Solution Properties and in Vitro Anti-Tumor Activities of Polysaccharides from Longan Pulp
Abstract
:1. Introduction
2. Results and Discussion
2.1. Aqueous Solution Properties of Longan Polysaccharides
2.1.1. Root-Mean-Square Radius of Gyration
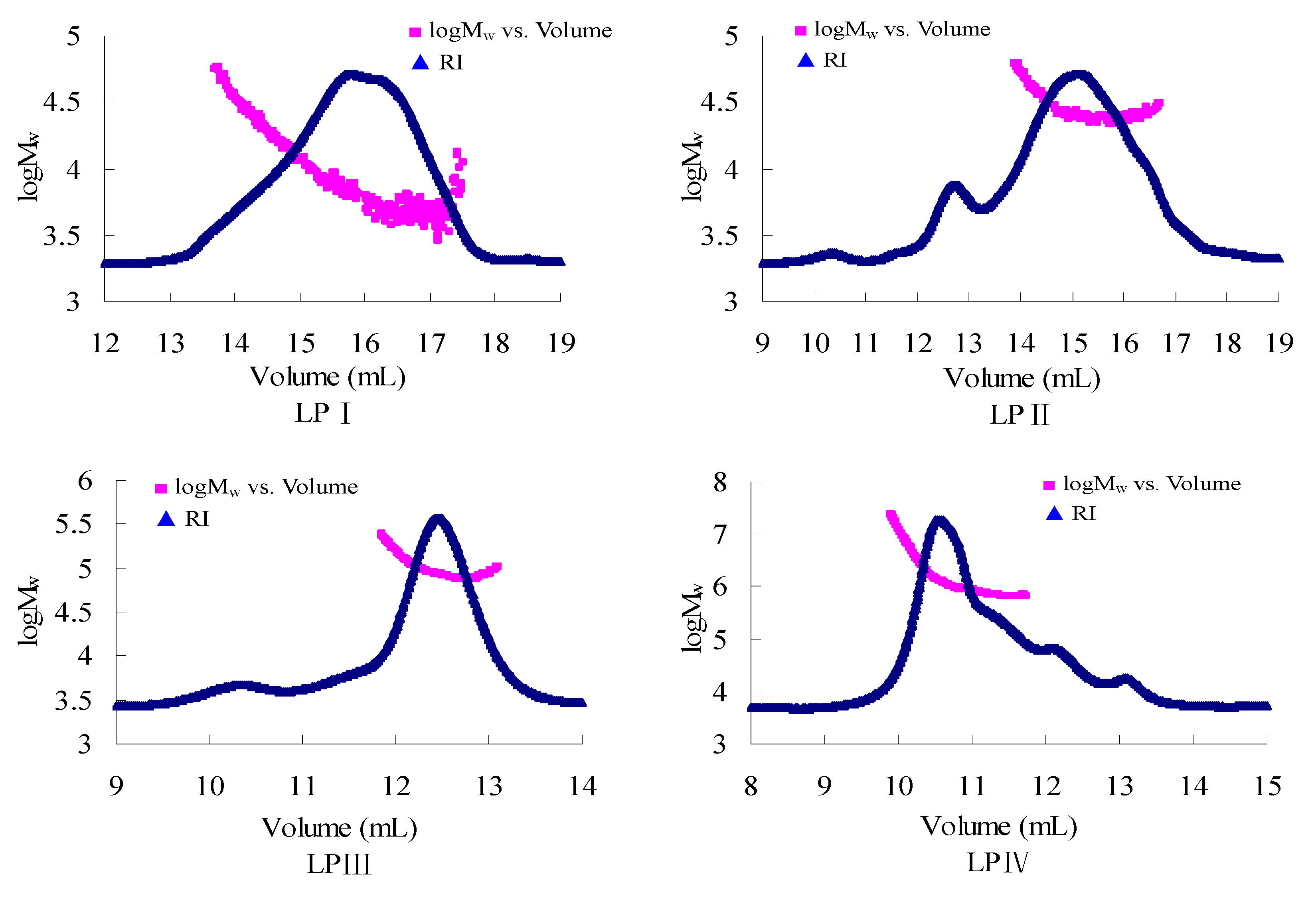
| Polysaccharide | <S2>z1/2 (nm) | v | [η] (mL/g) | k' |
|---|---|---|---|---|
| LPI | 43.3 | 0.04 ± 0.00 | 9.945 | 1.1879 |
| LPII | 62.6 | 0.50 ± 0.04 | 25.38 | 0.1951 |
| LPIII | 43.2 | 0.52 ± 0.03 | 308.2 | 0.0048 |
| LPIV | 77.3 | 0.02 ± 0.00 | 452.1 | 0.0047 |

2.1.2. Intrinsic Viscosity
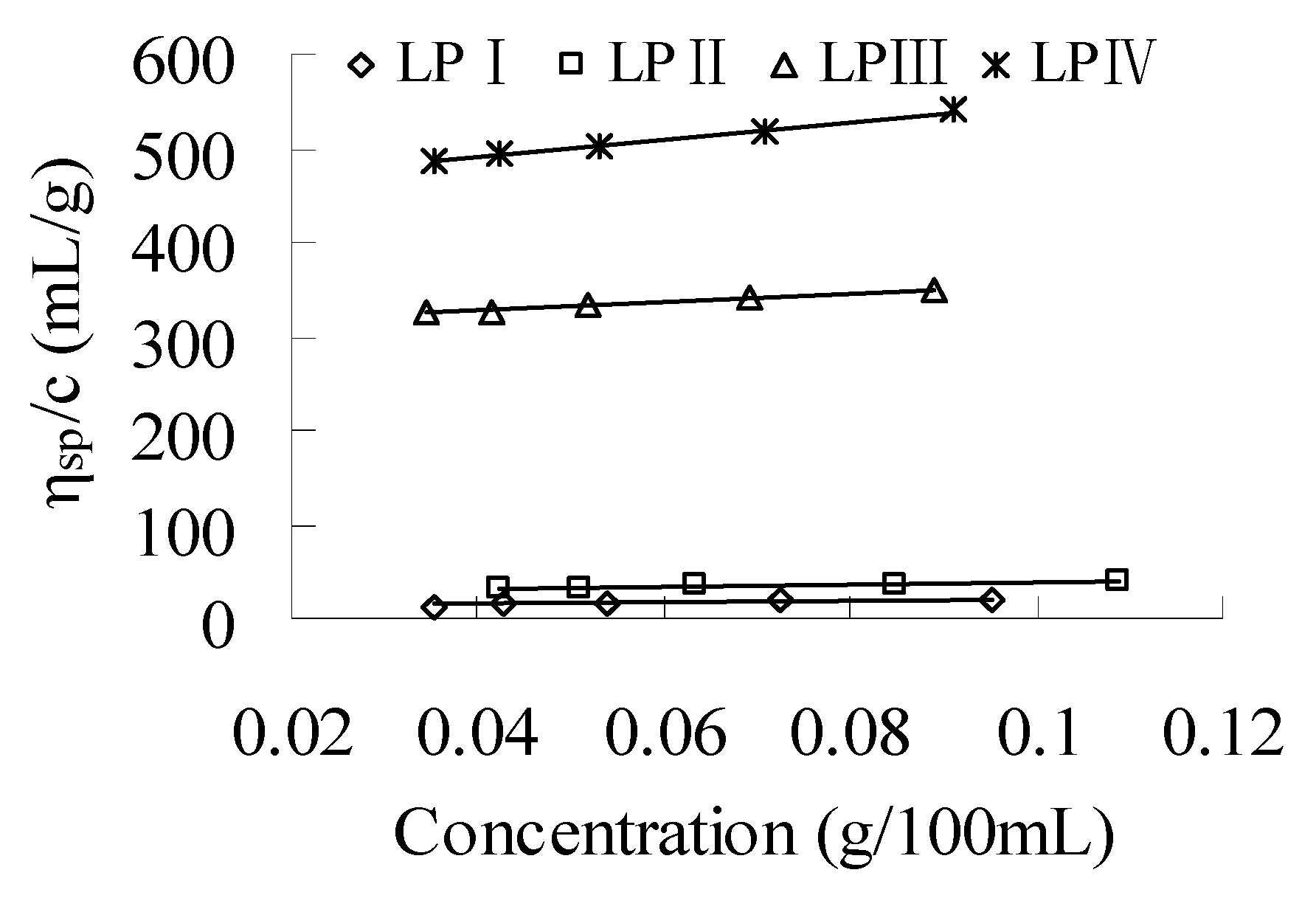






2.2. Helical Structures of Longan Polysaccharides

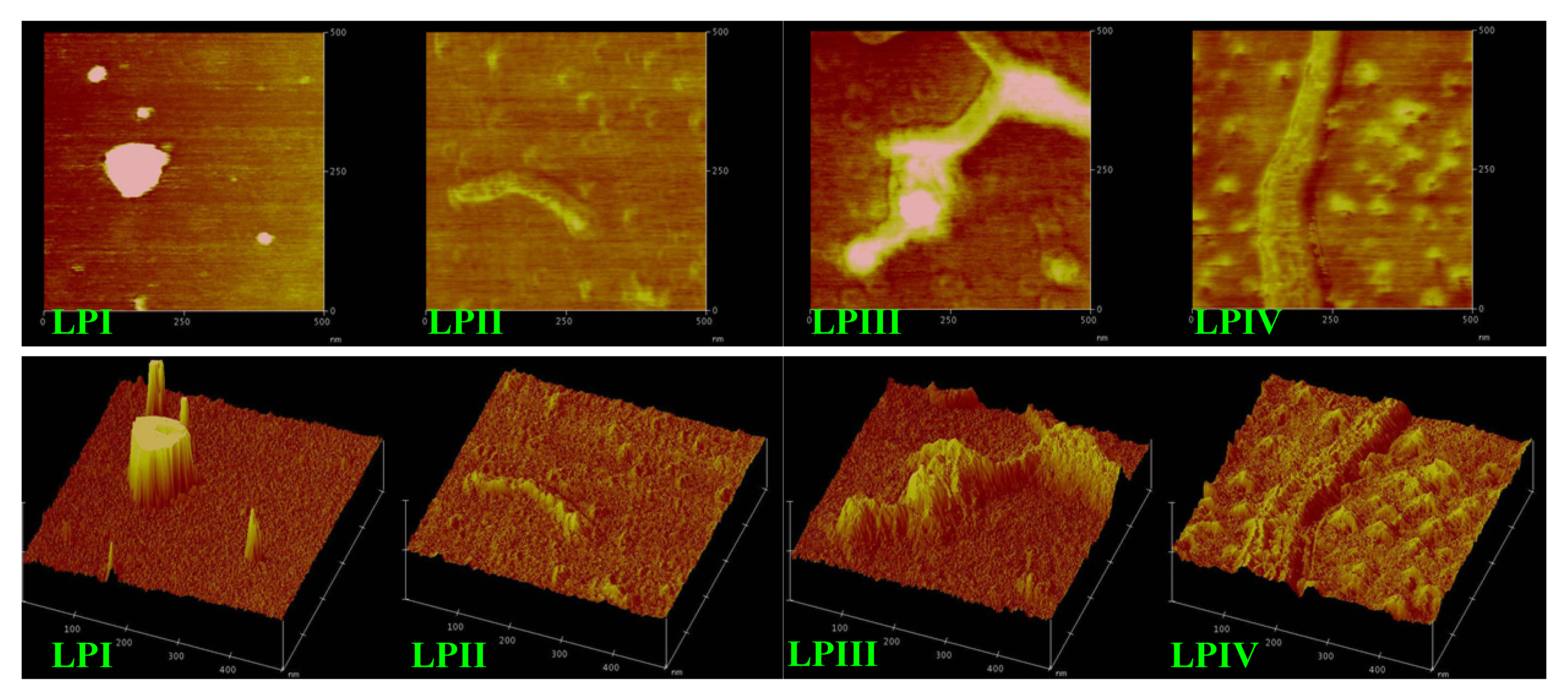
2.3. Atomic Force Microscopy Images of Longan Polysaccharides
2.4. Anti-Tumor Activities of Longan Polysaccharides
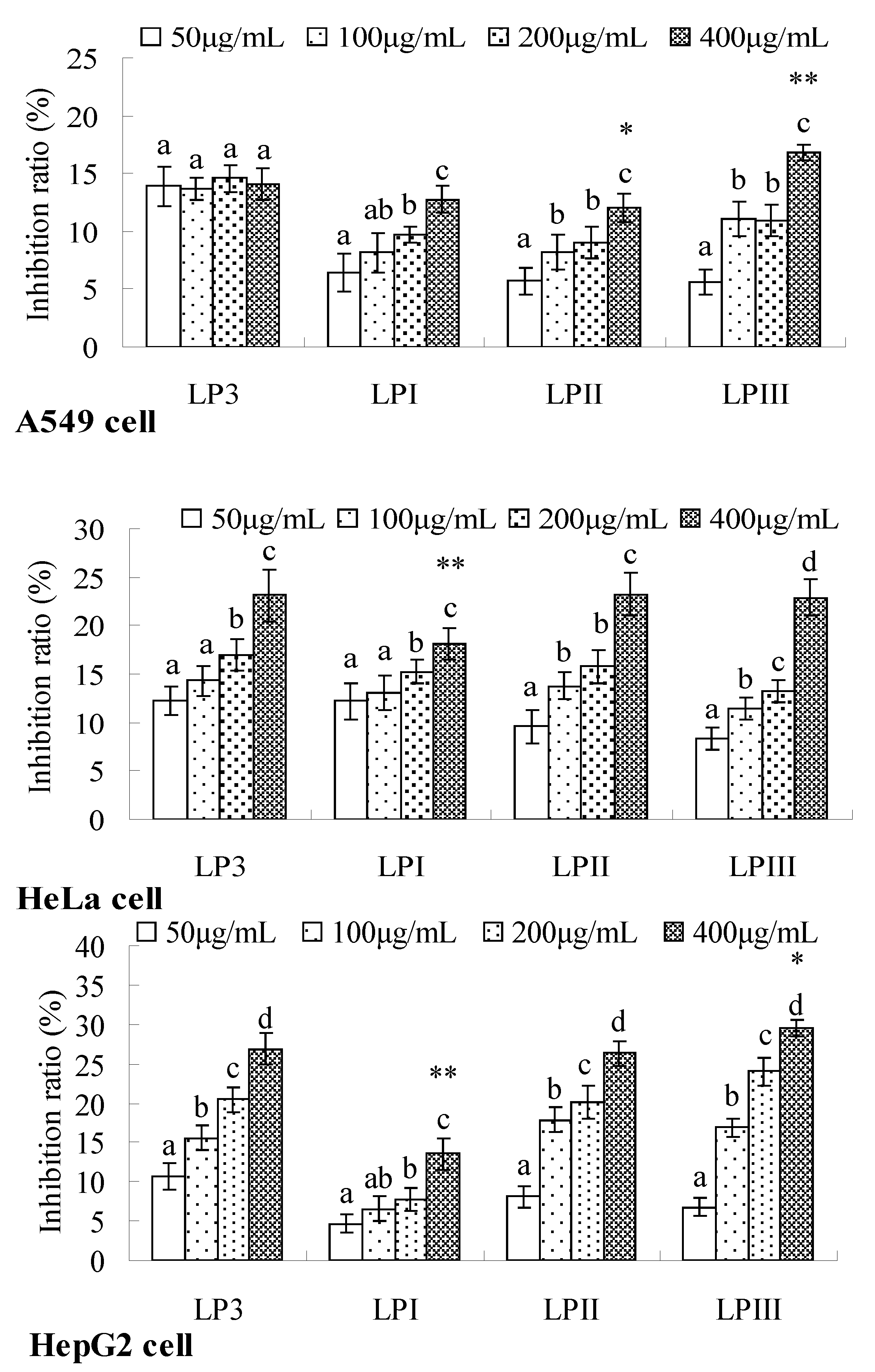
3. Experimental
3.1. Preparation of Longan Pulp Polysaccharide Fractions
3.2. Aqueous Solution Property Analysis
3.3. Anti-Tumor Activity Evaluation
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef]
- Lazareva, E.B.; Spiridonova, T.G.; Chernega, E.N.; Plesskaia, L.G.; Grunenkova, I.V.; Smirnov, S.V.; Men’shikov, D.D. Topical pectins for the treatment of burn wounds. Antibiot. Khimioter. 2002, 47, 9–13. [Google Scholar]
- Ovodov, I.S. Polysaccharides of flower plants: Structure and physiological activity. Bioorg. Khim. 1998, 24, 483–501. [Google Scholar]
- Yang, L.; Zhang, L.M. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr. Polym. 2009, 76, 349–361. [Google Scholar] [CrossRef]
