Synthesis and Antimicrobial Activity of Novel Substituted Ethyl 2-(Quinolin-4-yl)-propanoates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
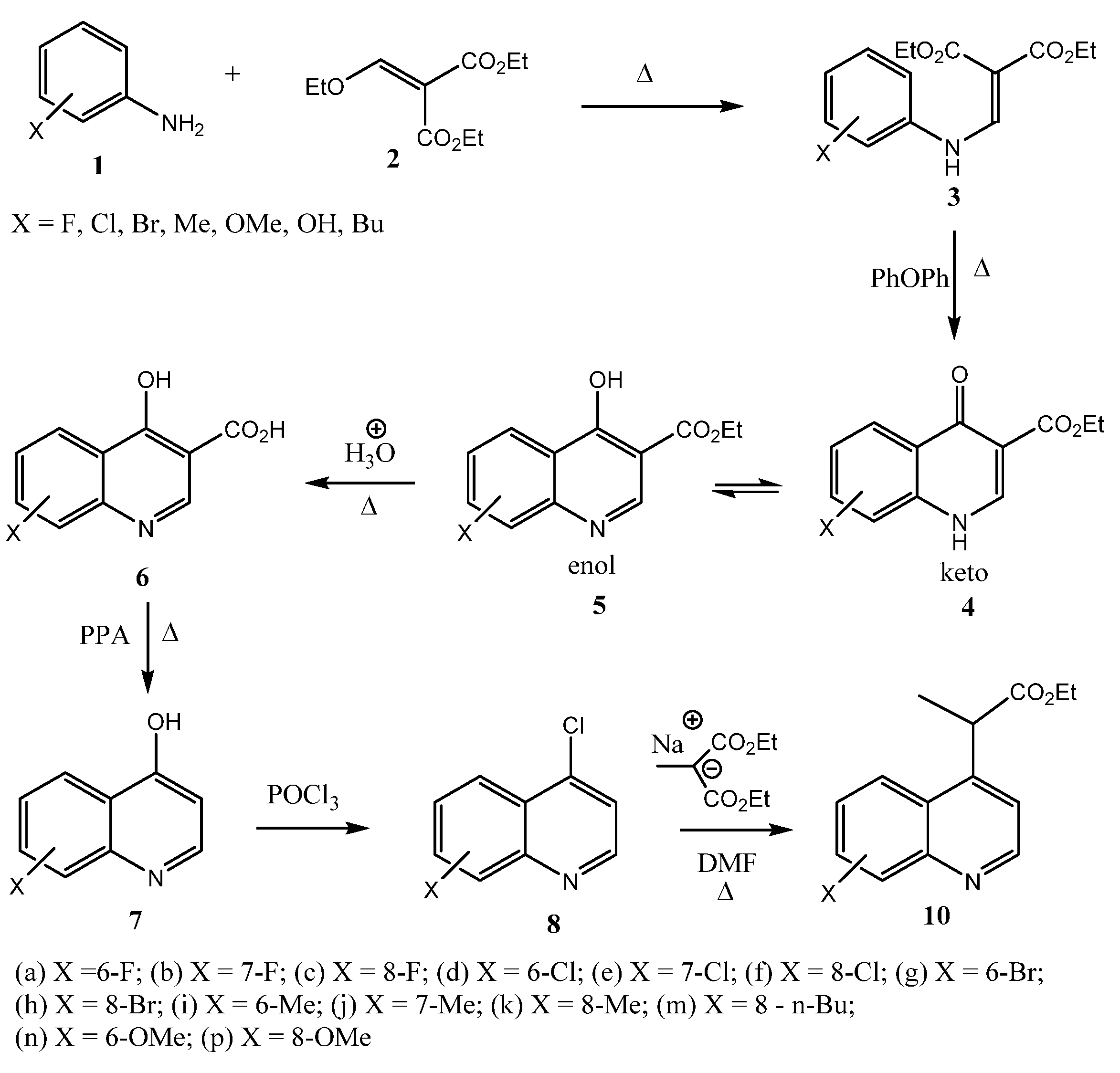
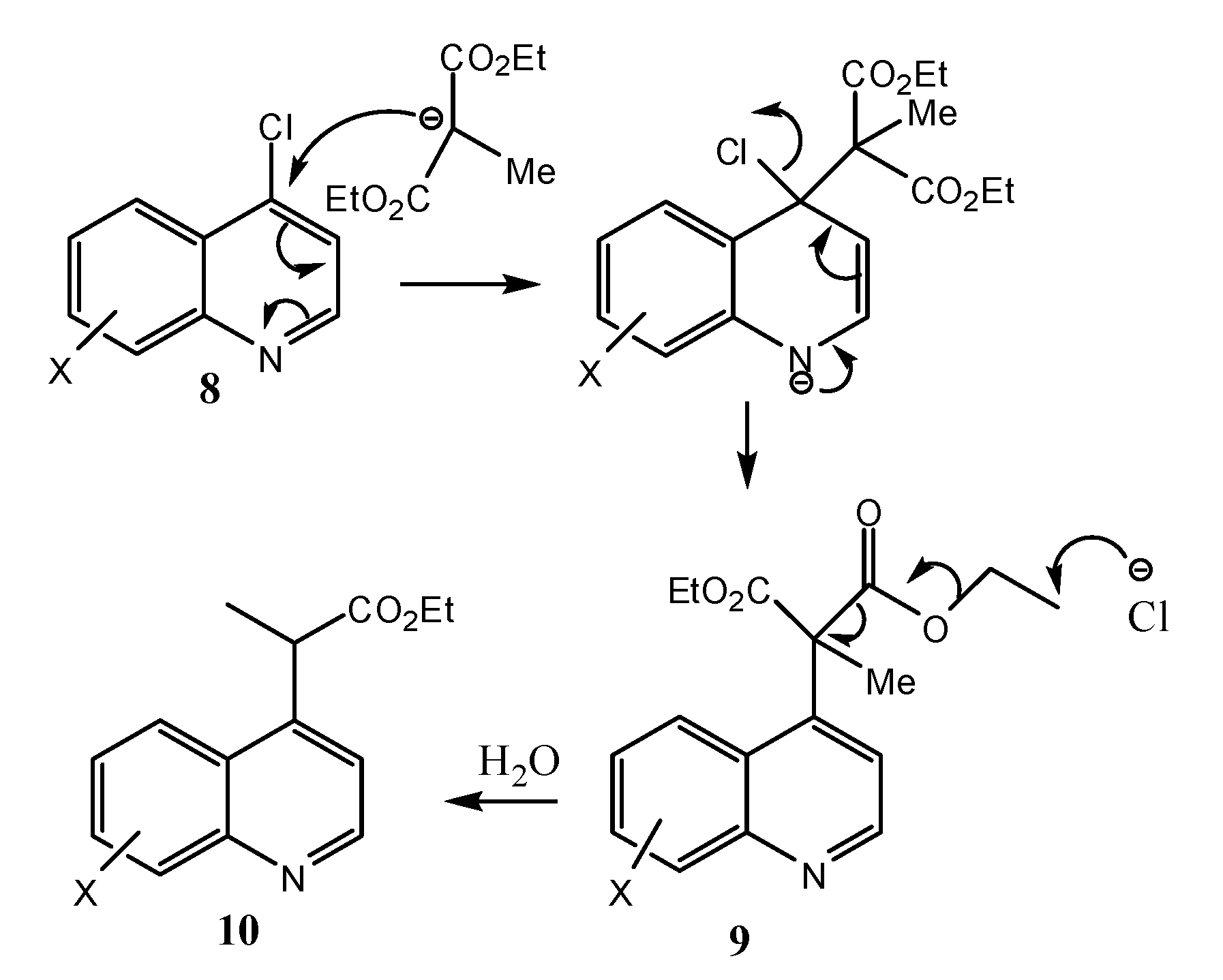
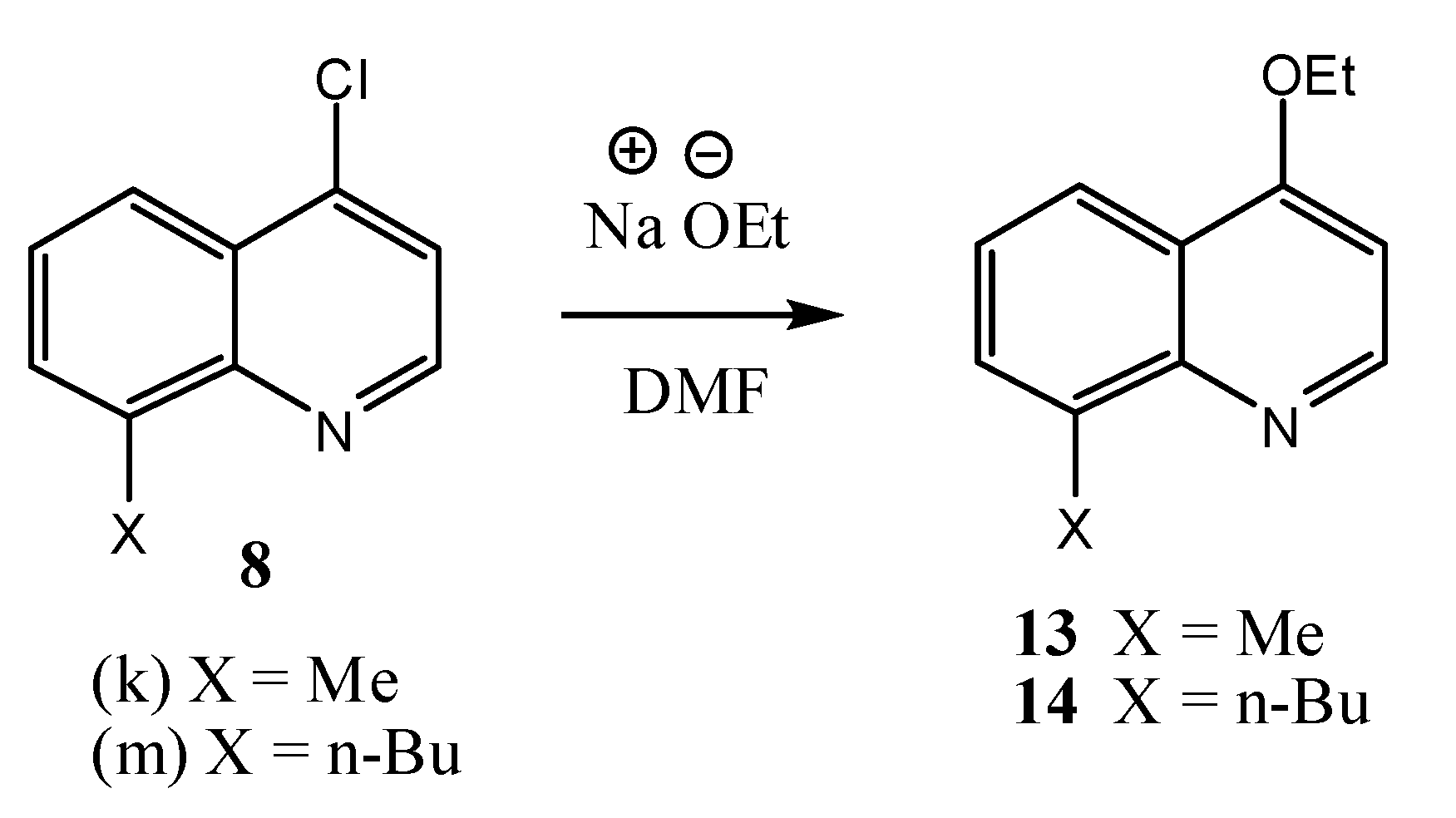
2.2. Microbiological Results and Discussion
| MIC (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gram positive bacteria | Gram negative bacteria | Fungi | ||||||
