Peripheral Antinociception of a Chalcone, Flavokawin B and Possible Involvement of the Nitric Oxide/Cyclic Guanosine Monophosphate/Potassium Channels Pathway
Abstract
:1. Introduction
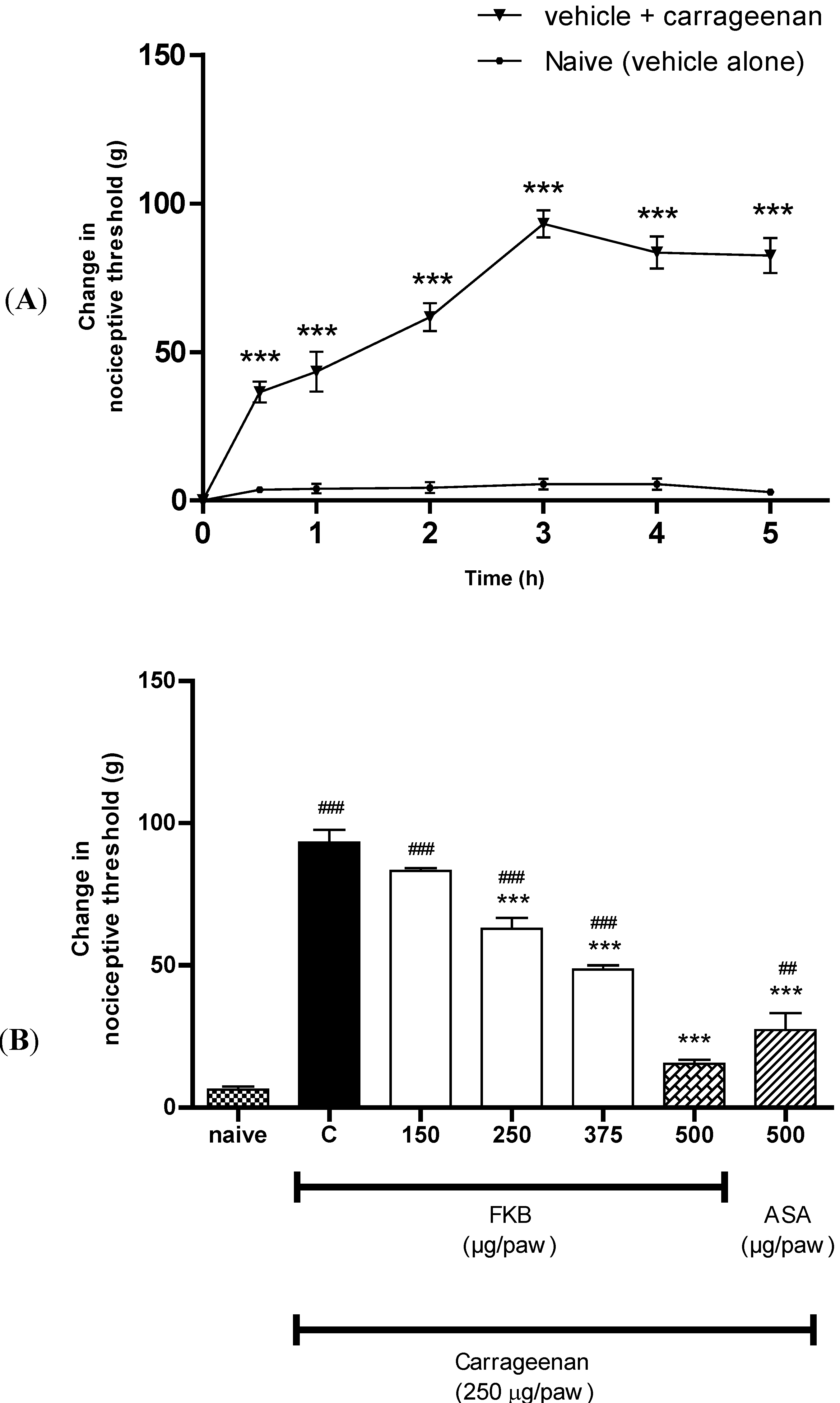
2. Results and Discussion



3. Experimental
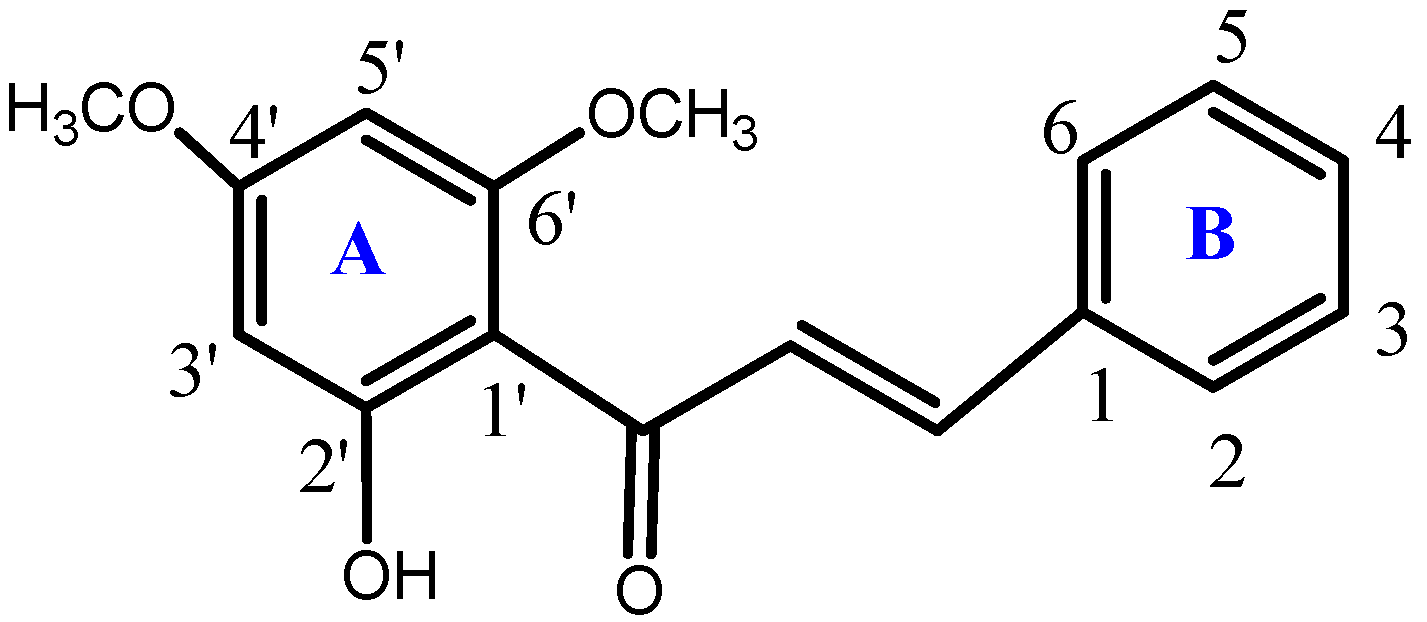
3.1. Synthesis of the Compound
3.2. Animals
3.3. Drugs
3.4. Measurement of Hyperalgesia
3.5. Experimental Protocols
3.5.1. Peripheral Anti-Hyperalgesic Effect of FKB
3.5.2. Participation of the l-Arginine/NO/cGMP Pathway
3.5.3. Participation of the K+ Channels
3.6. Data Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Yadav, V.R.; Prasad, S.; Sung, B.; Aggarwal, B.B. The role of chalcones in suppression of NF-kappaB-mediated inflammation and cancer. Int. Immunopharmacol. 2011, 11, 295–309. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Liu, Z.; Simoneau, A.R.; Xie, J.; Zi, X. Flavokawain B, A kava chalcone, Induces apoptosis via up-regulation of death-receptor 5 and Bim expression in androgen receptor negative, hormonal refractory prostate cancer cell lines and reduces tumor growth. Int. J. Cancer 2010, 127, 1758–1768. [Google Scholar] [CrossRef]
- Mohamad, A.S.; Akhtar, M.N.; Zakaria, Z.A.; Perimal, E.K.; Khalid, S.; Mohd, P.A.; Khalid, M.H.; Israf, D.A.; Lajis, N.H.; Sulaiman, M.R. Antinociceptive activity of a synthetic chalcone, Flavokawin B on chemical and thermal models of nociception in mice. Eur. J. Pharmacol. 2010, 647, 103–109. [Google Scholar] [CrossRef]
- Mohamad, A.S.; Akhtar, M.N.; Khalivulla, S.I.; Perimal, E.K.; Khalid, M.H.; Ong, H.M.; Zareen, S.; Akira, A.; Israf, D.A.; Lajis, N.; et al. Possible participation of nitric oxide/cyclic guanosine monophosphate/protein kinase C/ATP-sensitive K(+) channels pathway in the systemic antinociception of Flavokawin B. Basic Clin. Pharmacol. Toxicol. 2011, 108, 400–405. [Google Scholar] [CrossRef]
- Lin, C.T.; Senthil Kumar, K.J.; Tseng, Y.H.; Wang, Z.J.; Pan, M.Y.; Xiao, J.H.; Chien, S.C.; Wang, S.Y. Anti-inflammatory activity of Flavokawain B from Alpinia pricei Hayata. J. Agric. Food Chem. 2009, 57, 6060–6065. [Google Scholar] [CrossRef]
