Main Alkaloids of Peganum harmala L. and Their Different Effects on Dicot and Monocot Crops
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytotoxic Assays of Different Plant Parts of P. harmala
| Wheat | Lettuce | |||
|---|---|---|---|---|
| Root length (cm) | Shoot length (cm) | Root length (cm) | Shoot length (cm) | |
| Control | 6.01 ± 0.45 a | 2.12 ± 0.10 a | 3.55 ± 0.08 a | 1.38 ± 0.05 a |
| Leaf | 1.54 ± 0.06 b | 1.11 ± 0.02 bc | 0.31 ± 0.01 b | 0.53 ± 0.04 bc |
| Stem | 0.96 ± 0.31 b | 1.55 ± 0.03 b | 0.28 ± 0.06 bc | 0.87 ± 0.23 b |
| Root | 1.01 ± 0.25 b | 0.99 ± 0.29 c | 0.15 ± 0.01 c | 0.45 ± 0.01 cd |
| Seed | 0.61 ± 0.09 b | 0.78 ± 0.03 c | 0.14 ± 0.01 c | 0.13 ± 0.01 d |
2.2. Isolation and Identification of Two Toxic Alkaloids from Seeds of P. Harmala
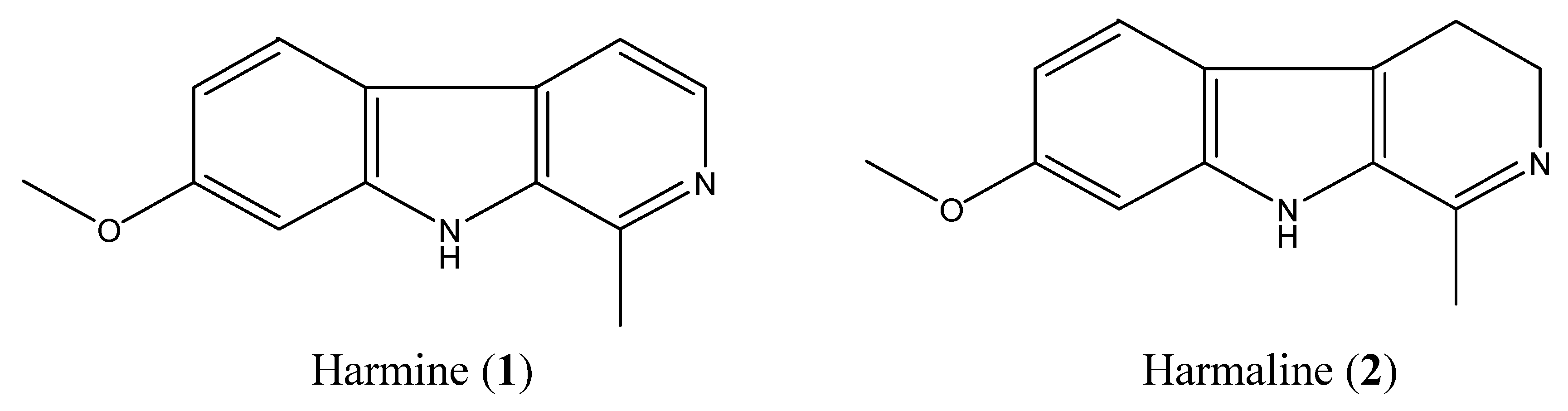
2.3. High-Performance Liquid Chromotography (HPLC) Analysis of Harmine and Harmaline
2.4. Phytotoxic Effects of Total Alkaloids, Harmine and Harmaline
| Concentration | Amaranth | Lettuce | Wheat | Ryegrass | |||||
|---|---|---|---|---|---|---|---|---|---|
| (µg/mL) | Root length | Shoot length | Root length | Shoot length | Root length | Shoot length | Root length | Shoot length | |
| Total alkaloids | 0 | 3.49 ± 0.16 a | 2.60 ± 0.09 a | 4.71 ± 0.18 a | 2.23 ± 0.07 a | 9.04 ± 0.28 a | 3.34 ± 0.15 a | 3.80± 0.19 a | 3.03 ± 0.15 a |
| 1 | 3.12 ± 0.16 b | 1.98 ± 0.10 b | 4.61 ± 0.21 a | 1.76 ± 0.06 b | 8.37 ± 0.24 ab | 3.42 ± 0.16 a | 3.85 ± 0.20 a | 3.04 ± 0.15 a | |
| 5 | 3.10 ± 0.18 b | 1.97 ± 0.10 b | 4.58 ± 0.17 a | 1.80 ± 0.06 b | 8.24 ± 0.35 b | 3.19 ± 0.15 ab | 4.05 ± 0.26 a | 2.70 ± 0.16 ab | |
| 20 | 2.00 ± 0.15 c | 1.94 ± 0.10 b | 3.29 ± 0.13 b | 1.54 ± 0.05 c | 7.67 ± 0.28 b | 2.99 ± 0.15 abc | 3.95 ± 0.20 a | 3.03± 0.13 a | |
| 100 | 0.48 ± 0.03 d | 0.84± 0.06 c | 1.14 ± 0.06 c | 0.93 ± 0.05 d | 5.44 ± 0.28 c | 2.88 ± 0.13 bc | 2.07 ± 0.14 b | 2.31 ± 0.15 b | |