| Inhibitor | SA | SE | BS | EC | PA | KP | HP | CA |
| 10b | 64 | 512 | 512 | 16 | 64 | >512 | 8 | 128 |
| 10c | 64 | >512 | >512 | 32 | 64 | >512 | 32 | >512 |
| 10e | 128 | 256 | 512 | 64 | 64 | 512 | 16 | 256 |
| 10f | 64 | 512 | 512 | 16 | 128 | >512 | 32 | 256 |
| 10g | 64 | 256 | 512 | 64 | 64 | 512 | 16 | 256 |
| 10i | 128 | 256 | 512 | 16 | 16 | 512 | 64 | 256 |
| 10j | 128 | 512 | 512 | 64 | 128 | 512 | 32 | 256 |
| 10k | 16 | 512 | 256 | 64 | 64 | 512 | 32 | 128 |
| 10n | 32 | 512 | 512 | 16 | 64 | 512 | 64 | 256 |
| 10p | 128 | >512 | 64 | 64 | 128 | >512 | 16 | 256 |
| Ampicillin | 0.032 | 0.032 | 8 | 4 | >512 | 32 | 2 | ND |
| Clarithromycin | 0.5 | 0.5 | ND | ND | ND | ND | 0.25 | ND |
| Fluconazole | ND | ND | ND | ND | ND | ND | ND | 1 |
| Tetracycline | 2 | 2 | 8 | 1 | 32 | 2 | 8 | 512 |


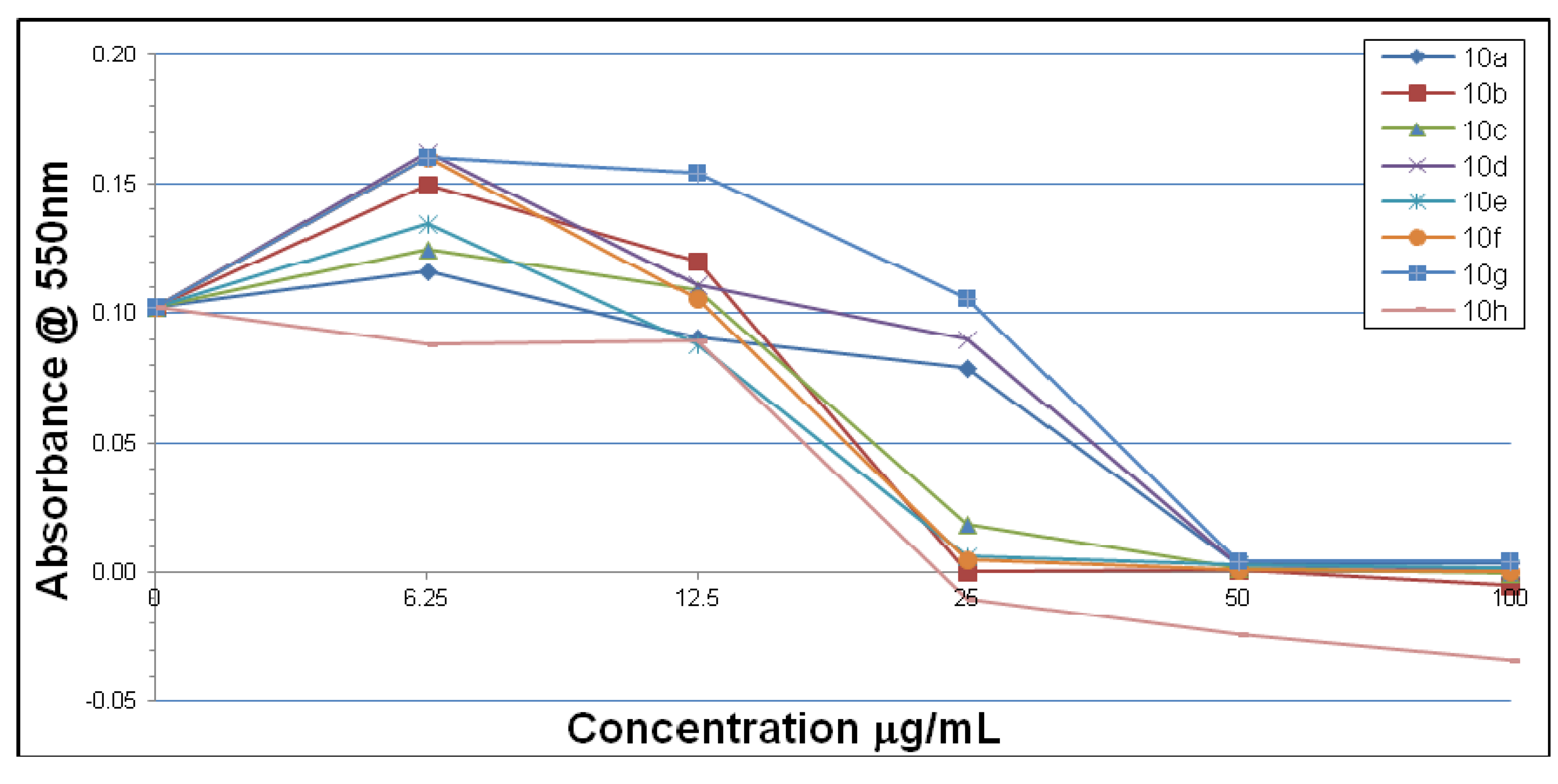

3. Experimental
3.1. Chemistry
3.2. Microbiology
3.2.1. Determination of Susceptibility to Antimicrobial Agents
3.2.2. Culture of H. pylori
3.2.3. Determination of H. pylori Susceptibility to Antimicrobial Agents
3.2.4. Fixed Time-Point Measurements of Membrane Damage
4. Conclusions
Acknowledgments
References
- Anti-Inflammatory Compounds; Williamson, W.R.N. (Ed.) Marcel Dekker: New York, NY, USA, 1987; p. 250.