- Syahida, A.; Israf, D.A.; Lajis, N.H.; Khozirah, S.; Habsah, M.; Permana, D.; Norhadiani, I. Effect of compounds isolated from natural products on IFN-γ/LPS-induced nitric oxide production in RAW 264. 7 macrophages. Pharm. Biol. 2006, 44, 50–59. [Google Scholar] [CrossRef]
- Duarte, I.D.; dos Santos, I.R.; Lorenzetti, B.B.; Ferreira, S.H. Analgesia by direct antagonism of nociceptor sensitization involves the arginine-nitric oxide-cGMP pathway. Eur. J. Pharmacol. 1992, 217, 225–227. [Google Scholar]
- Romero, T.R.; Duarte, I.D. alpha(2)-Adrenoceptor agonist xylazine induces peripheral antinociceptive effect by activation of the l-arginine/nitric oxide/cyclic GMP pathway in rat. Eur. J. Pharmacol. 2009, 613, 64–67. [Google Scholar] [CrossRef]
- Negrete, R.; Hervera, A.; Leanez, S.; Martin-Campos, J.M.; Pol, O. The antinociceptive effects of JWH-015 in chronic inflammatory pain are produced by nitric oxide-cGMP-PKG-KATP pathway activation mediated by opioids. PLoS One 2011, 6, e26688. [Google Scholar]
- Ferreira, S.H.; Duarte, I.D.; Lorenzetti, B.B. The molecular mechanism of action of peripheral morphine analgesia: Stimulation of the cGMP system via nitric oxide release. Eur. J. Pharmacol. 1991, 201, 121–122. [Google Scholar] [CrossRef]
- Reis, G.M.; Pacheco, D.; Perez, A.C.; Klein, A.; Ramos, M.A.; Duarte, I.D. Opioid receptor and NO/cGMP pathway as a mechanism of peripheral antinociceptive action of the cannabinoid receptor agonist anandamide. Life Sci. 2009, 85, 351–356. [Google Scholar] [CrossRef]
- Ventura-Martinez, R.; Deciga-Campos, M.; Diaz-Reval, M.I.; Gonzalez-Trujano, M.E.; Lopez-Munoz, F.J. Peripheral involvement of the nitric oxide-cGMP pathway in the indomethacin-induced antinociception in rat. Eur. J. Pharmacol. 2004, 503, 43–48. [Google Scholar] [CrossRef]
- Morris, C.J. Carrageenan-induced paw edema in the rat and mouse. Methods Mol. Biol. 2003, 225, 115–121. [Google Scholar]