| 500 | 0.26± 0.01 d | 0.43 ± 0.03 d | 0.50 ± 0.04 d | 0.52 ± 0.03 e | 2.70± 0.19 d | 2.58 ± 0.12 c | 0.67 ± 0.10 c | 2.64 ± 0.21 ab | |
| Harmine | 0 | 3.49± 0.16 a | 2.60± 0.09 a | 4.71 ± 0.18 a | 2.23 ± 0.07 a | 9.04 ± 0.28 a | 3.34 ± 0.15 a | 3.80± 0.19 ab | 3.03 ± 0.15 ab |
| 1 | 3.67 ± 0.19 a | 2.36 ± 0.16 ab | 4.95 ± 0.18 a | 2.10 ± 0.06 ab | 9.21 ± 0.28 a | 3.53± 0.15 a | 4.30 ± 0.27 ab | 3.10 ± 0.14 ab | |
| 5 | 3.76 ± 0.28 a | 2.27 ± 0.09 b | 4.87 ± 0.22 a | 2.10 ± 0.06 ab | 9.11 ± 0.33 a | 3.22 ± 0.14 ab | 4.19 ± 0.19 a | 3.19 ± 0.17 a | |
| 20 | 2.95 ± 0.19 b | 2.23 ± 0.11 b | 4.09± 0.18 b | 1.94 ± 0.07 b | 8.44 ± 0.34 a | 3.16± 0.13 ab | 3.33 ± 0.23 b | 2.60 ± 0.14 c | |
| 100 | 1.19 ± 0.08 c | 1.74 ± 0.09 c | 3.41± 0.18 c | 1.43 ± 0.04 c | 6.16 ± 0.38 b | 2.84 ± 0.16 b | 3.29 ± 0.17 b | 2.38± 0.14 c | |
| 500 | 0.17 ± 0.05 d | 1.30 ± 0.08 d | 2.20 ± 0.24 d | 1.20± 0.05 d | 3.83 ± 0.19 c | 2.70 ± 0.11 bc | 2.11 ± 0.15 c | 2.72± 0.12 bc | |
| Harmaline | 0 | 3.49± 0.16 a | 2.60± 0.09 a | 4.71 ± 0.18 a | 2.23 ± 0.07 a | 9.04 ± 0.28 a | 3.34 ± 0.15 a | 3.80± 0.19 a | 3.03 ± 0.15a |
| 1 | 1.89 ± 0.18 b | 2.16 ± 0.11 b | 4.18 ± 0.23 b | 1.87 ± 0.06 b | 8.31 ± 0.36 a | 3.13 ± 0.12 ab | 3.93 ± 0.23 a | 3.26± 0.14 a | |
| 5 | 1.84 ± 0.17 b | 2.19 ± 0.14 b | 3.26 ± 0.12 c | 1.85 ± 0.07 b | 7.30 ± 0.26 b | 3.07 ± 0.14 ab | 3.78 ± 0.19 a | 3.28 ± 0.16 a | |
| 20 | 0.94 ± 0.08 c | 1.40 ± 0.08 c | 1.78 ± 0.11 d | 1.46 ± 0.03 c | 6.45 ± 0.25 c | 2.82 ± 0.14 bc | 2.22 ± 0.14 b | 3.14 ± 0.14 a | |
| 100 | 0.40 ± 0.02 d | 0.29 ± 0.03 d | 0.54 ± 0.16 e | 0.48 ± 0.07 d | 3.43 ± 0.20 d | 2.54 ± 0.15 c | 0.50± 0.07 c | 2.58 ± 0.13 b | |
| 500 | 0.22 ± 0.02 d | 0.20 ± 0.02 d | 0.20 ± 0.01 e | 0.00 ± 0.00 e | 1.58 ± 0.19 e | 1.88 ± 0.16 d | 0.02 ± 0.01 d | 0.56 ± 0.17 c | |
