Synthesis of Esters of Ginsenoside Metabolite M1 and Their Cytotoxicity on MGC80-3 Cells
Abstract
:1. Introduction
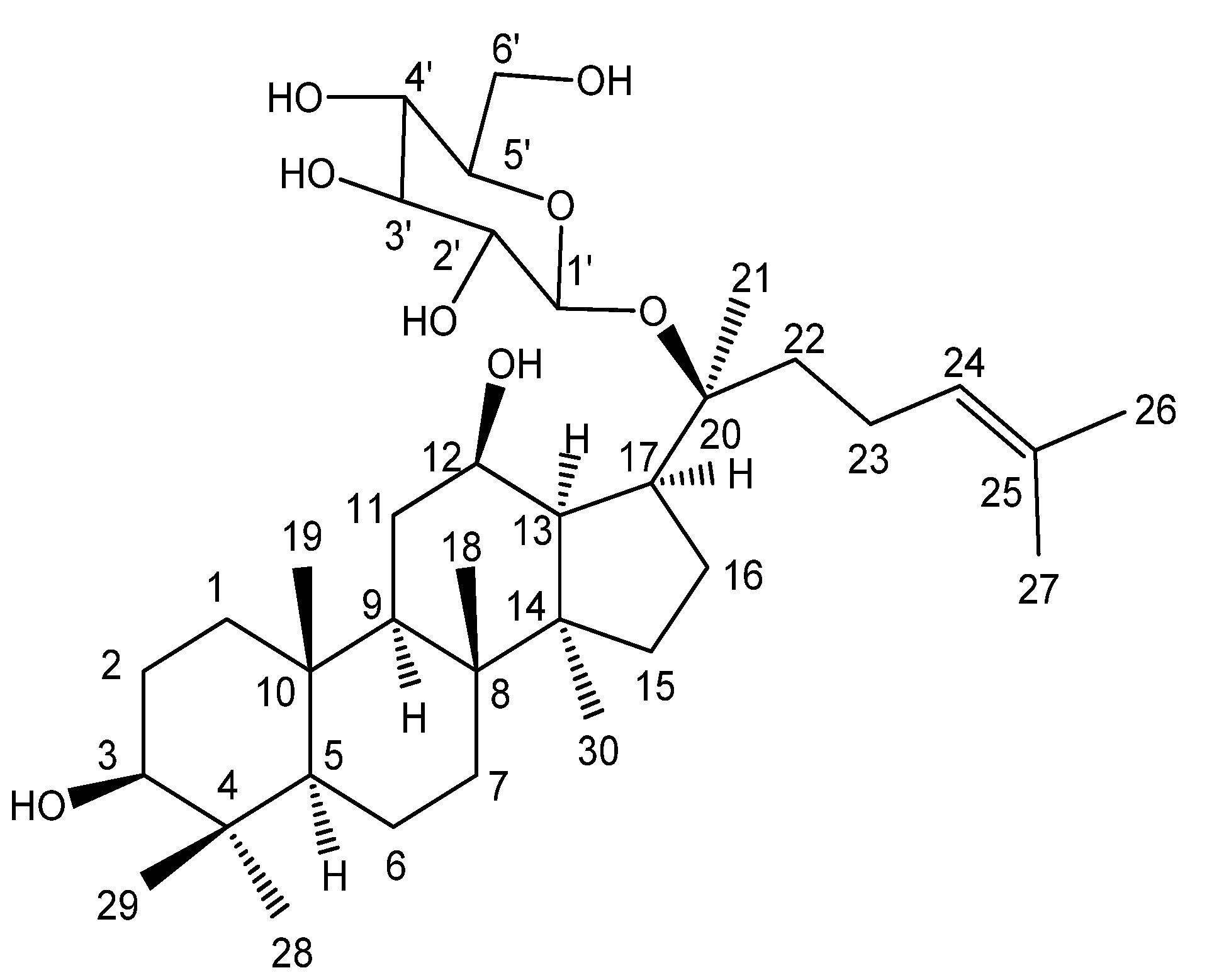

2. Results and Discussion
2.1. Synthesis
| Entry | Acylating reagent | Cat. | M1: Acylating reagent: Cat. molar ratio | Time (h) | Yield 2C (%) |
|---|---|---|---|---|---|
| 1 | C11H23CO2H-DIC | DMAP | 1:1:0.5 | 6 | 12.5 |
| 2 | C11H23CO2H-DIC | DMAP | 0.5:1:0.3 | 6 | 4.6 |
| 3 | C11H23CO2H-DIC | DMAP | 1:2:1 | 9 | 5.1 |
| 4 | C11H23CO2H-DIC | DPAP | 1:1:0.5 | 6 | 8.7 |
| 5 | C11H23COCl | DMAP | 1:1:0.5 | 0.5 | 54.1 |
| Entry | RCOCl | Time (h) | Prod. | Yield (%) |
|---|---|---|---|---|
| 1 | C11H23COCl | 0.6 | 2C | 48 b |
| 2 | C9H19COCl | 0.5 | 3C | 43 b |
| 3 | C7H15COCl | 0.5 | 4C | 48 b |
| 4 | C5H11COCl | 0.5 | 5C | 41 b |
| 5 | t-C4H9COCl | 2.0 | 6C | 48 b |
| 6 | C6H5COCl | 0.5 | 7C | 54 c |
2.2. Structure Characterization of the New Monoesters 2A and 2B
2.3. Cytotoxicity of the Synthesized Monoesters
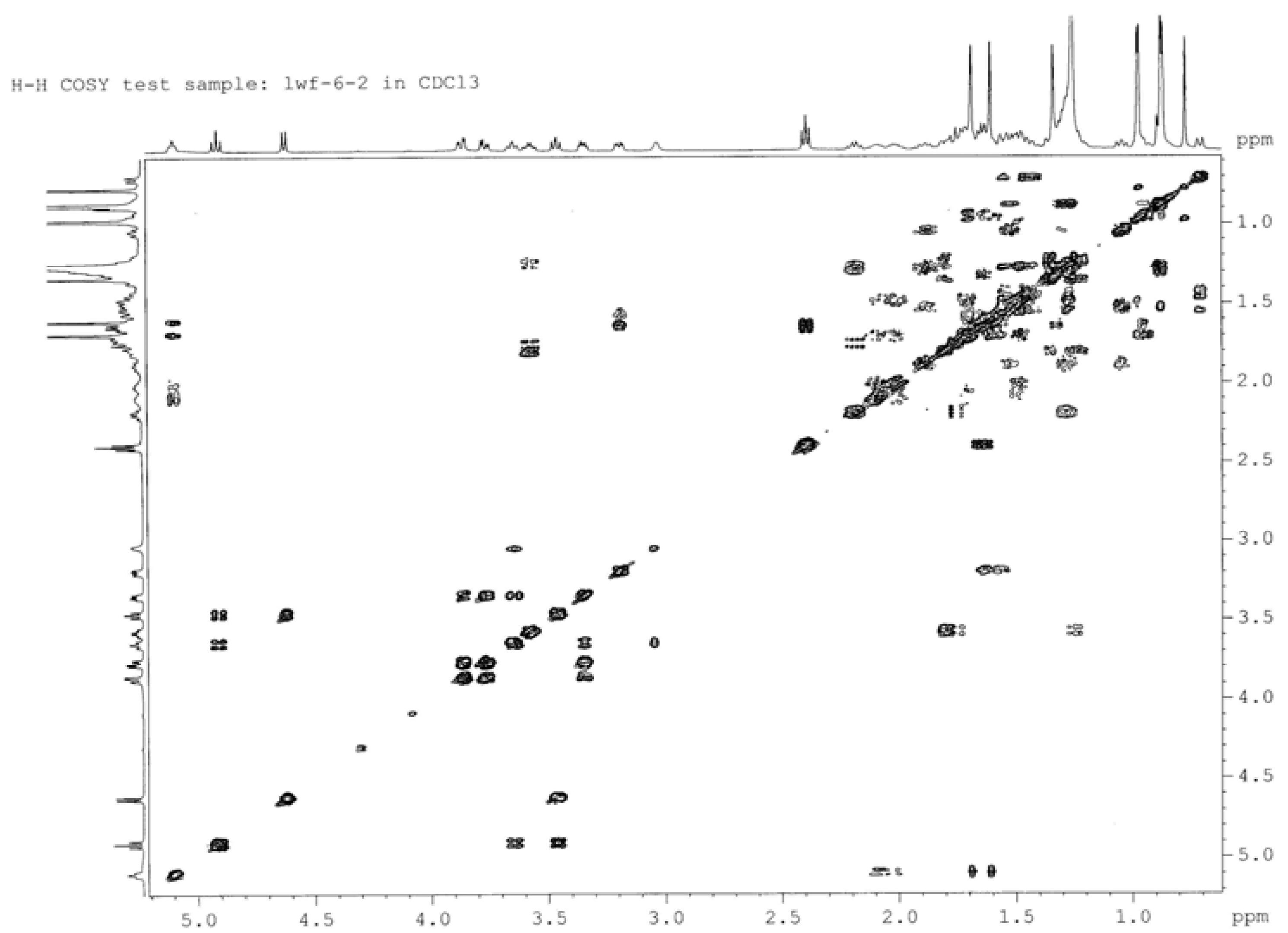
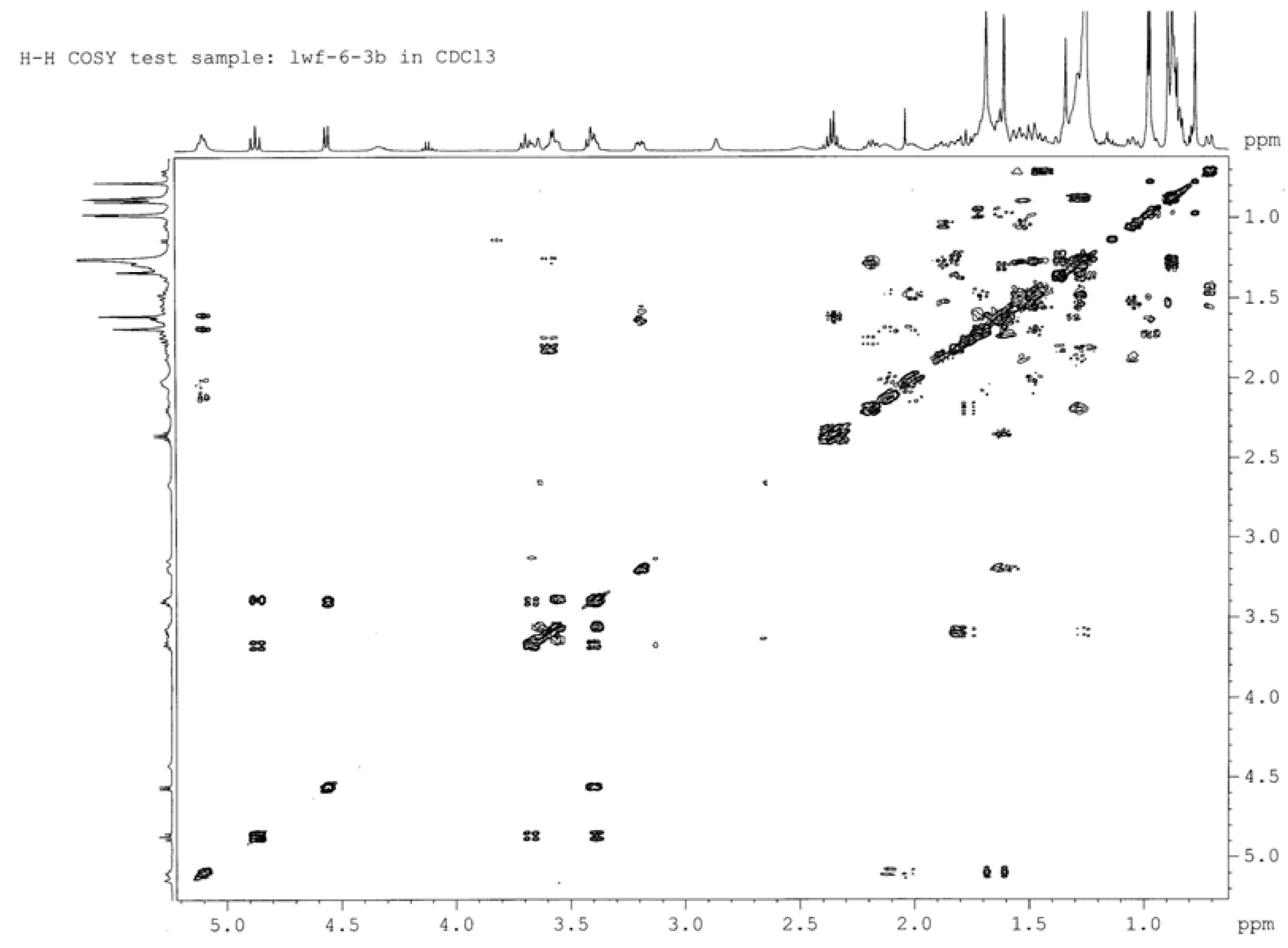
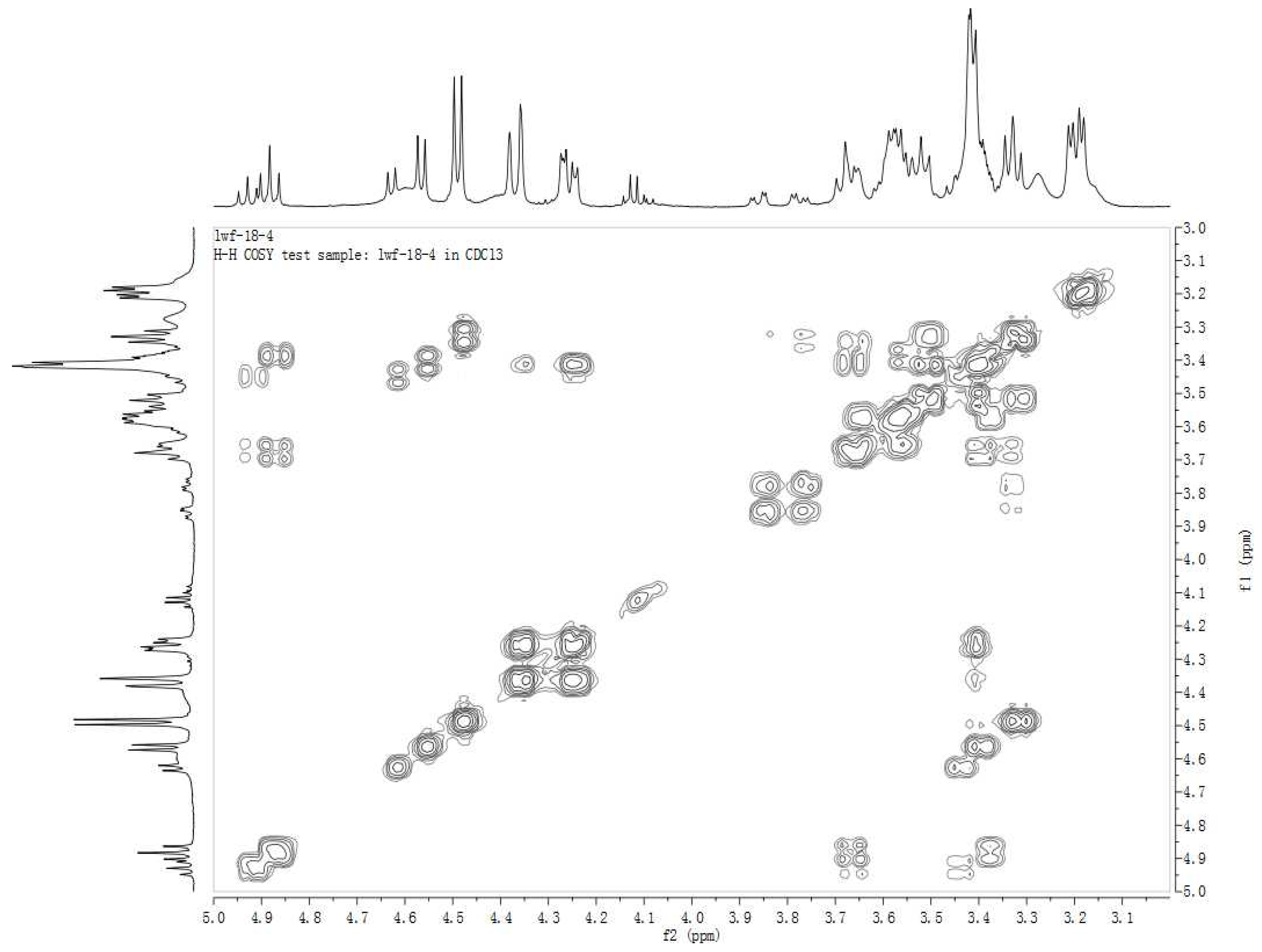

| Position | δM1 | δ2A (C-3' ester) | δ2B (C-4' ester) | δ2C (C-6' ester) |
|---|---|---|---|---|
| 1' | 4.57 (d, 7.3) | 4.62 (d, 7.8) | 4.56 (d, 7.7) | 4.49 (d, 7.7) |
| 2' | 3.21 (dd, 8.0) | 3.46 (t, 8.9) | 3.41 (t, 8.4) | 3.31 (m) |
| 3' | 3.46 (m) | 4.91 (t, 9.3) | 3.69 (t, 9.4) | 3.42 (m) |