- Lavrado, J.; Moreira, R.; Paulo, A. Indoloquinolines as scaffolds for drug discovery. Curr. Med. Chem. 2010, 17, 2348–2370. [Google Scholar] [CrossRef]
- Price, C.C.; Boekelheide, V. A synthesis of substituted 4-aminoquinolines. J. Am. Chem. Soc. 1946, 68, 1246–1250. [Google Scholar] [CrossRef]
- Elderfield, R.C.; Gensler, W.J.; Birstein, O.; Kreysa, F.J.; Maynard, J.T.; Galbreath, J. Synthesis of certain simple 4-aminoquinoline derivatives. J. Am. Chem. Soc. 1946, 68, 1250–1251. [Google Scholar] [CrossRef]
- Draker, N.L.; Creech, H.J.; Draper, D.; Garman, J.A.; Haywood, S.; Peck, R.M.; Walton, E; van Hook, J.O. Synthetic antimalarials. The preparation and properties of 7-chloro-4-(4-diethyamino-1-methylbutylamino)-quinoline. J. Am. Chem. Soc. 1946, 68, 1214–1240. [Google Scholar] [CrossRef]
- Fuson, R.C.F.; Parham, W.E.; Reed, L.J. The synthesis of 7-chloro-4-(1-ethyl-4-piperidylamino)-quinoline. J. Am. Chem. Soc. 1946, 68, 1239–1240. [Google Scholar] [CrossRef]
- Ratheesh, M.; Sindhu, G.; Helen, A. Anti-inflammatory effect of quinoline alkaloid skimmianine isolated from Ruta graveolens L. Inflamm. Res. 2013. [Google Scholar] [CrossRef]
- Mukherjee, S.; Pal, M. Quinolines: A new hope against inflammation. Drug Discov. Today 2012. [Google Scholar] [CrossRef]
- Rajanarendar, E.; Nagi, R.M.; Rama Krishna, S.; Rama Murthy, K.; Reddy, Y.N.; Rajam, M.V. Design, synthesis, antimicrobial, anti-inflammatory and analgesic activity of novel isoxazolyl pyrimido[4,5-b]quinolines and isoxazolyl chromeno[2,3-d]pyrimidin-4-ones. Eur. J. Med. Chem. 2012, 55, 273–283. [Google Scholar] [CrossRef]
- Chopra, I. The 2012 Garrod Lecture: Discovery of antibacterial drugs in the 21st century. J. Antimicrob. Chemoth. 2013, in press. [Google Scholar]
- Marshall, B.; Warren, J. Unidentified curved bacilli in the stomach of 99 patients with gastritis and peptic ulceration. Lancet 1984, 1, 1311–1315. [Google Scholar]
- Warren, J.R. Gastric pathology associated with Helicobacter pylori. Gastroenterol. Clin. North Am. 2000, 29, 705–751. [Google Scholar] [CrossRef]
- Warren, J.R. Helicobacter: The ease and difficulty of a new discovery (Nobel lecture). Chem. Med. Chem. 2006, 1, 672–685. [Google Scholar]
- Leung, W.K.; Graham, D.Y. Rescue therapy for Helicobacter pylori. Curr. Treat. Options Infect. Dis. 2002, 5, 133–138. [Google Scholar] [CrossRef]
- Li, H.C.; Stoicov, C.; Cai, X.; Wang, T.C.; Houghton, J. Helicobacter and gastric cancer disease mechanisms: Host response and disease susceptibility. Curr. Gastroenterol. Rep. 2003, 5, 459–467. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer Working Group. Schistosomes, liver flukes and Helicobacter pylori. IARC working group on the evaluation of carcinogenic risks to humans. IARC Monogr. Eval. Carcinog. Risks Hum. 1994, 61, 1–124.
