1H-MAS-NMR Chemical Shifts in Hydrogen-Bonded Complexes of Chlorophenols (Pentachlorophenol, 2,4,6-Trichlorophenol, 2,6-Dichlorophenol, 3,5-Dichlorophenol, and p-Chlorophenol) and Amine, and H/D Isotope Effects on 1H-MAS-NMR Spectra
Abstract
:1. Introduction
| Pentachloro- phenol | 2,4,6-Trichloro phenol | 2,6-Dichloro phenol | 3,5-Dichloro phenol | p-Chloro phenol | ||
|---|---|---|---|---|---|---|
| Symbols | PCP (5.26) | TCP (6.59) | 26DCP (7.02) | 35DCP (8.04) | p-CP (9.47) | |
| Pyridine | PYR (0.65) | 10.85 | 9.20 | 8.31 | 8.00 | 9.80 |
| 3-Cyanopyridine | 3CP (1.35) | 12.18 | ||||
| 4-Cyanopyridine | 4CP (1.86) | 11.40 | 9.63 | 9.14 | 9.20 | 10.80 |
| 3-Bromopyridine | 3BP (2.85) | 12.54 | 9.87 | |||
| 4-Acethylpyridine | 4AP (12.02) | 12.02 | 11.51 | 12.59 | ||
| Quinoline | QL (14.11) | 14.11 | 11.13 | |||