| 4' | 3.61 (m) | 3.65 (t, 9.1) | 4.87 (t, 9.6) | 3.62 (m) |
| 5' | 3.33 (m) | 3.35 (m) | 3.38 (m) | 3.35 (m) |
| 6' | 3.77 (dd, 7.5, 11.2) | 3.87 (dd, 3.3, 11.9) 3.77 (dd, 4.7, 11.9) | 3.65 (m) 3.56 (m) | 4.24 (dd, 11.0, 5.4) 4.37 (d, 11.2) |
| Position | δM1 | δ2A | δ2A-δM1 | δ2B | δ2B-δM1 | δ2C | δ2C-δM1 |
|---|---|---|---|---|---|---|---|
| 1' | 98.0 | 97.3 | −0.7 | 96.8 | −1.2 | 97.0 | −1.0 |
| 2' | 73.7 | 72.0 | −1.7 | 74.3 | 0.6 | 73.4 | −0.3 |
| 3' | 77.0 | 78.3 | 1.3 | 74.7 | −2.3 | 76.8 | −0.2 |
| 4' | 70.1 | 69.6 | −0.5 | 70.5 | −0.4 | 70.1 | 0.0 |
| 5' | 76.0 | 75.6 | −0.4 | 74.1 | −1.9 | 73.6 | −2.4 |
| 6' | 61.5 | 62.4 | 0.9 | 61.7 | 0.2 | 63.2 | 1.7 |
| Samples | Inhibitory in 24 h (%) (%) | Inhibitory in 48 h (%) |
|---|---|---|
| 2B | 45.9 | 98.1 |
| 3C | 97.7 | 78.9 |
| 4C | 53.1 | 54.6 |
| 5C | 32.4 | 86.4 |
| 6C | 42.4 | 57.7 |
| 7C | 82.0 | 90.4 |
| 5-FU b | 14.8 | 37.0 |
3. Experimental
3.1. General
3.2. Synthesis of Monoesters of M1
3.3. Bioassay
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hasegawa, H.; Sung, J.H.; Matsumiya, S.; Uchiyama, M. Main ginseng saponin metabolites formed by intestinal bacteria. Planta Med. 1996, 62, 453–457. [Google Scholar] [CrossRef]
- Wakabayashi, C.; Hasegawa, H.; Murata, J.; Saiki, I. In vivo antimetastatic action of ginseng protopanaxadiol saponins is based on their intestinal bacterial metabolites after oral administration. Oncol. Res. 1997, 9, 411–417. [Google Scholar]
