, S.; Dück, R.; Bongartz, A.; Schmitz, O.J.; Hayen, H. High-Performance Liquid Chromatography-Mass Spectrometry profiling of phenolic compounds for evaluation of olive oil bitterness and pungency. J. Agric. Food Chem. 2012, 60, 7597–7606. [Google Scholar] [CrossRef]
- ICH Topic Q2 (R1) (1995) Validation of analytical procedures: Text and methodology. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf. (accessed on 10 November 2013).
- Tuberoso, C.I.G.; Rosa, A.; Bifulco, E.; Melis, M.P.; Atzeri, A.; Pirisi, F.M.; Dessì, M.A. Chemical composition and antioxidant activities of Myrtus. communis L. berries extracts. Food Chem. 2010, 123, 1242–1251. [Google Scholar] [CrossRef]
- Sample Availability: The samples of the oils are available for limited time from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Šarolić, M.; Gugić, M.; Tuberoso, C.I.G.; Jerković, I.; Šuste, M.; Marijanović, Z.; Kuś, P.M. Volatile Profile, Phytochemicals and Antioxidant Activity of Virgin Olive Oils from Croatian Autochthonous Varieties Mašnjača and Krvavica in Comparison with Italian Variety Leccino. Molecules 2014, 19, 881-895. https://doi.org/10.3390/molecules19010881
Šarolić M, Gugić M, Tuberoso CIG, Jerković I, Šuste M, Marijanović Z, Kuś PM. Volatile Profile, Phytochemicals and Antioxidant Activity of Virgin Olive Oils from Croatian Autochthonous Varieties Mašnjača and Krvavica in Comparison with Italian Variety Leccino. Molecules. 2014; 19(1):881-895. https://doi.org/10.3390/molecules19010881
Chicago/Turabian StyleŠarolić, Mladenka, Mirko Gugić, Carlo Ignazio Giovanni Tuberoso, Igor Jerković, Marko Šuste, Zvonimir Marijanović, and Piotr Marek Kuś. 2014. "Volatile Profile, Phytochemicals and Antioxidant Activity of Virgin Olive Oils from Croatian Autochthonous Varieties Mašnjača and Krvavica in Comparison with Italian Variety Leccino" Molecules 19, no. 1: 881-895. https://doi.org/10.3390/molecules19010881
APA StyleŠarolić, M., Gugić, M., Tuberoso, C. I. G., Jerković, I., Šuste, M., Marijanović, Z., & Kuś, P. M. (2014). Volatile Profile, Phytochemicals and Antioxidant Activity of Virgin Olive Oils from Croatian Autochthonous Varieties Mašnjača and Krvavica in Comparison with Italian Variety Leccino. Molecules, 19(1), 881-895. https://doi.org/10.3390/molecules19010881