| Isoquinoline | IQL (5.40) | 13.35 | ||||
| 2-Methylpyridine | 2MP (5.94) | 15.49, 12.69 | ||||
| 4-Methylpyridine | 4MP (6.03) | 17.85, 12.91 | ||||
| Imidazole | IMID (16.92) | 16.92 | 18.62, 15.84 | |||
| 2-Amino-3-methyl-pyridine | 2A3MP (7.21) | 16.10 | 13.97 | |||
| 2-Amino-4-methyl-pyridine | 2A4MP (7.38) | 15.06 | 15.71, 9.77 | 14.14 | ||
| Triethylenediamine | TEDA (8.82) | 14.36 | 13.73 | 12.74 | 14.05 | 11.90 |
| 4-Dimethlamino-pyridine | 4DMAP (9.61) | 13.18 | 15.64 | 14.29 | ||
| Triethylamine | TEA (10.75) | 13.11, 10.49 | 10.40 |
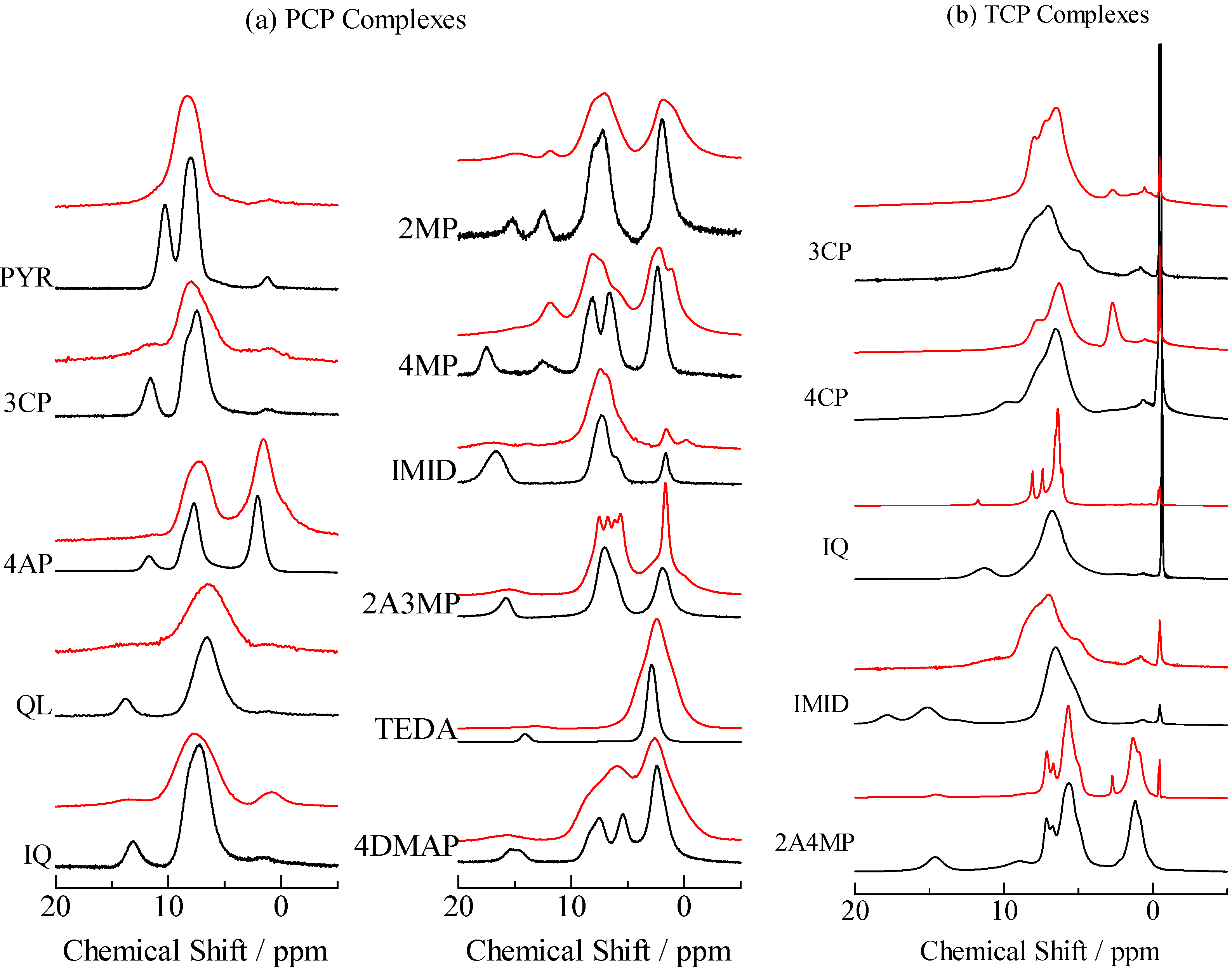
2. Results and Discussion
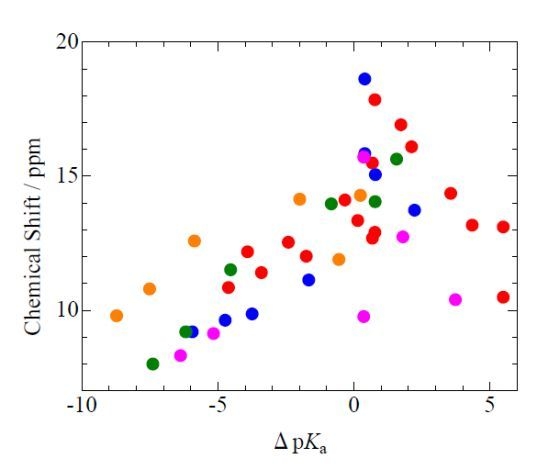
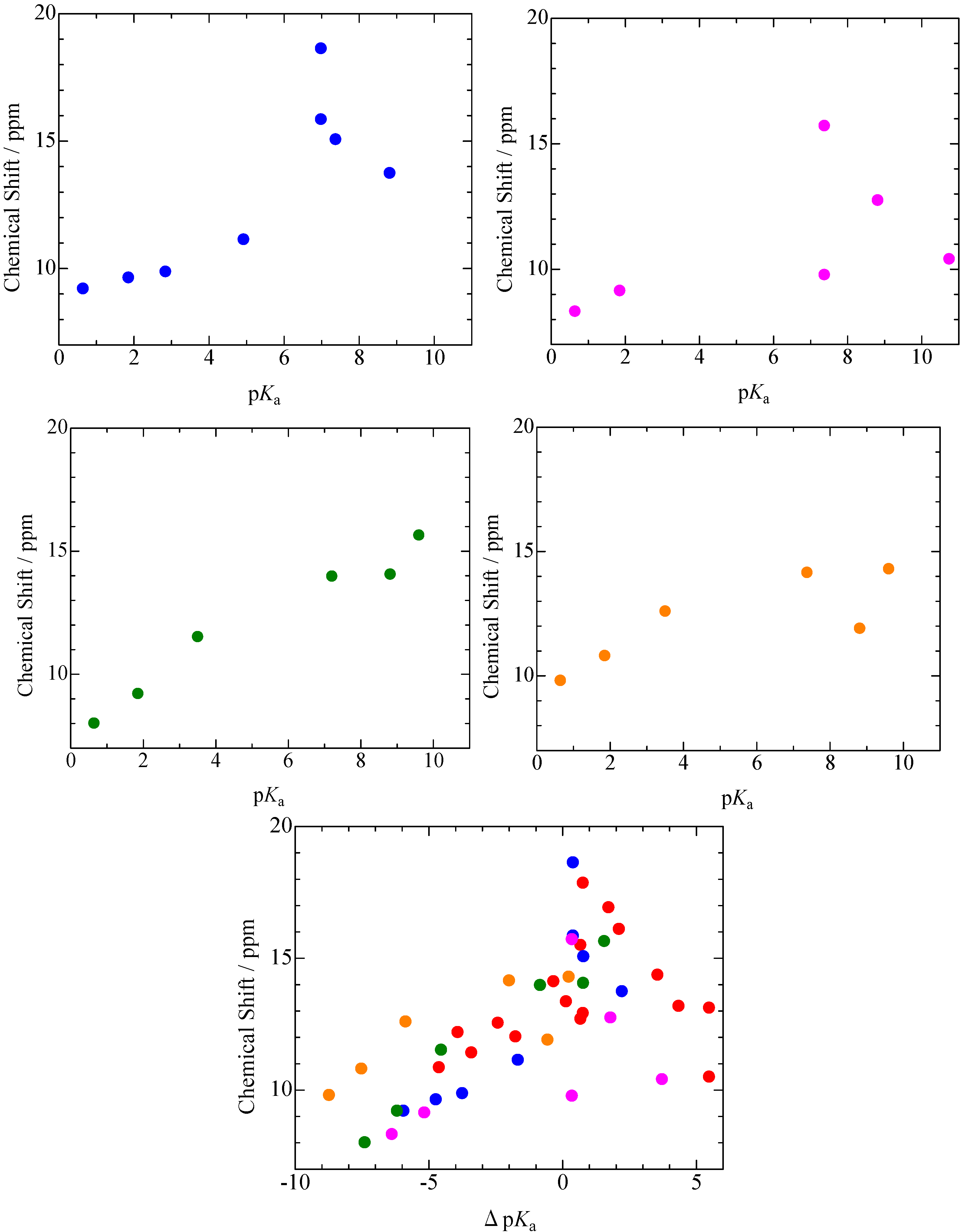
2.1. pKa Dependences of 1H-MAS-NMR Chemical Shift
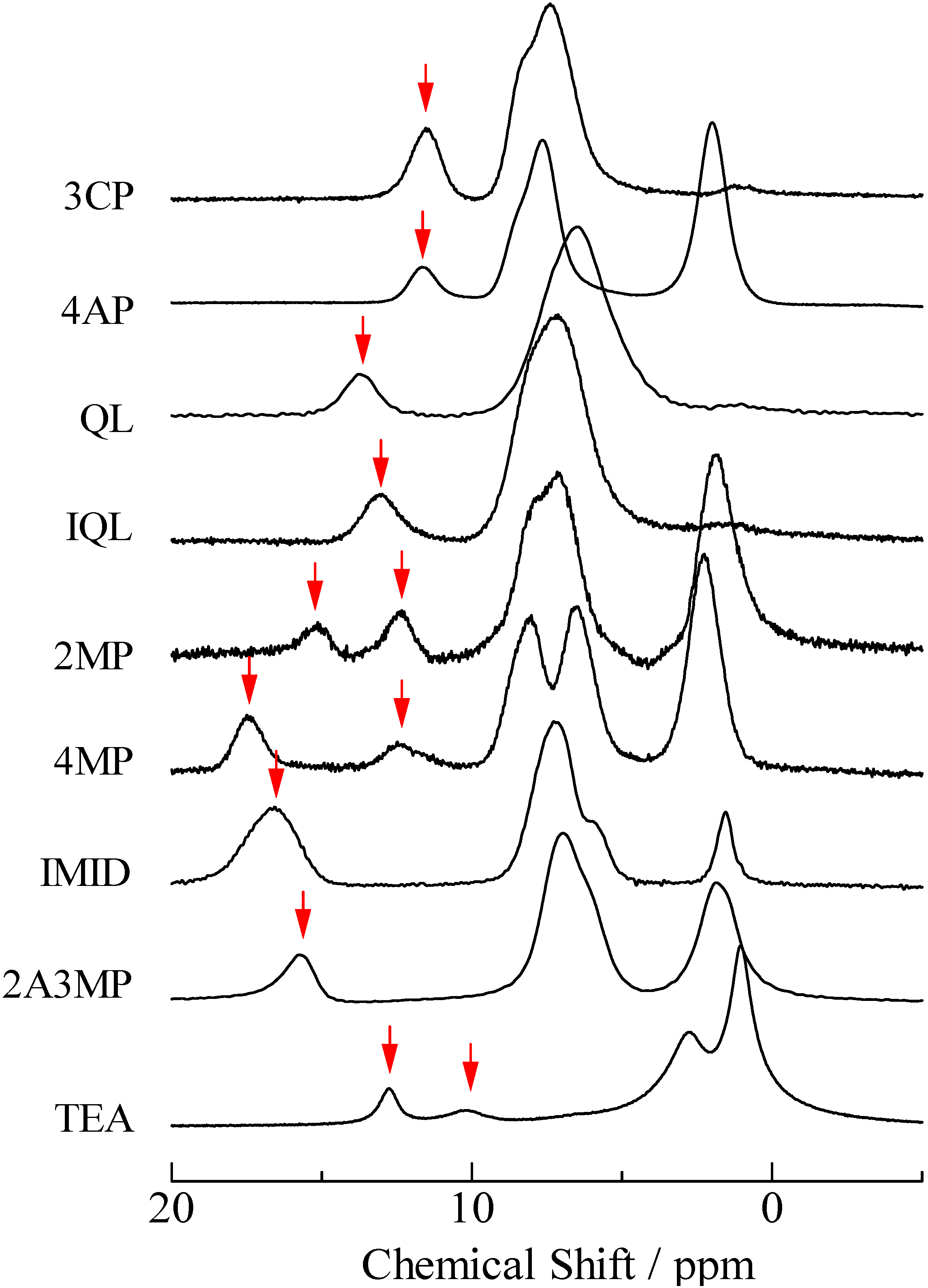
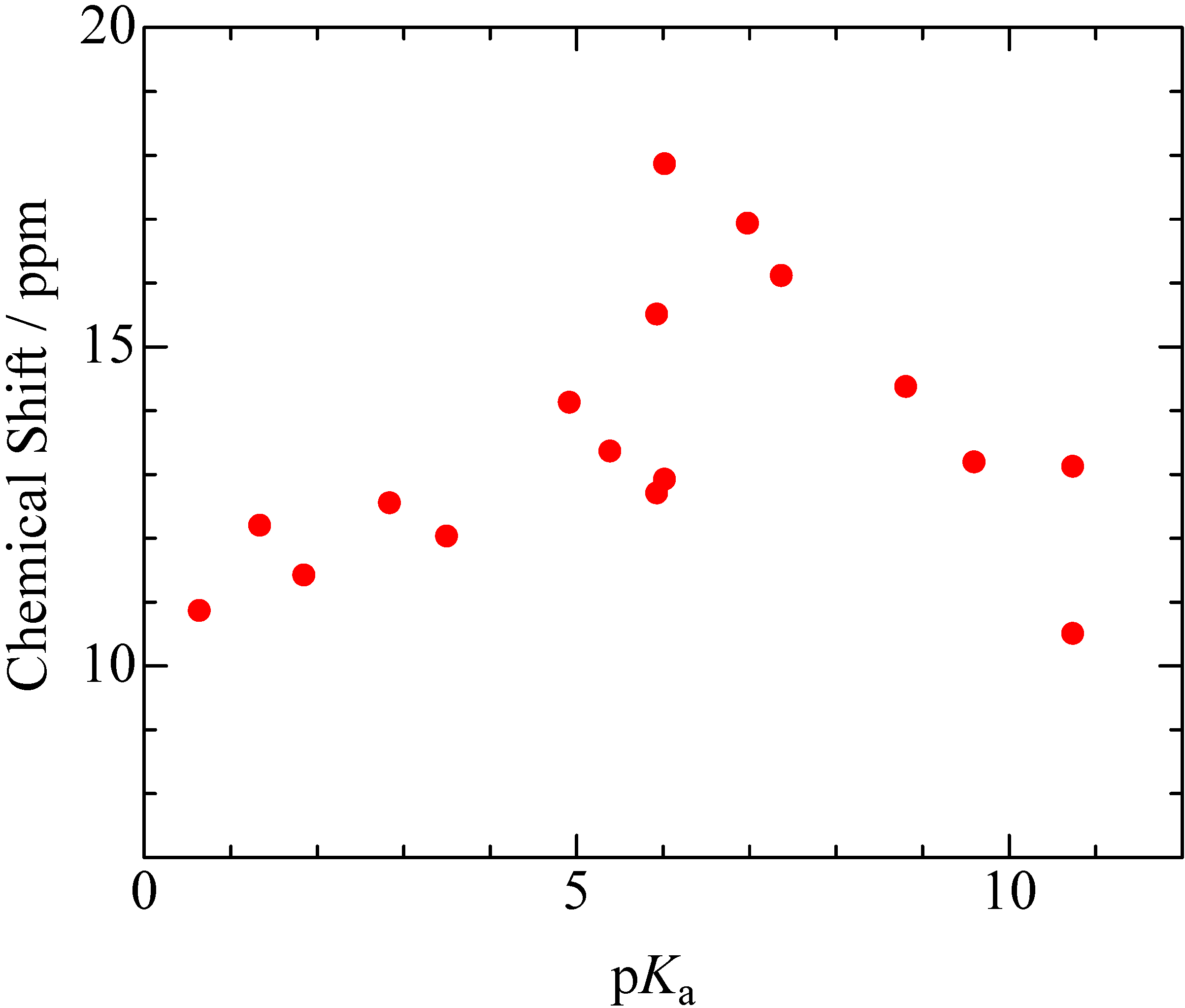