- Hasegawa, H.; Lee, K.S.; Nagaoka, T.; Tezuka, Y.; Uchiyama, M.; Kadota, S.; Saiki, I. Pharmacokinetics of ginsenoside deglycosylated by intestinal bacteria and its transformation to biologically active fatty acid esters. Biol. Pharm. Bull. 2000, 23, 298–304. [Google Scholar] [CrossRef]
- Hasegawa, H.; Sung, J.H.; Benno, Y. Role of human intestinal prevotella oris in hydrolyzing ginseng saponins. Planta Med. 1997, 63, 436–440. [Google Scholar]
- Akao, T.; Kida, H.; Kanaoka, M.; Hattori, M.; Kobashi, K. Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J. Pharm. Pharmacol. 1998, 50, 1155–1160. [Google Scholar] [CrossRef]
- Bae, E.A.; Park, S.Y.; Kim, D.H. Constitutive beta-glucosidases hydrolyzing ginsenoside Rb1 and Rb2 from human intestinal bacteria. Biol. Pharm. Bull. 2000, 23, 1481–1485. [Google Scholar] [CrossRef]
- Bae, E.; Choo, M.K.; Park, E.K.; Park, S.Y.; Shin, H.Y.; Kim, D.H. Metabolism of ginsenoside R(C) by human intestinal bacteria and its related antiallergic activity. Biol. Pharm. Bull. 2002, 25, 743–747. [Google Scholar]
- Tawab, M.A.; Bahr, U.; Karas, M.; Wurglics, M.; Schubert-Zsilavecz, M. Degradation of ginsenosides in humans after oral administration. Drug Metab. Dispos. 2003, 31, 1065–1071. [Google Scholar] [CrossRef]