- Bao, X.F.; Wang, X.S.; Dong, Q.; Fang, J.N.; Li, X.Y. Structural features of immunologically active polysaccharides from Ganoderma lucidum. Phytochemistry 2002, 59, 175–181. [Google Scholar]
- Im, S.A.; Oh, S.T.; Song, S.; Kim, M.R.; Kim, D.S.; Woo, S.S.; Jo, T.H.; Park, Y.I.; Lee, C.K. Identification of optimal molecular size of modified Aloe polysaccharides with maximum immunomodulatory activity. Int. Immunopharmacol. 2005, 5, 271–279. [Google Scholar] [CrossRef]
- Surenjav, U.; Zhang, L.; Xu, X.; Zhang, X.; Zeng, F. Effects of molecular structure on antitumor activities of (1→3)-β-d-glucans from different Lentinus edodes. Carbohydr. Polym. 2006, 63, 97–104. [Google Scholar] [CrossRef]
- Ohno, N.; Miura, N.N.; Chiba, N.; Adachi, Y.; Yadomae, T. Comparison of the immunopharmacological activities of triple and single-helical schizophyllan in mice. Biol. Pharm. Bull. 1995, 18, 1242–1247. [Google Scholar] [CrossRef]
- Kishida, E.; Sone, Y.; Misaki, A. Effects of branch distribution and chemical modifications of antitumor (1→3)-β-glucans. Carbohydr. Polym. 1992, 17, 89–95. [Google Scholar] [CrossRef]
- Falch, B.H.; Espevik, T.; Ryan, L.; Stokke, B.T. The cytokine stimulating activity of (1→3)-β-d-glucans is dependent on the triple helix conformation. Carbohydr. Res. 2000, 329, 587–596. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Xu, X.; Zeng, F. Correlation between antitumor activity, molecular weight, and conformation of lentinan. Carbohydr. Res. 2005, 340, 1515–1521. [Google Scholar] [CrossRef]
- Tao, Y.; Xu, W. Microwave-assisted solubilization and solution properties of hyperbranched polysaccharide. Carbohydr. Res. 2008, 343, 3071–3078. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, L.; Cheung, P.C. Physicochemical properties and antitumor activities of water-soluble native and sulfated hyperbranched mushroom polysaccharides. Carbohydr. Res. 2006, 341, 2261–2269. [Google Scholar] [CrossRef]