 ), 2,6-dichlorophenol(
), 2,6-dichlorophenol(  ), 3,5-dichlorophenol(
), 3,5-dichlorophenol(  ), and p-chlorophenol(
), and p-chlorophenol(  ) complexes plotted as a function of pKa and ΔpKa = pKa (N-Bases) - pKa (chlorophenols) (pentachlorophenol(
) complexes plotted as a function of pKa and ΔpKa = pKa (N-Bases) - pKa (chlorophenols) (pentachlorophenol(  )).
)).
 ), 2,6-dichlorophenol(
), 2,6-dichlorophenol(  ), 3,5-dichlorophenol(
), 3,5-dichlorophenol(  ), and p-chlorophenol(
), and p-chlorophenol(  ) complexes plotted as a function of pKa and ΔpKa = pKa (N-Bases) - pKa (chlorophenols) (pentachlorophenol(
) complexes plotted as a function of pKa and ΔpKa = pKa (N-Bases) - pKa (chlorophenols) (pentachlorophenol(  )).
)). 
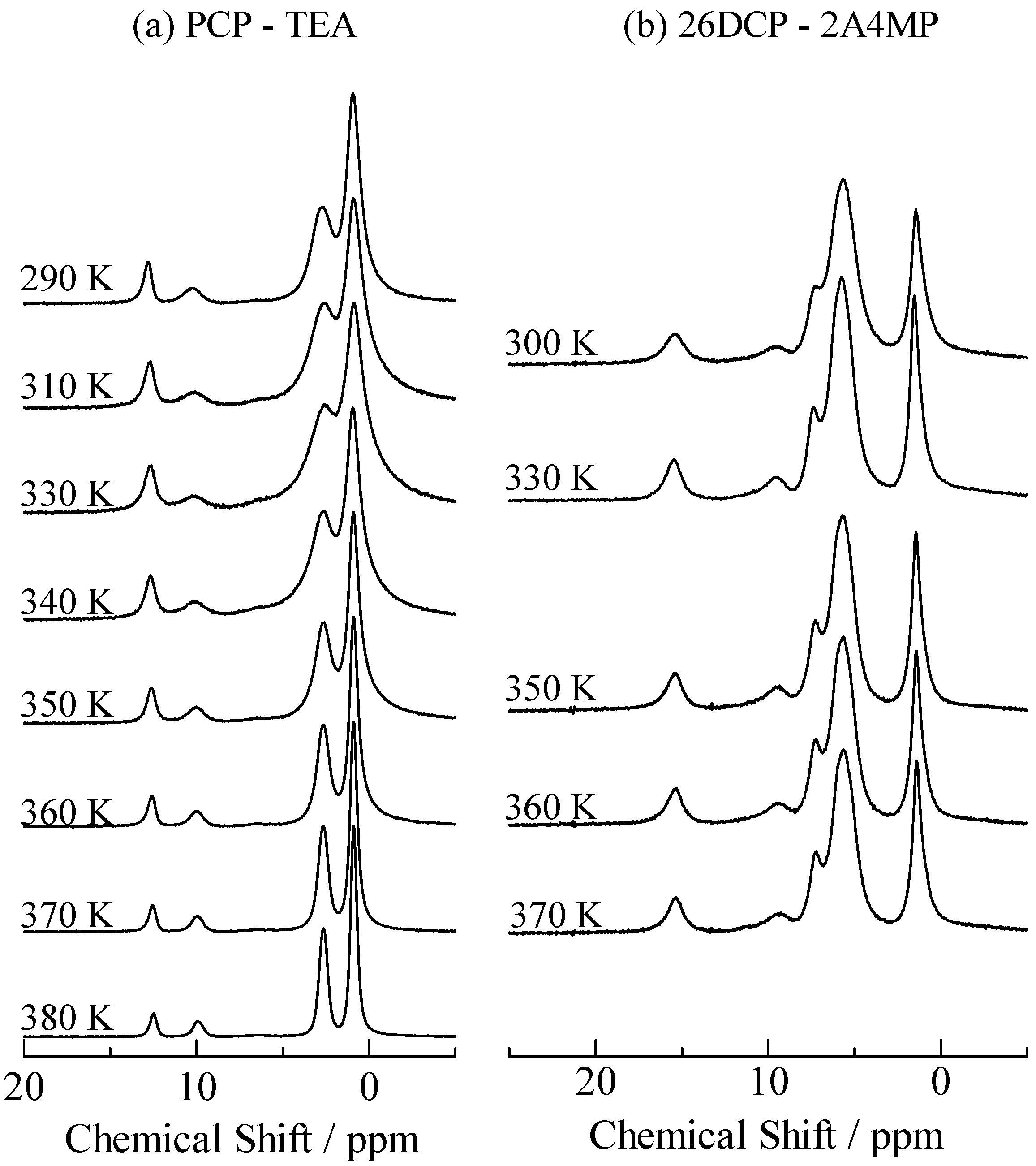
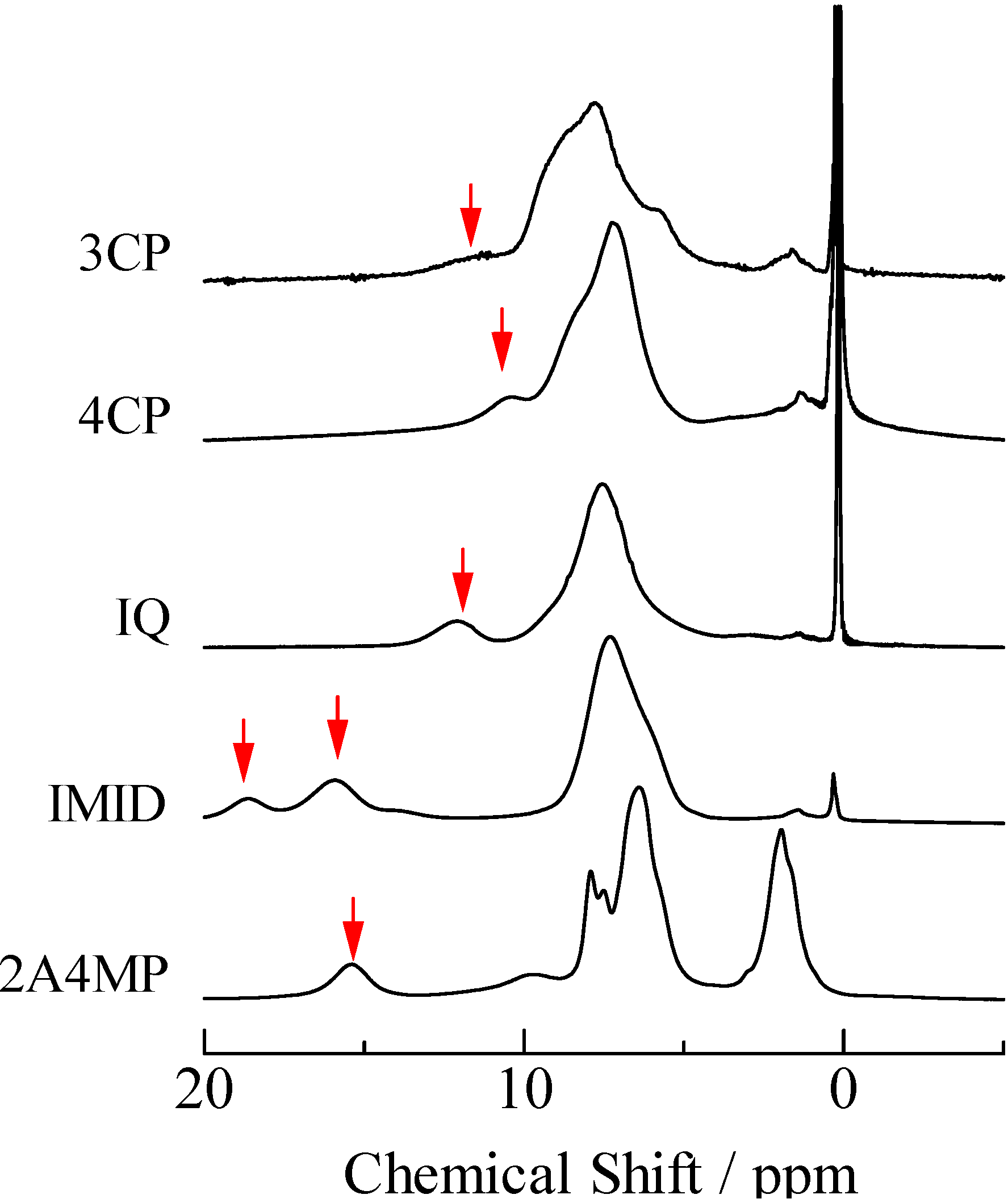