- Shin, H.Y.; Lee, J.H.; Lee, J.Y.; Han, Y.O.; Han, M.J.; Kim, D.H. Purification and characterization of ginsenoside Ra-hydrolyzing β-D-xylosidase from bifidobacterium Breve K-110, a human intestinal anaerobic bacterium. Biol. Pharm. Bull. 2003, 26, 1170–1173. [Google Scholar]
- Hasegawa, H.; Sung, J.H.; Huh, J.H. Ginseng intestinal bacterial metabolite IH901 as a new anti-metastatic agent. Arch. Pharm. Res. 1997, 20, 539–544. [Google Scholar]
- Hasegawa, H. Proof of the mysterious efficacy of ginseng: Basic and clinical trials: Metabolic activation of ginsenoside: Deglycosylation by intestinal bacteria and esterification with fatty Acid. J. Pharmacol. Sci. 2004, 95, 153–157. [Google Scholar] [CrossRef]
- Shin, J.E.; Park, E.Y.; Kim, E.J.J.; Hong, Y.H.; Lee, K.T.; Kim, D.H. Cytotoxicity of compound K (IH-901) and ginsenoside Rh2, main biotransformatants of ginseng saponins by bifidobacteria, against some tumor cells. J. Ginseng Res. 2003, 27, 129–134. [Google Scholar] [CrossRef]
- Ming, Y.; Chen, Z.; Chen, L.; Lin, D.; Tong, Q.; Zheng, Z.; Song, G. Ginsenoside compound K attenuates metastatic growth of hepatocellular carcinoma, which is associated with the translocation of nuclear factor-κB p65 and reduction of matrix metalloproteinase-2/9. Planta Med. 2011, 77, 428–433. [Google Scholar] [CrossRef]
- Jeong, A.; Lee, H.J.; Jeong, S.J.; Lee, H.J.; Lee, E.O.; Bae, H.; Kim, S.H. Compound K inhibits basic fibroblast growth factor-induced angiogenesis via regulation of P38 mitogen activated protein kinase and AKT in human umbilical vein endothelial cells. Biol. Pharm. Bull. 2010, 33, 945–950. [Google Scholar] [CrossRef]