- Sulaiman, M.R.; Perimal, E.K.; Akhtar, M.N.; Mohamad, A.S.; Khalid, M.H.; Tasrip, N.A.; Mokhtar, F.; Zakaria, Z.A.; Lajis, N.H.; Israf, D.A. Anti-inflammatory effect of zerumbone on acute and chronic inflammation models in mice. Fitoterapia 2010, 81, 855–858. [Google Scholar] [CrossRef]
- da Silva, K.A.; Manjavachi, M.N.; Paszcuk, A.F.; Pivatto, M.; Viegas, C., Jr.; Bolzani, V.S.; Calixto, J.B. Plant derived alkaloid (−)-cassine induces anti-inflammatory and anti-hyperalgesics effects in both acute and chronic inflammatory and neuropathic pain models. Neuropharmacology 2012, 62, 967–977. [Google Scholar] [CrossRef]
- Millan, M.J. The induction of pain: An integrative review. Prog. Neurobiol. 1999, 57, 1–164. [Google Scholar] [CrossRef]
- Amarante, L.H.; Alves, D.P.; Duarte, I.D. Study of the involvement of K+ channels in the peripheral antinociception of the kappa-opioid receptor agonist bremazocine. Eur. J. Pharmacol. 2004, 494, 155–160. [Google Scholar] [CrossRef]
- Lozano-Cuenca, J.; Castaneda-Hernandez, G.; Granados-Soto, V. Peripheral and spinal mechanisms of antinociceptive action of lumiracoxib. Eur. J. Pharmacol. 2005, 513, 81–91. [Google Scholar] [CrossRef]
- Perimal, E.K.; Akhtar, M.N.; Mohamad, A.S.; Khalid, M.H.; Ming, O.H.; Khalid, S.; Tatt, L.M.; Kamaldin, M.N.; Zakaria, Z.A.; Israf, D.A.; et al. Zerumbone-induced antinociception: Involvement of the l-arginine-nitric oxide-cGMP -PKC-K+ ATP channel pathways. Basic Clin. Pharmacol. Toxicol. 2011, 108, 155–162. [Google Scholar]
- Khalid, M.H.; Akhtar, M.N.; Mohamad, A.S.; Perimal, E.K.; Akira, A.; Israf, D.A.; Lajis, N.; Sulaiman, M.R. Antinociceptive effect of the essential oil of Zingiber zerumbet in mice: Possible mechanisms. J. Ethnopharmacol. 2011, 137, 345–351. [Google Scholar] [CrossRef]
- Jesse, C.R.; Rocha, J.B.; Nogueira, C.W.; Savegnago, L. Further analysis of the antinociceptive action caused by p-methoxyl-diphenyl diselenide in mice. Pharmacol. Biochem. Behav. 2009, 91, 573–580. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar]
- Moncada, S. Nitric oxide in the vasculature: Physiology and pathophysiology. Ann. NY Acad. Sci. 1997, 811, 60–69. [Google Scholar] [CrossRef]