- Huang, Q.; Jin, Y.; Zhang, L.N.; Cheung, P.C.K.; Kennedy, J.F. Structure, molecular size and antitumor activities of polysaccharides from Poria cocos mycelia produced in fermenter. Carbohydr. Polym. 2007, 70, 324–333. [Google Scholar] [CrossRef]
- Lee, J.S.; Kwon, J.S.; Yun, J.S.; Pahk, J.W.; Shin, W.C.; Lee, S.Y.; Hong, E.K. Structural characterization of immunostimulating polysaccharide from cultured mycelia of Cordyceps militaris. Carbohydr. Polym. 2010, 80, 1011–1017. [Google Scholar] [CrossRef]
- Yang, C.; He, N.; Ling, X.; Ye, M.; Zhang, C.; Shao, W.; Yao, C.; Wang, Z.; Li, Q. The isolation and characterization of polysaccharides from longan pulp. Sep. Purif. Technol. 2008, 63, 226–230. [Google Scholar] [CrossRef]
- Park, S.J.; Park, D.H.; Kim, D.H.; Lee, S.; Yoon, B.H.; Jung, W.Y.; Lee, K.T.; Cheong, J.H.; Ryu, J.H. The memory-enhancing effects of Euphoria longan fruit extract in mice. J. Ethnopharmacol. 2010, 128, 160–165. [Google Scholar] [CrossRef]
- Zhong, K.; Wang, Q.; He, Y.; He, X. Evaluation of radicals scavenging, immunity-modulatory and antitumor activities of longan polysaccharides with ultrasonic extraction on in S180 tumor mice models. Int. J. Biol. Macromol. 2010, 47, 356–360. [Google Scholar] [CrossRef]