- Park, S.; Lee, H.J.; Jeong, S.J.; Song, H.S.; Kim, M.; Lee, H.J.; Lee, E.O.; Kim, D.H.; Ahn, K.S.; Kim, S.H. Inhibition of JAK1/STAT3 signaling mediates compound K-induced apoptosis in human multiple myeloma U266 cells. Food Chem. Toxicol. 2011, 49, 1367–1372. [Google Scholar] [CrossRef]
- Cho, S.H.; Chung, K.S.; Choi, J.H.; Kim, D.H.; Lee, K.T. Compound K, a metabolite of ginseng saponin, induces apoptosis via caspase-8-dependent pathway in HL-60 human leukemia cells. BMC Cancer 2009, 9, 449. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Du, G.-J.; Zhang, Z.Y.; Wen, X.-D.; Calway, T.; Zhen, Z.; Musch, M.W.; Bissonnette, M.; Chang, E.B; Yuan, C.-S. Ginsenoside compound K, not Rb1, possesses potential chemopreventive activities in human colorectal cancer. Int. J. Oncol. 2012, 40, 1970–1976. [Google Scholar]
- Joh, E.H.; Lee, I.A.; Jung, I.H.; Kim, D.H. Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1 activation--the key step of inflammation. Biochem. Pharmacol. 2011, 82, 278–286. [Google Scholar] [CrossRef]
- Han, G.C.; Ko, S.K.; Sung, J.H.; Chung, S.H. Compound K Enhances Insulin Secretion with Beneficial Metabolic Effects In Db/Db Mice. J. Agric. Food Chem. 2007, 55, 10641–10648. [Google Scholar] [CrossRef]