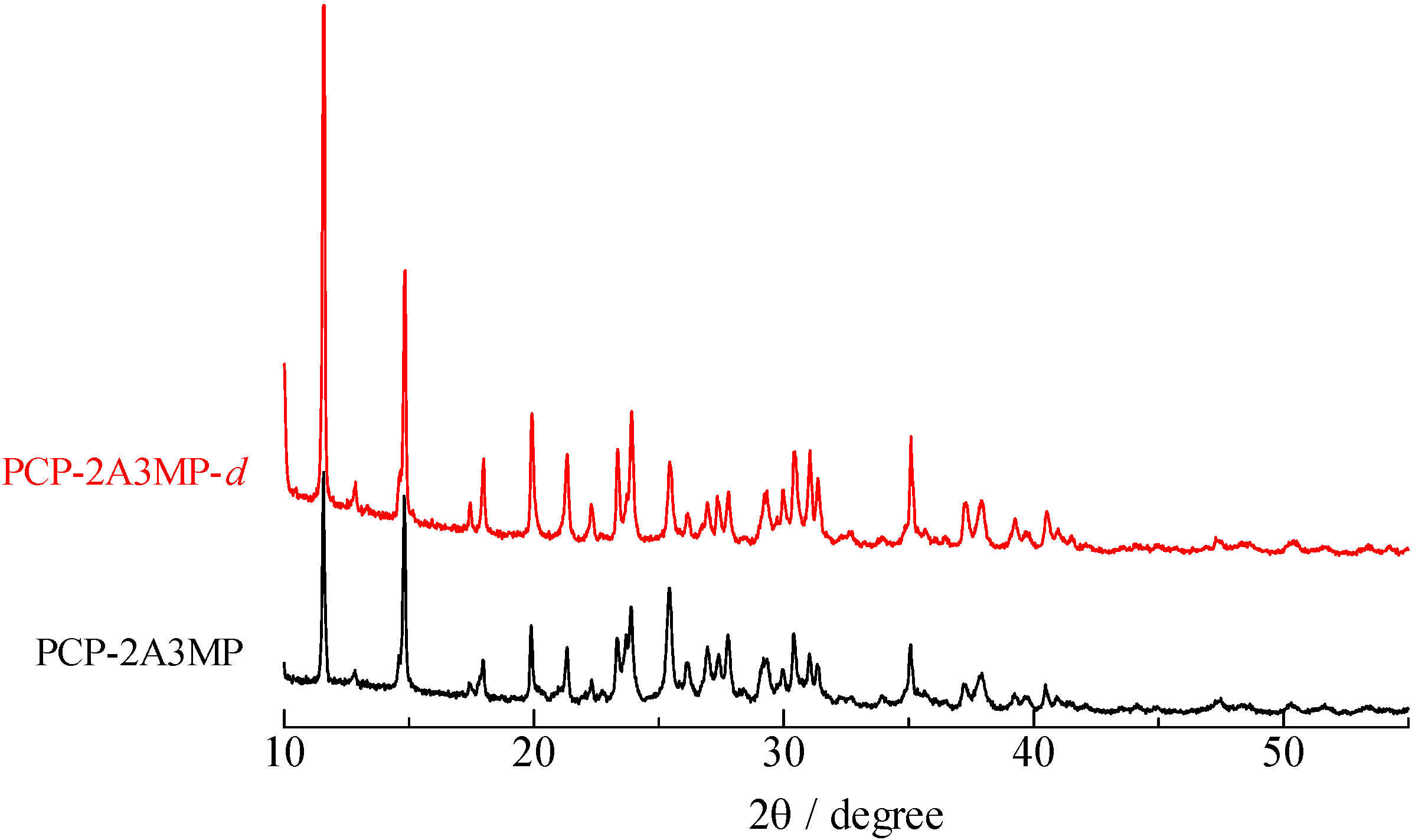
2.2. H/D Isotope Effects
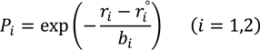
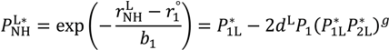

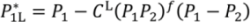

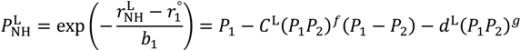
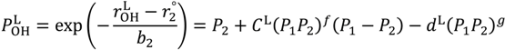
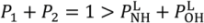

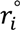 are lengths and equilibrium distances, respectively. The parameter of bi characterizes the decrease of the bond orders with increasing bond separation.
are lengths and equilibrium distances, respectively. The parameter of bi characterizes the decrease of the bond orders with increasing bond separation.  and
and 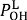 are bond orders corrected by anharmonic quantum zero point vibrational effects, where L = H or D.