- Yi, Y.; Liao, S.T.; Zhang, M.W.; Shi, J.; Zhang, R.F.; Deng, Y.Y.; Wei, Z.C. Physicochemical characteristics and immunomodulatory activities of three polysaccharide-protein complexes of longan pulp. Molecules 2011, 16, 6148–6164. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, M.W.; Liao, S.T.; Zhang, R.F.; Deng, Y.Y.; Wei, Z.C.; Tang, X.J.; Zhang, Y. Structural features and immunomodulatory activities of polysaccharides of longan pulp. Carbohydr. Polym. 2012, 87, 636–643. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, Y.; Li, X.; Zhang, L. Molecular mass and chain conformations of Rhizoma Panacis Japonici polysaccharides. Carbohydr. Polym. 2009, 78, 596–601. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Y.; Zhang, L. Chemical modification and antitumor activities of two polysaccharide-protein complexes from Pleurotus tuber-regium. Int. J. Biol. Macromol. 2009, 45, 109–115. [Google Scholar] [CrossRef]
- Hanselmann, R.; Burchard, W.; Ehrat, M.; Widmer, H.M. Structural properties of fractionated starch polymers and their dependence on the dissolution process. Macromolecules 1996, 29, 3277–3282. [Google Scholar] [CrossRef]
- Badiger, M.V.; Gupta, N.R.; Eckelt, J.; Wolf, B.A. Intrinsic viscosity of aqueous solutions of carboxymethyl guar in the presence and in the absence of salt. Macromol. Chem. Phys. 2008, 209, 2087–2093. [Google Scholar] [CrossRef]
- Ma, C.; Guan, S.H.; Yang, M.; Liu, X.; Guo, D.A. Differential protein expression in mouse splenic mononuclear cells treated with polysaccharides from spores of Ganoderma lucidum. Phytomedicine 2008, 15, 268–276. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, L.; Zhang, Y.; Xu, X.; Kennedy, J.F. Solution properties of water-insoluble polysaccharides from the mycelium of Ganoderma tsugae. Carbohydr. Polym. 2005, 59, 351–356. [Google Scholar] [CrossRef]