- Li, W.; Zhang, M.; Gu, J.; Meng, Z.J.; Zhao, L.C.; Zheng, Y.N.; Chen, L.; Yang, G.L. Hypoglycemic effect of protopanaxadiol-type ginsenosides and compound K on Type 2 diabetes mice induced by high-fat diet combining with streptozotocin via suppression of hepatic gluconeogenesis. Fitoterapia 2012, 83, 192–198. [Google Scholar] [CrossRef]
- Park, J.S.; Shin, J.A.; Jung, J.S.; Hyun, J.W.; Van, L.T.K.; Kim, D.H.; Park, E.M.; Kim, H.S. Anti-inflammatory mechanism of compound K in activated microglia and its neuroprotective effect on experimental stroke in mice. J. Pharmacol. Exp. Ther. 2012, 341, 59–67. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, Q.; Li, J.Y.; Zhang, X.C.; Zhou, P. Biotransformation of Panax notoginseng saponins into ginsenoside compound K production by Paecilomyces bainier sp. 229. J. Appl. Microbiol. 2008, 104, 699–706. [Google Scholar] [CrossRef]
- Han, Y.; Sun, B.; Hu, X.; Zhang, H.; Jiang, B.; Spranger, M.I.; Zhao, Y. Transformation of bioactive compounds by fusarium sacchari fungus isolated from the soil-cultivated ginseng. J. Agric. Food Chem. 2007, 55, 9373–9379. [Google Scholar]
- Yoo, M.H.; Yeom, S.J.; Park, C.S.; Lee, K.W.; Oh, D.K. Production of aglycon protopanaxadiol via compound K by a thermostable β-glycosidase from Pyrococcus furiosus. Appl. Microbiol. Biotechnol. 2011, 89, 1019–1028. [Google Scholar] [CrossRef]