- Kearney, D.J. Helicobacter pylori infection. Curr. Treat. Options Infect. Dis. 2003, 5, 197–206. [Google Scholar]
- Ribeiro, M.L.; Vitiello, L.; Miranda, M.C.; Benvengo, Y.H.; Godoy, A.P.; Mendonca, S.; Pedrazzoli, J., Jr. Mutations in 23s rRNA gene are associated with clarithromycin resistance in H. pylori isolates in Brazil. Ann. Clin. Microbiol. Antimicrob. 2003, 2, 25–30. [Google Scholar]
- Bazzoli, F.; de luca, L.; Graham, D.Y. Helicobacter pylori infection and the use of NSAIDs. Best Pract. Res. Clin. Gastroenterol. 2001, 15, 775–785. [Google Scholar] [CrossRef]
- Wu, C.Y.; Wu, M.S.; Chen, C.J.; Li, M.C.; Lin, J.T.; Chen, G.H. The interaction of H. pylori infection and NSAIDs in cyclooxygenase-2 mRNA expression in gastric antral, corpus mucosa, and gastric ulcer. J. Clin. Gastroenterol. 2005, 39, 50–55. [Google Scholar]
- Brzozowski, T.; Konturek, P.C.; Sliwowski, Z.; Kwiecień, S.; Drozdowicz, D.; Pawlik, M; Mach, K.; Konturek, S.J.; Pawlik, W.W. Interaction of nonsteroidal anti-inflammatory drugs (NSAID) with Helicobacter pylori in the stomach of humans and experimental animals. J. Physiol. Pharmacol. 2006, 57, 67–79. [Google Scholar]
- Smith, M.B. Organic Synthesis; McGraw-Hill: New York, NY, USA, 1994; pp. 1334–1338. [Google Scholar]
- Gould, R.G.; Jacobs, W.A. The synthesis of certain substtituted quinolines and 5,6-benzoquinolines. J. Am. Chem. Soc. 1939, 61, 2890–2895. [Google Scholar] [CrossRef]
- Price, C.C.; Roberts, R.M. The Synthesis of 4-Hydroxyquinolines. I. Through Ethoxymethylenemalonic Esters. J. Am. Chem. Soc. 1946, 68, 1204–1208. [Google Scholar] [CrossRef]
- Price, C.C.; Roberts, R.N. Organic Syntheses Collective; Wiley: New York, NY, 1955; Volume III, p. 272. [Google Scholar]
- Riegel, B.; Lappin, G.R.; Albisetti, C.J., Jr.; Adelson, B.H.; Dodson, R.M.; Ginger, L.G.; Baker, R.H. The Preparation of Some 4-Aminoquinolines. J. Am. Chem. Soc. 1946, 68, 1229–1232. [Google Scholar] [CrossRef]
- Krapcho, A.P.; Glynn, G.A.; Grenon, B.J. The decarbethoxylation of geminal dicarethoxy componds. Tetrahedron Lett. 1967, 8, 215–217. [Google Scholar] [CrossRef]
- Davies, D.T. Aromatic Heterocyclic Chemistry; Oxford University Press: Oxford, UK, 1992; pp. 40–45. [Google Scholar]
- Fuchs, P.; Hess, U.; Holst, H.H.; Lund, H. Electrochemical carboxylation of some heteroaromatic compounds. Acta Chem. Scand. 1981, B35, 185–192. [Google Scholar] [CrossRef]
- Comins, D.L.; Brown, J.D. Regioselective addition of titanium enolates to 1-acylpyridinium salts. A convenient synthesis of 4-(2-oxoalkyl)pyridines. Tetrahedron Lett. 1984, 25, 3297–3300. [Google Scholar] [CrossRef]
- Adger, B.M.; Bannister, N.J.L.; O’Farrell, C. Synthesis of 2-substituted 4-pyridylpropionates. Part 1. Claisen condensation approach. J. Chem. Soc. Perkin Trans. 1 1988, 10, 2785–2789. [Google Scholar]
- Raychaudhuri, A.; Basu, U.P. Side products in the preparation of ethyl 7-chloro-4-hydroxyquinoline-3-carboxylate from diethyl ethoxymalonate and m-chloroaniline. J. Indian Chem. Soc. 1970, 47, 25–32. [Google Scholar]