- Park, I.I.H.; Choi, E.J. Characterization of branched polyethyleneimine by laser light scattering and viscometry. Polymer 1996, 37, 313–319. [Google Scholar] [CrossRef]
- Yamakawa, H.; Fujii, M. Intrinsic viscosity of wormlike chains. Determination of the shift factor. Macromolecules 1974, 7, 128–135. [Google Scholar] [CrossRef]
- Li, B.; Xie, B.; Kennedy, J.F. Studies on the molecular chain morphology of konjac glucomannan. Carbohydr. Polym. 2006, 64, 510–515. [Google Scholar] [CrossRef]
- Nakata, M.; Kawaguchi, T.; Kodama, Y.; Konno, A. Characterization of curdlan in aqueous sodium hydroxide. Polymer 1998, 39, 1475–1481. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, L. Solution properties of (1→3)-α-D-glucan and its sulfated derivative from Poria cocos mycelia via fermentation tank. Biopolymers 2005, 79, 28–38. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Dong, J.; Guo, J.; Song, Y.; Cheung, P.C.K. Chemical structure and chain conformation of the water-insoluble glucan isolated from Pleurotus tuber-regium. Biopolymers 2001, 59, 457–464. [Google Scholar] [CrossRef]
- Kido, S.; Nakanishi, T.; Norisuye, T. Ordered conformation of succinoglycan in aqueous sodium chloride. Biomacromolecules 2001, 2, 952–957. [Google Scholar] [CrossRef]
- Hara, C.; Kiho, T.; Ukai, S. A branched (1→3)-β-d-glucan from a sodium carbonate extract of Dictyophora indusiata fisch. Carbohydr. Res. 1983, 117, 201–213. [Google Scholar] [CrossRef]
- Ogawa, K.; Wanatabe, T.; Tsurugi, J.; Ono, S. Conformational behavior of a gel-forming (1→3)-β-d-glucan in alkaline solution. Carbohydr. Res. 1972, 23, 399–405. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, M.W.; Liao, S.T.; Zhang, R.F.; Deng, Y.Y.; Wei, Z.C.; Tang, X.J.; Zhang, Y. Effects of alkali dissociation on the molecular conformation and immunomodulatory activity of longan pulp polysaccharide LPI. Carbohydr. Polym. 2012, 87, 1311–1317. [Google Scholar] [CrossRef]