- Duarte, I.D.; Ferreira, S.H. The molecular mechanism of central analgesia induced by morphine or carbachol and the l-arginine-nitric oxide-cGMP pathway. Eur. J. Pharmacol. 1992, 221, 171–174. [Google Scholar] [CrossRef]
- Duarte, I.D.; Ferreira-Alves, D.L.; Nakamura-Craig, M. Possible participation of endogenous opioid peptides on the mechanism involved in analgesia induced by vouacapan. Life Sci. 1992, 50, 891–897. [Google Scholar] [CrossRef]
- Alves, D.; Duarte, I. Involvement of ATP-sensitive K(+) channels in the peripheral antinociceptive effect induced by dipyrone. Eur. J. Pharmacol. 2002, 444, 47–52. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Randall, L.O.; Selitto, J.J. A method for measurement of analgesic activity on inflamed tissue. Arch. Int. Pharmacodyn. Ther. 1957, 111, 409–419. [Google Scholar]
- Sample Availability: Samples are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kamaldin, M.N.; Akhtar, M.N.; Mohamad, A.S.; Lajis, N.; Perimal, E.K.; Akira, A.; Ming-Tatt, L.; Israf, D.A.; Sulaiman, M.R. Peripheral Antinociception of a Chalcone, Flavokawin B and Possible Involvement of the Nitric Oxide/Cyclic Guanosine Monophosphate/Potassium Channels Pathway. Molecules 2013, 18, 4209-4220. https://doi.org/10.3390/molecules18044209
Kamaldin MN, Akhtar MN, Mohamad AS, Lajis N, Perimal EK, Akira A, Ming-Tatt L, Israf DA, Sulaiman MR. Peripheral Antinociception of a Chalcone, Flavokawin B and Possible Involvement of the Nitric Oxide/Cyclic Guanosine Monophosphate/Potassium Channels Pathway. Molecules. 2013; 18(4):4209-4220. https://doi.org/10.3390/molecules18044209
Chicago/Turabian StyleKamaldin, Mohd Nasier, Muhammad Nadeem Akhtar, Azam Shah Mohamad, Nordin Lajis, Enoch Kumar Perimal, Ahmad Akira, Lee Ming-Tatt, Daud Ahmad Israf, and Mohd Roslan Sulaiman. 2013. "Peripheral Antinociception of a Chalcone, Flavokawin B and Possible Involvement of the Nitric Oxide/Cyclic Guanosine Monophosphate/Potassium Channels Pathway" Molecules 18, no. 4: 4209-4220. https://doi.org/10.3390/molecules18044209