are bond orders corrected by anharmonic quantum zero point vibrational effects, where L = H or D.  and
and 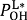 , are modified bond orders of
, are modified bond orders of  and
and 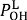 for weak H-bonds. The parameters of CH = 360, CD = 30, f = 5, dH = 0.45, dD = 0.45, g = 2,
for weak H-bonds. The parameters of CH = 360, CD = 30, f = 5, dH = 0.45, dD = 0.45, g = 2, 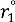 = 94.2 pm,
= 94.2 pm, 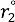 = 99.2 pm,
= 99.2 pm, 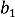 = 37.1 pm, and
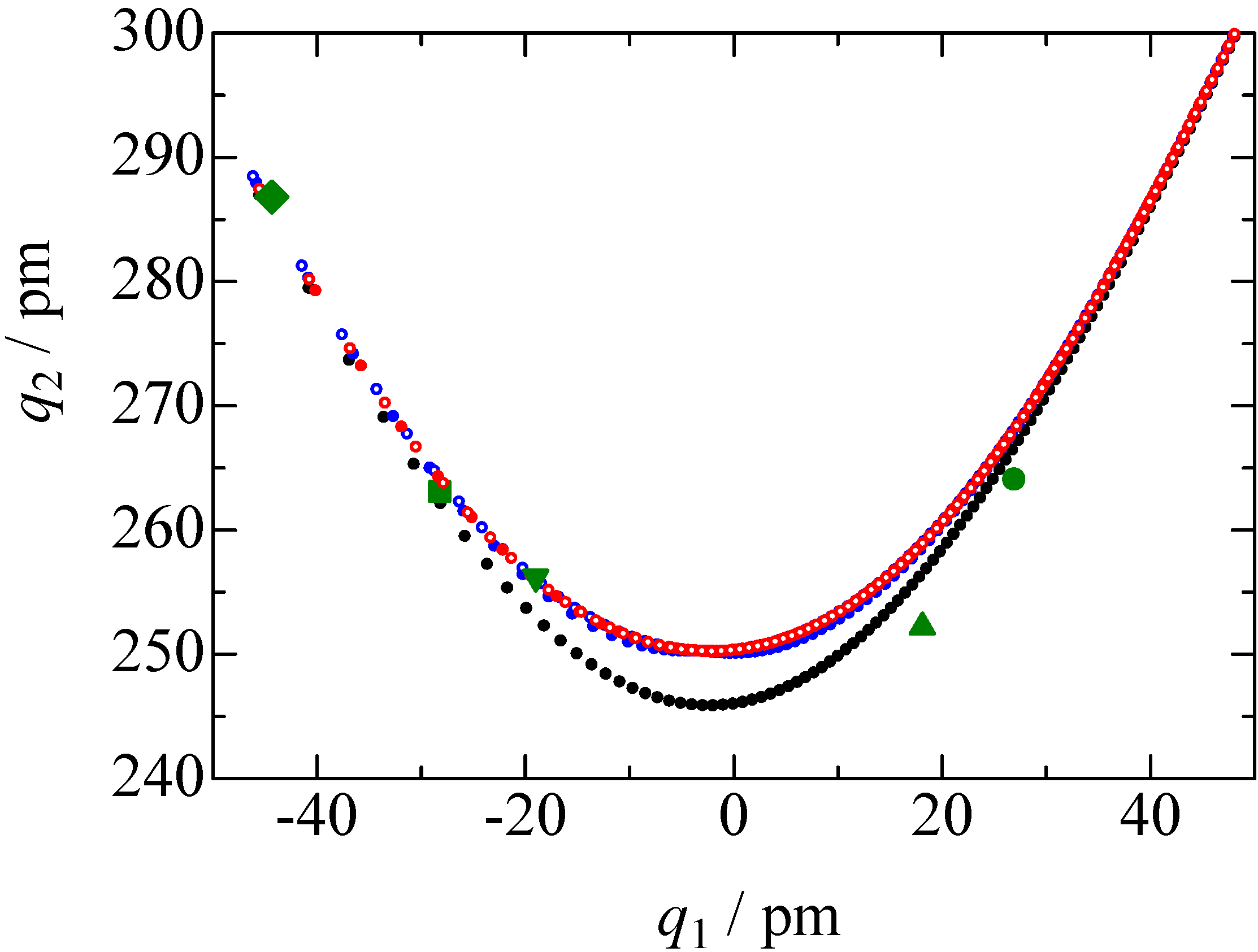
= 37.1 pm, and 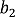 = 38.5 pm have been reported for pyridine – acid complexes [33]. Since Limbach et al. explain H-bond characters well employing the above relations and the parameters, the same calculations were performed for some complexes treated in this study. The obtained data are displayed in Figure 11. In the case of PCP–2A3MP, the q1 and q2 values were located at an area of weak quantum effects as displayed in Figure 11, therefore, it can be considered that the H/D isotope effects on the 1H-MAS-NMR spectrum can be explained by classical quantum models as shown in Figure 10. Based on these results, H/D isotope effects on the 1H-MAS-NMR line-shapes of the PCP–2A3MP-d complex can be explained by shorting the N–D length as compared with the N–H distance.
= 38.5 pm have been reported for pyridine – acid complexes [33]. Since Limbach et al. explain H-bond characters well employing the above relations and the parameters, the same calculations were performed for some complexes treated in this study. The obtained data are displayed in Figure 11. In the case of PCP–2A3MP, the q1 and q2 values were located at an area of weak quantum effects as displayed in Figure 11, therefore, it can be considered that the H/D isotope effects on the 1H-MAS-NMR spectrum can be explained by classical quantum models as shown in Figure 10. Based on these results, H/D isotope effects on the 1H-MAS-NMR line-shapes of the PCP–2A3MP-d complex can be explained by shorting the N–D length as compared with the N–H distance. ), H(NH2) (
), H(NH2) (  ), H(CH3) (
), H(CH3) (  ), 4-H(p) (
), 4-H(p) (  ), 5-H(m) (
), 5-H(m) (  ), 6-H(o) (
), 6-H(o) (  ), H(N–H···O) (
), H(N–H···O) (  ).
).
 ), H(NH2) (
), H(NH2) (  ), H(CH3) (
), H(CH3) (  ), 4-H(p) (
), 4-H(p) (  ), 5-H(m) (
), 5-H(m) (  ), 6-H(o) (
), 6-H(o) (  ), H(N–H···O) (
), H(N–H···O) (  ).