- Bao, X.; Liu, C.; Fang, J.; Li, X. Structural and immunological studies of a major polysaccharide from spores of Ganoderma lucidum (Fr.) Karst. Carbohydr. Res. 2001, 332, 67–74. [Google Scholar] [CrossRef]
- Li, S.G.; Wang, D.G.; Tian, W.; Wang, X.X.; Zhao, J.X.; Liu, Z.; Chen, R. Characterization and anti-tumor activity of a polysaccharide from Hedysarum polybotrys Hand.-Mazz. Carbohydr. Polym. 2008, 73, 344–350. [Google Scholar] [CrossRef]
- Sheng, J.; Yu, F.; Xin, Z.; Zhao, L.; Zhu, X.; Hu, Q. Preparation, identification and their antitumor activities in vitro of polysaccharides from Chlorella pyrenoidosa. Food Chem. 2007, 105, 533–539. [Google Scholar] [CrossRef]
- Sun, Y.X.; Li, Y.J.; Li, M.Q.; Tong, H.B.; Yang, X.D.; Liu, J.C. Optimization of extraction technology of the Anemone raddeana polysaccharides (ARP) by orthogonal test design and evaluation of its anti-tumor activity. Carbohydr. Polym. 2009, 75, 575–579. [Google Scholar] [CrossRef]
- Cao, W.; Li, X.-Q.; Liu, L.; Yang, T.-H.; Li, C.; Fan, H.-T.; Jia, M.; Lu, Z.-G.; Mei, Q.-B. Structure of an anti-tumor polysaccharide from Angelica sinensis (Oliv.) Diels. Carbohydr. Polym 2006, 66, 149–159. [Google Scholar] [CrossRef]
- Wang, J.; Luo, J.; Zha, X.; Feng, B. Comparison of antitumor activities of different polysaccharide fractions from the stems of Dendrobium nobile Lindl. Carbohydr. Polym. 2010, 79, 114–118. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Chen, Z. Sulfated modification of the polysaccharides obtained from defatted rice bran and their antitumor activities. Int. J. Biol. Macromol. 2009, 44, 211–214. [Google Scholar] [CrossRef]