- Chae, S.; Kang, K.A.; Chang, W.Y.; Kim, M.J.; Lee, S.J.; Lee, Y.S.; Kim, H.S.; Kim, D.H.; Hyun, J.W. Effect of compound K, a metabolite of ginseng saponin, combined with gamma-ray radiation in human lung cancer cells in vitro and in vivo. J. Agric. Food Chem. 2009, 57, 5777–5782. [Google Scholar]
- Atopkin, L.N.; Denisenko, V.A. Synthesis of 20(S)-protopanaxadiol 20-O-β-D-glucopyranoside, a metabolite of Panax ginseng glycosides, and compounds related to it. Chem. Nat. Compd. 2006, 42, 452–458. [Google Scholar]
- Hasegawa, H.; Suzuki, R.; Wakabayashi, C.; Murata, J.; Tezuka, Y.; Saiki, I.; Kadota, S. Synthesis of a biologically active fluorescent derivative of GM1, a main Ginseng saponin metabolite formed by intestinal bacteria. Biol. Pharm. Bull. 1998, 21, 513–516. [Google Scholar] [CrossRef]
- Lei, J.; Li, X.; Gong, X.J.; Zheng, Y.N. Isolation, synthesis and structures of cytotoxic Ginsenoside derivatives. Molecules 2007, 12, 2140–2150. [Google Scholar]
- Smith, A.; Nobmann, P.; Henehan, G.; Bourke, P.; Dunne, J. Synthesis and antimicrobial evaluation of carbohydrate and polyhydroxylated non-carbohydrate fatty acid ester and ether derivatives. Carbohyd. Res. 2008, 343, 2557–2566. [Google Scholar] [CrossRef]
- Kurahashi, T.; Mizutani, T.; Yoshida, J.-I. Functionalized DMAP catalysts for regioselective acetylation of carbohydrates. Tetrahedron 2002, 58, 8669–8677. [Google Scholar] [CrossRef]