).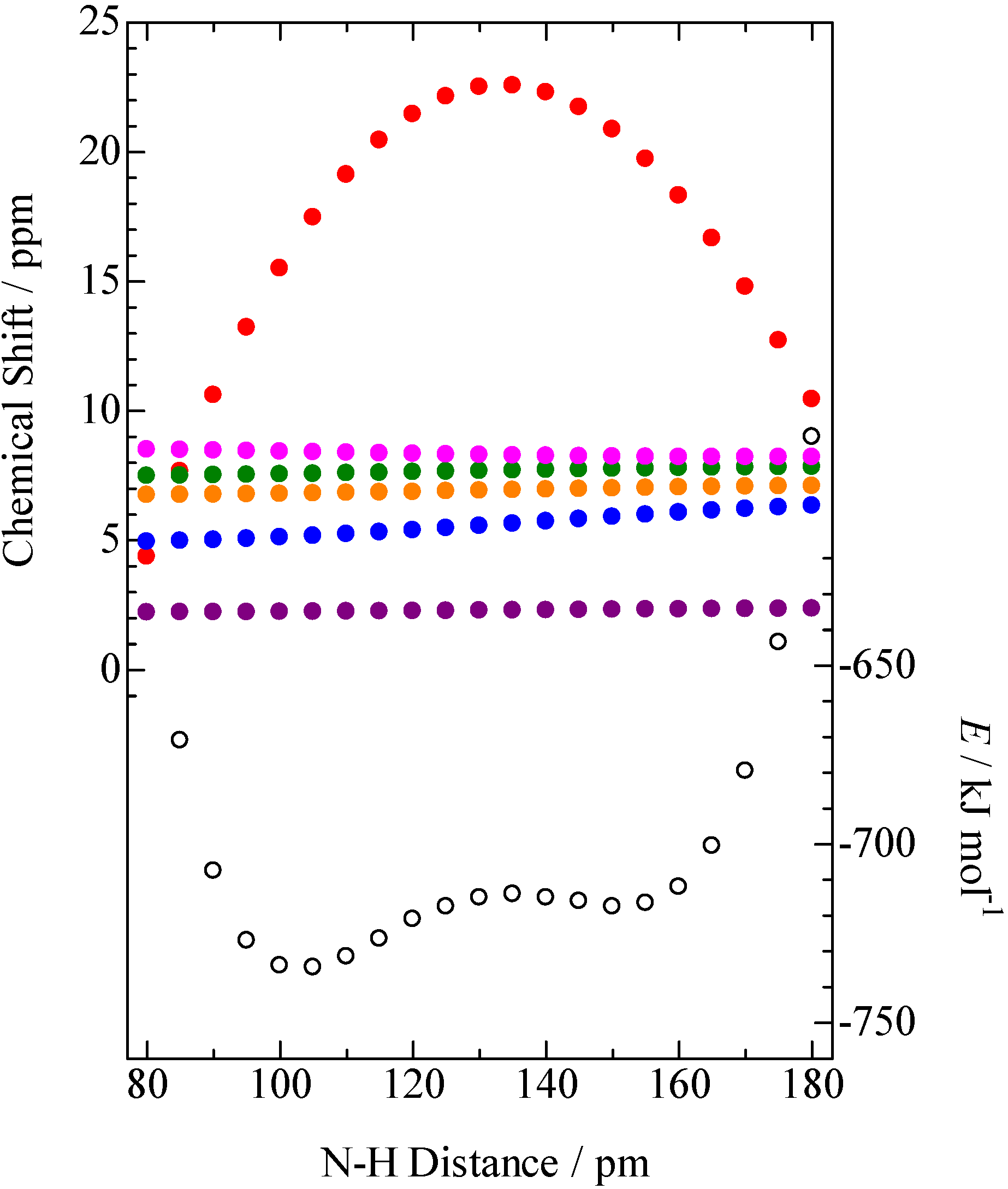
 =H) and (
=H) and (  =D) (anharmonic quantum zero point vibration model), and red circles were demonstrated by combination of equation (1) to (3) (
=D) (anharmonic quantum zero point vibration model), and red circles were demonstrated by combination of equation (1) to (3) (  =H) and (○=D) (modified quantum model). The green-colored symbols refer to PCP–2A3MP (
=H) and (○=D) (modified quantum model). The green-colored symbols refer to PCP–2A3MP (  ), PCP–3CP (
), PCP–3CP (  ), PCP–4DMAP (
), PCP–4DMAP (  ), PCP–4MP (
), PCP–4MP (  ), and PCP–TEA (
), and PCP–TEA (  ).
).
 =H) and (
=H) and (  =D) (anharmonic quantum zero point vibration model), and red circles were demonstrated by combination of equation (1) to (3) (
=D) (anharmonic quantum zero point vibration model), and red circles were demonstrated by combination of equation (1) to (3) (  =H) and (○=D) (modified quantum model). The green-colored symbols refer to PCP–2A3MP (
=H) and (○=D) (modified quantum model). The green-colored symbols refer to PCP–2A3MP (  ), PCP–3CP (
), PCP–3CP (  ), PCP–4DMAP (
), PCP–4DMAP (  ), PCP–4MP (
), PCP–4MP (  ), and PCP–TEA (
), and PCP–TEA (  ).
).
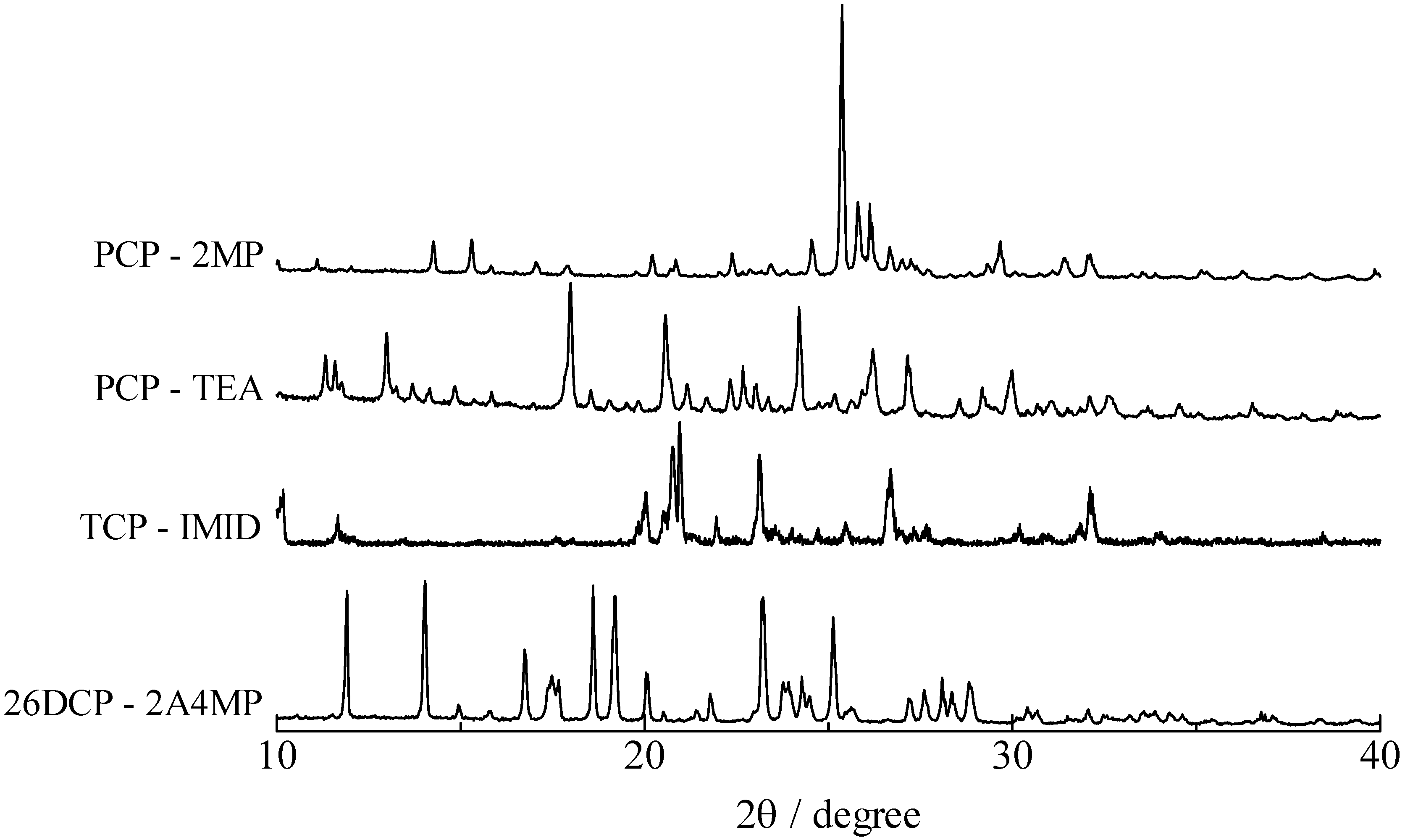
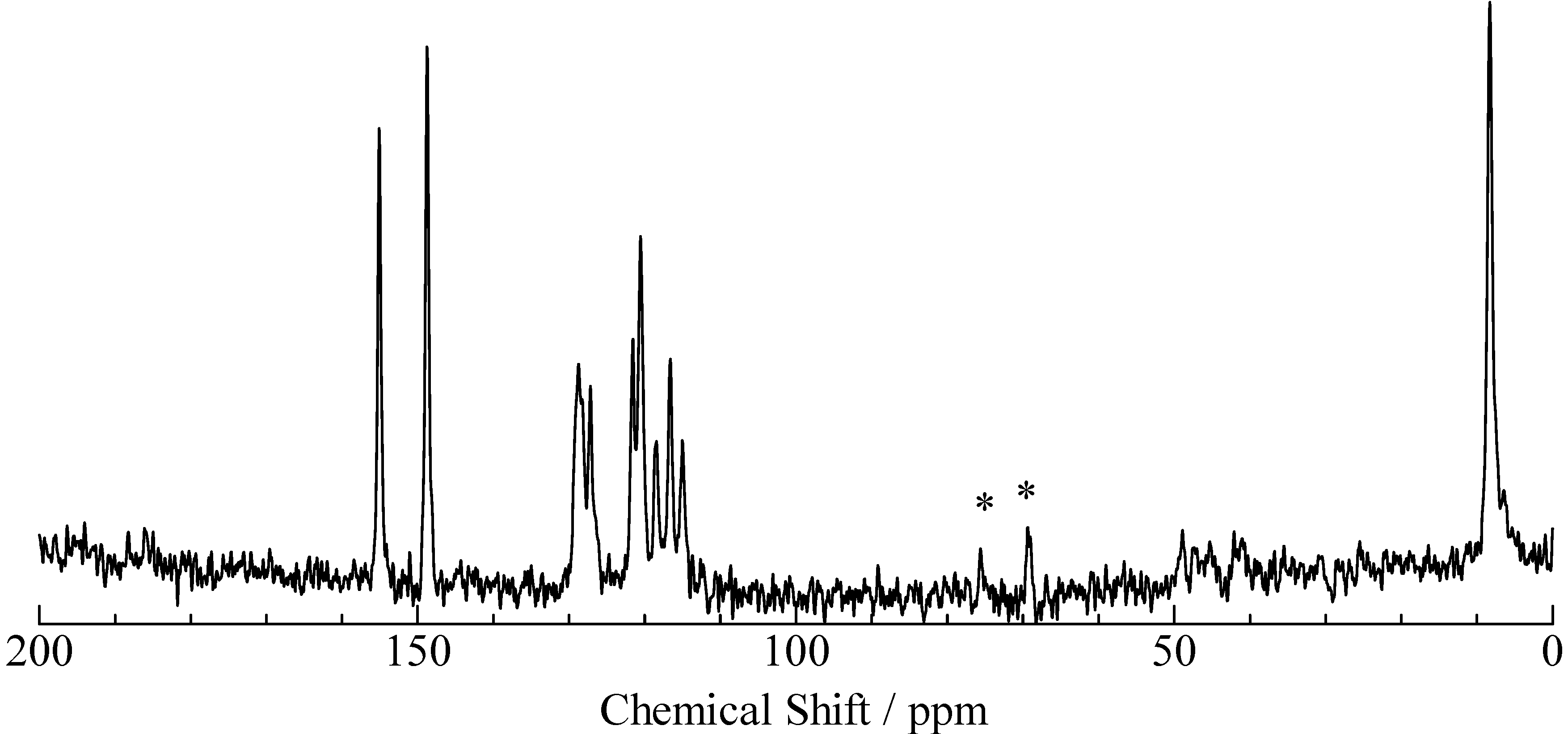
3. Experimental
4. Conclusions
Acknowledgments
References
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Scheiner, S. Hydrogen Bonding; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Marechal, Y. The Hydrogen Bond and the Water Molecule; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Matsushita, E.; Matsubara, T. Note on isotope effect in hydrogen bonded crystals. Prog. Theo. Phys. 1982, 67, 1–19. [Google Scholar] [CrossRef]
- Blinc, R.; Mali, M.; Trontelj, Z. 35Cl and 37Cl quadrupole resonance in normal and deuterated KH(CCl3COO)2 and NH4(CCl3COO)2. Phys. Lett. A 1967, 25, 289–290. [Google Scholar] [CrossRef]
- Pies, W.; Weiss, A. Advances in Nuclear Quadrupole Resonance; Heyden: London, UK, 1974; Volume 1, pp. 57–70. [Google Scholar]
- Lynch, R.J.; Waddington, T.C.; O’Shea, T.A.; Smith, J.A.S. Investigation of the hydrogen bonding in chlorocarboxylate anions using 35Cl quadrupole resonance spectroscopy. J. Chem. Soc. Faraday Trans. 2 1976, 72, 1980–1990. [Google Scholar] [CrossRef]
- Borchers, D.; Weiss, A. Structure, hydrogen bonds and phase transition in ethylenediammonium hexachlorometallates, [H3N(CH2)2NH3]2+ [XCl6]2−, X = tin, lead, tellurium,and platinum A chlorine-35 x-ray diffraction study. Ber. Bunsen-Gesellschaft 1986, 90, 718–725. [Google Scholar]
- Shirley, W.M. Nuclear quadrupole resonance studies of hydrogen bonding in trans-dichlorobis(ethylenediamine)cobalt(III) diaquahydrogen chloride. Spectrochim. Acta Part A 1987, 43A, 565–568. [Google Scholar] [CrossRef]
- Sasane, A.; Shinohara, H.; Mori, Y.; Kume, Y.; Asaji, T.; Nakamura, D. A temperature dependence study of chlorine-35 nuclear quadrupole resonance frequencies in some hexachloroplatinate(IV) hexahydrates. Z Naturforschung A 1987, 42, 611–616. [Google Scholar]
- Mackowiak, M.; Koziol, P. Effect of pressure on the symmetric hydrogen bond in (CCI3COO)2HK. Physica Status Solidi A Appl. Res. 1988, 108, 739–745. [Google Scholar] [CrossRef]
- Kalenik, J.; Majerz, I.; Malarski, Z.; Sobczyk, L. Isotopic effect on 35Cl NQR spectra of pentachlorophenol-amine hydrogen-bonded complexes. Chem. Phys. Lett. 1990, 165, 15–18. [Google Scholar] [CrossRef]
- Horiuchi, K. Temperature dependence of the effect of deuteriation on the chlorine-35 quadrupole resonance frequency in an Mg(H2O)6SnCl6 crystal. J. Chem. Soc. Faraday Trans. 1993, 89, 3359–3362. [Google Scholar] [CrossRef]
- Zdanowska-Fraczek, M. 35Cl NQR study of geometric isotope effect in hydrogen bonded chlorooctanes. J. Mol. Struct. 1994, 321, 53–56. [Google Scholar] [CrossRef]
- Zdanowska-Fraczek, M. NQR studies of charge distribution in hydrogen-bonded chloroacetates. Ber. Bunsen-Gesellschaft 1998, 102, 340–343. [Google Scholar]