- Phillips, I.; Andrews, J.M.; Bint, A.J.; Bridson, E.; Brown, D.F.J.; Cooke, E.M.; Greenwood, D.; Holt, H.A.; King, A.; Spencer, R.C.; et al. Guide to Sensitivity Testing. Report of the Working Party on Antibiotic Sensitivity Testing of the British Society for Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 1991, 27, 1–50. [Google Scholar]
- Zhou, Y.; Taylor, B.; Smith, T.J.; Liu, Z.-P.; Clench, M.; Davies, N.W.; Rainsford, K.D. A novel compound from celery seed with a bactericidal effect against Helicobacter pylori. J. Pharm. Pharmacol. 2009, 61, 1067–1077. [Google Scholar]
- Tomb, J.F.; White, O.; Kerlavage, A.R.; Clayton, R.A.; Sutton, G.G.; Fleischmann, R.D.; Ketchum, K.A.; Klenk, H.P.; Gill, S.; Dougherty, B.A.; et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 1997, 388, 539–547. [Google Scholar] [CrossRef]
- Morgan, D.R.; Freedman, R.; Depew, C.E.; Kraft, W.G. Growth of Campylobacter pylori in liquid media. J. Clin. Microbiol. 1987, 25, 2123–2125. [Google Scholar]
- Hilliard, J.J.; Goldschmidt, R.M.; Licata, L.; Baum, E.Z.; Bush, K. Multiple Mechanisms of Action for Inhibitors of Histidine Protein Kinases from Bacterial Two-Component Systems. Antimicrob. Agents Chemother. 1999, 43, 1693–1699. [Google Scholar]
- O’Neill, A.J.; Miller, K.; Oliva, B.; Chopra, I. Comparison of assays for detection of agents causing membrane damage in Staphylococcus aureus. J. Antimicrob. Chemother. 2004, 54, 1127–1129. [Google Scholar] [CrossRef]
- Hobbs, J.K.; Miller, K.; O’Neill, A.J.; Chopra, I. Consequences of daptomycin-mediated membrane damage in Staphylococcus aureus. J. Antimicrob. Chemother. 2008, 62, 1003–1008. [Google Scholar] [CrossRef]
- Dinakaran, M.; Senthilkumar, P.; Yogeeswari, P.; China, A.; Nagaraja, V.; Sriram, D. Antimycobacterial activities of novel 2-(sub)-3-fluoro/nitro-5,12-dihydro-5-oxobenzothiazolo[3,2-a]quinoline-6-carboxylic acid. Bioorg. Med. Chem. 2008, 16, 3408–3418. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 10a–k, 10m, 10n, 10p are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Khan, M.A.; Miller, K.; Rainsford, K.D.; Zhou, Y. Synthesis and Antimicrobial Activity of Novel Substituted Ethyl 2-(Quinolin-4-yl)-propanoates. Molecules 2013, 18, 3227-3240. https://doi.org/10.3390/molecules18033227
Khan MA, Miller K, Rainsford KD, Zhou Y. Synthesis and Antimicrobial Activity of Novel Substituted Ethyl 2-(Quinolin-4-yl)-propanoates. Molecules. 2013; 18(3):3227-3240. https://doi.org/10.3390/molecules18033227
Chicago/Turabian StyleKhan, M. Akram, Keith Miller, Kim Drummond Rainsford, and Yong Zhou. 2013. "Synthesis and Antimicrobial Activity of Novel Substituted Ethyl 2-(Quinolin-4-yl)-propanoates" Molecules 18, no. 3: 3227-3240. https://doi.org/10.3390/molecules18033227
APA StyleKhan, M. A., Miller, K., Rainsford, K. D., & Zhou, Y. (2013). Synthesis and Antimicrobial Activity of Novel Substituted Ethyl 2-(Quinolin-4-yl)-propanoates. Molecules, 18(3), 3227-3240. https://doi.org/10.3390/molecules18033227