| Glyphosate | 0 | 3.49± 0.16 a | 2.60± 0.09 a | 4.71 ± 0.18 a | 2.23 ± 0.07 a | 9.04 ± 0.28 a | 3.34 ± 0.15 bc | 3.80± 0.19 b | 3.03 ± 0.15 c |
| 1 | 3.81 ± 0.17 a | 2.11 ± 0.06 b | 4.29 ± 0.27 b | 2.24± 0.06 a | 10.13 ± 0.34 b | 4.44 ± 0.34 a | 4.63 ± 0.20 a | 4.21 ± 0.12 a | |
| 5 | 3.47 ± 0.19 a | 2.19 ± 0.07 b | 4.02 ± 0.18 b | 2.17 ± 0.06 ab | 7.99± 0.31 c | 3.81 ± 0.20 b | 2.32 ± 0.13 c | 3.52 ± 0.13 b | |
| 20 | 2.57 ± 0.11 b | 2.50 ± 0.09 a | 1.63 ± 0.06 c | 1.91 ± 0.09 c | 4.34 ± 0.21 d | 3.10 ± 0.20 c | 1.33 ± 0.09 d | 3.25 ± 0.11 bc | |
| 100 | 1.46 ± 0.08 c | 2.48 ± 0.06 a | 1.10 ± 0.06 d | 2.01 ± 0.07 bc | 2.72 ± 0.15 e | 1.84 ± 0.13 d | 0.87 ± 0.05 e | 2.63± 0.11 d | |
| 500 | 0.95 ± 0.06 d | 2.61 ± 0.09 a | 0.70 ± 0.04 e | 1.46 ± 0.04 d | 1.45 ± 0.19 f | 0.82 ± 0.12 e | 0.10 ± 0.03 f | 0.19 ± 0.07 e | |
3. Experimental
3.1. Instrumentation
3.2. Materials and Reagents
3.3. Phytotoxic Effects of Ethanol Extracts of Different Plant Parts
3.4. Extraction and Isolation of Phytotoxins
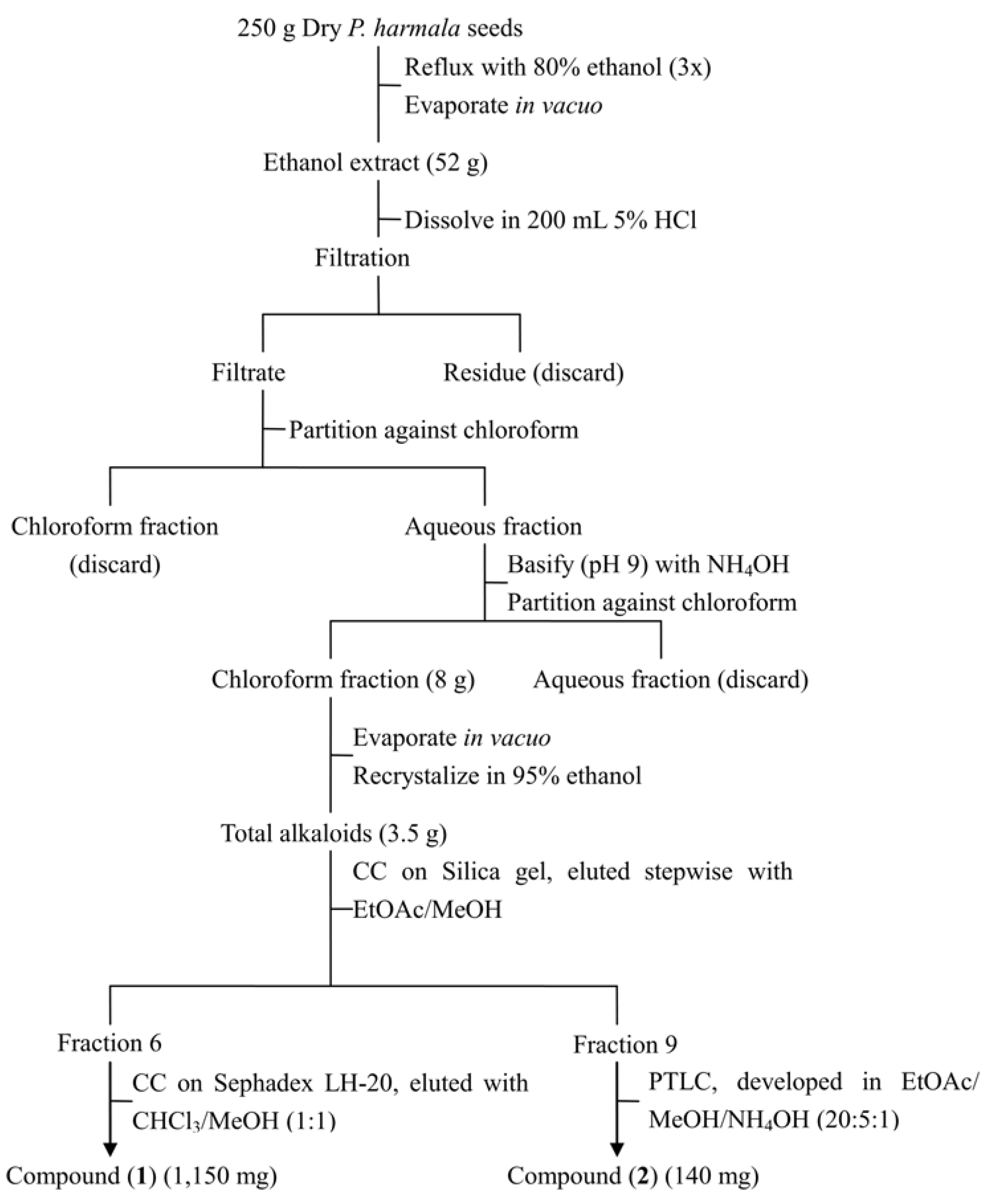
3.5. HPLC Analysis of Harmine and Harmaline
3.6. Phytotoxic Effects of Total Alkaloids, Purified Harmine and Harmaline
3.7. Statistical Analyses
4. Conclusions
Acknowledgments
References
- Kartal, M.; Altun, M.L.; Kurucu, S. HPLC method for the analysis of harmol, harmalol, harmin and harmaline in the seeds of Peganum harmala L. J. Pharm. Biomed. Anal. 2003, 31, 263–269. [Google Scholar] [CrossRef]
- Abbott, L.B.; Bettmann, G.T.; Sterling, T.M. Physiology and recovery of african rue (Peganum harmala) seedlings under water-deficit stress. Weed Sci. 2008, 56, 52–57. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, Z.T.; Branford-White, C.J.; Xu, H.; Wang, C.H. Classification and differentiation of the genus Peganum indigenous to China based on chloroplast trnL-F and psbA-trnH sequences and seed coat morphology. Plant Biol. (Stuttg). 2011, 13, 940–947. [Google Scholar] [CrossRef]