- Ren, M.; Yan, W.; Yao, W.; Jin, L.; Gao, X. Enzymatic degradation products from a marine polysaccharide YCP with different immunological activity and binding affinity to macrophages, hydrolyzed by α-amylases from different origins. Biochimie 2010, 92, 411–417. [Google Scholar] [CrossRef]
- Zhang, W. Biochemical Technology of Carbohydrate Complexes; Zhejiang University Press: Hangzhou, China, 1994; pp. 193–200. [Google Scholar]
- Felicea, D.L.; Sun, J.; Liu, R.H. A modified methylene blue assay for accurate cell counting. J. Funct. Foods 2009, 1, 109–118. [Google Scholar] [CrossRef]
- Sample Availability:Samples of the polysaccharides are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yi, Y.; Huang, F.; Zhang, M.-W.; Zhang, R.-F.; Deng, Y.-Y.; Wei, Z.-C.; He, J.-R. Solution Properties and in Vitro Anti-Tumor Activities of Polysaccharides from Longan Pulp. Molecules 2013, 18, 11601-11613. https://doi.org/10.3390/molecules180911601
Yi Y, Huang F, Zhang M-W, Zhang R-F, Deng Y-Y, Wei Z-C, He J-R. Solution Properties and in Vitro Anti-Tumor Activities of Polysaccharides from Longan Pulp. Molecules. 2013; 18(9):11601-11613. https://doi.org/10.3390/molecules180911601
Chicago/Turabian StyleYi, Yang, Fei Huang, Ming-Wei Zhang, Rui-Fen Zhang, Yuan-Yuan Deng, Zhen-Cheng Wei, and Jing-Ren He. 2013. "Solution Properties and in Vitro Anti-Tumor Activities of Polysaccharides from Longan Pulp" Molecules 18, no. 9: 11601-11613. https://doi.org/10.3390/molecules180911601