- Jansson, P.-E.; Kenne, L.; Schweda, E. Nuclear magnetic resonance and conformational studies on monoacetylated methyl D-Gluco- and D-Galacto-pyranosides. J. Chem. Soc. Perkin Trans. I 1987, 377–383. [Google Scholar] [CrossRef]
- Kawabata, T.; Muramatsu, W.; Nishio, T.; Shibata, T.; Schedel, H. A catalytic one-step process for the chemo- and regioselective acylation of monosaccharides. J. Am. Chem. Soc. 2007, 129, 12890–12895. [Google Scholar]
- Sample Availability: Samples of the 2A, 2B and 2C–7C are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, W.-F.; Chen, L.-R.; Gong, X.-J.; Li, Z.-N.; Li, K.-K. Synthesis of Esters of Ginsenoside Metabolite M1 and Their Cytotoxicity on MGC80-3 Cells. Molecules 2013, 18, 3689-3702. https://doi.org/10.3390/molecules18043689
Li W-F, Chen L-R, Gong X-J, Li Z-N, Li K-K. Synthesis of Esters of Ginsenoside Metabolite M1 and Their Cytotoxicity on MGC80-3 Cells. Molecules. 2013; 18(4):3689-3702. https://doi.org/10.3390/molecules18043689
Chicago/Turabian StyleLi, Wen-Fang, Li-Rong Chen, Xiao-Jie Gong, Zheng-Ning Li, and Ke-Ke Li. 2013. "Synthesis of Esters of Ginsenoside Metabolite M1 and Their Cytotoxicity on MGC80-3 Cells" Molecules 18, no. 4: 3689-3702. https://doi.org/10.3390/molecules18043689
APA StyleLi, W.-F., Chen, L.-R., Gong, X.-J., Li, Z.-N., & Li, K.-K. (2013). Synthesis of Esters of Ginsenoside Metabolite M1 and Their Cytotoxicity on MGC80-3 Cells. Molecules, 18(4), 3689-3702. https://doi.org/10.3390/molecules18043689