- Honda, H. Even-Odd Effect of 35Cl Quadrupole Coupling Constants in Solid n-Alkylammonium Chlorides (C5–C10). Z. Naturforschung A 2003, 58, 623–630. [Google Scholar]
- Nakano, R.; Honda, H.; Kimura, T.; Nakata, E.; Takamizawa, S.; Noro, S.; Ishimaru, S. Anomalous H/D Isotope Effect on 35Cl NQR frequencies in piperidinium p-Chlorobenzoate. Hyperfine Interact. 2008, 181, 59–68. [Google Scholar] [CrossRef]
- Nakano, R.; Honda, H.; Kimura, T.; Kyo, S.; Ishimaru, S.; Miyake, R.; Nakata, E.; Takamizawa, S.; Noro, S. Anomalous H/D isotope effect on 35Cl NQR frequencies and h/d isotope effect on 1H MAS NMR Spectra in pyrrolidinium p-chlorobenzoate. Bull. Chem. Soc. Jpn. 2010, 83, 1019–1029. [Google Scholar] [CrossRef]
- Honda, H.; Kyo, S.; Akaho, Y.; Takamizawa, S.; Terao, H. H/D isotope effect of 1H MAS NMR spectra and 79Br NQR frequencies of piperidinium p-bromobenzoate and pyrrolidinium p-bromobenzoate. Hyperfine Interact. 2011, 197, 275–285. [Google Scholar]
- Kashino, S.; Sumida, Y.; Haisa, M. The crystal and molecular structures of the 1:1 addition compounds of piperidine with p-bromobenzoic acid and p-chlorobenzoic acid. Acta Cryst. B 1972, 28, 1374–1383. [Google Scholar] [CrossRef]
- Kashino, S.; Kataoka, S.; Haisa, M. The crystal and molecular structures of pyrrolidinium p-chlorobenzoate and pyrrolidinium p-toluate. Bull. Chem Soc. Jpn. 1978, 51, 1717–1722. [Google Scholar] [CrossRef]
- Hansen, P.E.; Bolvig, S.; Wozniak, K. Steric compression and twist in o-hydroxy acyl aromatics with intramolecular hydrogen bonding. J. Mol. Struct. 2005, 749, 155–168. [Google Scholar] [CrossRef]
- Hansen, P.E. Isotope effect on chemical shifts in hydrogen-bonded systems. J. Labelled Compd. Radiopharm. 2007, 50, 967–981. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Le, T.N.; Duus, F.; Hansen, B.K. V.; Hansen, P.E. Intramolecular hydrogen bonding of novel o-hydroxythioacetophenones and related compounds evaluated by deuterium isotope effects on 13C chemical shifts. Magn. Reson. Chem. 2007, 45, 245–252. [Google Scholar] [CrossRef]
- Hofmann, J.P.; Duus, F.; Bond, A.D.; Hansen, P.E. A spectrochemometric approach to tautomerism and hydrogen-bonding in 3-acyltetronic acids. J. Mol. Struct. 2006, 790, 80–88. [Google Scholar] [CrossRef]
- Hansen, P.E.; Kamounah, F.S.; Hansen, B.K. V.; Spanget-Larsen, J. Conformational and tautomeric eccentricities of 2-acetyl-1,8-dihydroxynaphthalenes. Magn. Reson. Chem. 2007, 45, 106–117. [Google Scholar] [CrossRef]
- Nakano, R.; Honda, H.; Ishimaru, S.; Noro, S. 1H MAS, 13C CP/MAS, and 2H NMR spectra studies of piperidinium p-chlorobenzoate. Hyperfine Interact. 2012. [Google Scholar] [CrossRef]
- Grech, E.; Kalenik, J.; Sobczyk, L. 35Cl nuclear quadruplore resonance studies of pentachlorophenol-amine hydrogen-bonded complexes. J. Chem. Soc. Faraday 1979, 75, 1587–1592. [Google Scholar] [CrossRef]
- Steiner, T.; Majerz, I.; Wilson, C.C. First O–H–N Hydrogen bond with a centered proton obtained by thermally induced proton migration. Angew. Chem. Int. Ed. 2001, 40, 2651–2654. [Google Scholar] [CrossRef]
- Steiner, T.; Wilson, C.C.; Majerz, I. Neutron diffraction study of a very short O–H···N hydrogen bond: Crystalline adduct of 2-methylpyridine and pentachlorophenol. Chem. Commun. 2000, 1231–1232. [Google Scholar] [CrossRef]
- Malarski, Z.; Majerz, I.; Lis, T. The crystal structures at 80 K and IR spectra of the complex of 4-methylpyridine with pentachlorophenol and its deuterated analogue. J. Mol. Struct. 1996, 380, 249–256. [Google Scholar] [CrossRef]
- Majerz, I.; Malarski, Z.; Lis, T. An unusual geometrical and spectroscopic isotopic effect in the solid complex of 4-methylpyridine with pentachlorophenol. J. Mol. Struct. 1990, 240, 47–58. [Google Scholar] [CrossRef]
- Ip, B.C.K.; Shenderovich, I.G.; Tolstoy, P.M.; Frydel, J.; Denisov, G.S.; Buntkowsky, G.; Limbach, H. NMR studies of solid pentachlorophenol-4-methylpyridine complexes exhibiting strong OHN hydrogen bonds: Geometric H/D isotope effects and hydrogen bond coupling cause isotopic polymorphism. J. Phys. Chem. A 2012, 116, 11370–11387. [Google Scholar]
- Zhou, J.; Kye, Y.; Harbison, G.S. Isotopometric polymorphism. J. Am. Chem. Soc. 2004, 126, 8392–8393. [Google Scholar] [CrossRef]
- Majerz, I.; Olovsson, I. Influence of proton transfer degree on the potential energy surface for two very short hydrogen bonds. Acta Chim. Slov. 2011, 58, 379–384. [Google Scholar]
- Zhou, J.; Kye, Y.; Kolesnikov, A.I.; Harbison, G.S. Origins of isotopomeric polymorphism. Isotopes Environ. Health Stud. 2006, 42, 271–277. [Google Scholar] [CrossRef]
- Limbach, H.; Pietrzak, M.; Sharif, S.; Tolstoy, P.M.; Shenderovich, I.G.; Smirnov, S.N.; Golubev, N.S.; Denisov, G.S. NMR parameters and geometries of ohn and odn hydrogen bonds of pyridine-acid complexes. Chem. Euro. J. 2004, 10, 5195–5204. [Google Scholar] [CrossRef]
- Koll, A.; Majerz, I. Internal and external implications of the structure of hydrogen bonded complexes. II. MNDO-PM3 studies on the structure of the 4-methylpyridine complex with pentachlorophenol. Bull. Soc. Chim. Belg. 1994, 103, 629–640. [Google Scholar]
- Majerz, I.; Sobczyk, L. UV Absorption spectra of pentachlorophenol and amine hydrogen bonded complexes in the solid state. J. Chim. Phys. Phys. Chim. Biolog. 1993, 90, 1657–1666. [Google Scholar]
- Habeeb, M.M. Interaction site and proton transfer equilibrium in the 4-aminoantipyrine-pentaclorophenol hydrogen-bonded adduct. J. Chem. Res. (S) 2002, 255–256. [Google Scholar]
- Malarski, Z.; Majerz, I.; Lis, T. Structure and spectroscopic properties of the 1:1 complex of 2-methylpyridine with pentachlorophenol. J. Mol. Struct. 1987, 158, 369–377. [Google Scholar] [CrossRef]
- Malarski, Z.; Majerz, I.; Lis, T. Structure of the 1:1 complex of 3-pyridinecarbonitrile with pentachlorophenol. Acta Cryst. 1987, C43, 1766–1769. [Google Scholar]
- Majerz, I.; Malarski, Z.; Dobrowolska, S. Structure and IR spectroscopic behavior of the molecular complex of 4-acetylpyridine with pentachlorophenol. J. Mol. Struct. 1991, 249, 109–116. [Google Scholar] [CrossRef]
- Majerz, I.; Koll, A. Internal and external implications of the structure of hydrogen bonded complexes. I. MNDO-PM3 and AM1 studies on structure of 4-acethylpyridine complex with pentaclorophenol. Polish J. Chem. 1994, 68, 2109–2119. [Google Scholar]
- Schmidtmann, M.; Wilson, C.C. Hydrogen transfer in pentachlorophenol-dimethylpyridine complexes. CrstEngComm 2008, 10, 177–183. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03; Revision B.04; Gaussian, Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Guan, X.; Stark, R.E. A general protocol for temperature calibration of MAS NMR probes at arbitrary spinning speeds. Solid State Nucl. Magn. Reson. 2010, 38, 74–76. [Google Scholar] [CrossRef]
- Metz, G.; Wu, X.; Smith, S.O. Ramped-amplitude cross polarization in magic-angle-spinning NMR. J. Magn. Reson. Ser. A 1994, 110, 219–227. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Honda, H. 1H-MAS-NMR Chemical Shifts in Hydrogen-Bonded Complexes of Chlorophenols (Pentachlorophenol, 2,4,6-Trichlorophenol, 2,6-Dichlorophenol, 3,5-Dichlorophenol, and p-Chlorophenol) and Amine, and H/D Isotope Effects on 1H-MAS-NMR Spectra. Molecules 2013, 18, 4786-4802. https://doi.org/10.3390/molecules18044786
Honda H. 1H-MAS-NMR Chemical Shifts in Hydrogen-Bonded Complexes of Chlorophenols (Pentachlorophenol, 2,4,6-Trichlorophenol, 2,6-Dichlorophenol, 3,5-Dichlorophenol, and p-Chlorophenol) and Amine, and H/D Isotope Effects on 1H-MAS-NMR Spectra. Molecules. 2013; 18(4):4786-4802. https://doi.org/10.3390/molecules18044786
Chicago/Turabian StyleHonda, Hisashi. 2013. "1H-MAS-NMR Chemical Shifts in Hydrogen-Bonded Complexes of Chlorophenols (Pentachlorophenol, 2,4,6-Trichlorophenol, 2,6-Dichlorophenol, 3,5-Dichlorophenol, and p-Chlorophenol) and Amine, and H/D Isotope Effects on 1H-MAS-NMR Spectra" Molecules 18, no. 4: 4786-4802. https://doi.org/10.3390/molecules18044786