- Duan, J.A.; Zhou, R.H.; Zhao, S.X.; Wang, M.S.; Che, C.T. Studies on the chemical constituents of Peganum multisectum Maxim II. The alkaloids from seeds and antitumour activity. J. Chin. Pharma. Univ. 1998, 29, 21–23. [Google Scholar]
- Chatterjee, A.; Ganguly, M. Alkaloidal constituents of Peganum harmala and synthesis of the minor alkaloid deoxyvascinone. Phytochemistry 1968, 7, 307–311. [Google Scholar] [CrossRef]
- Sharaf, M.; El-Ansari, M.A.; Matlin, S.A.; Saleh, N.A.M. Four flavonoid glycosides from Peganum harmala. Phytochemistry 1997, 44, 533–536. [Google Scholar]
- Movafeghi, A.; Abedini, M.; Fathiazad, F.; Aliasgharpour, M.; Omidi, Y. Floral nectar composition of Peganum harmala L. Nat. Prod. Res. 2009, 23, 301–308. [Google Scholar] [CrossRef]
- Xing, M.; Shen, F.; Liu, L.; Chen, Z.; Guo, N.; Wang, X.; Wang, W.; Zhang, K.; Wu, X.; Wang, X.; et al. Antimicrobial efficacy of the alkaloid harmaline alone and in combination with chlorhexidine digluconate against clinical isolates of Staphylococcus aureus grown in planktonic and biofilm cultures. Lett. Appl. Microbiol. 2012, 54, 475–482. [Google Scholar] [CrossRef]
- Herraiz, T.; Gonzaleza, D.; Ancin-Azpilicuetac, C.; Aranb, V.J.; Guillena, H. β-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO). Food Chem. Toxicol. 2010, 48, 839–845. [Google Scholar] [CrossRef]
- Farouk, L.; Laroubi, A.; Aboufatima, R.; Benharref, A.; Chait, A. Evaluation of the analgesic effect of alkaloid extract of Peganum harmala L.: Possible mechanisms involved. J. Ethnopharmacol. 2008, 115, 449–454. [Google Scholar] [CrossRef]
- Lamchouri, F.; Settaf, A.; Cherra, Y.; Hassar, M.; Zemzami, M.; Atif, N.; Nadori, E.B.; Zaid, A.; Lyoussi, B. In vitro cell-toxicity of Peganum harmala alkaloids on cancerous cell-lines. Fitoterapia 2000, 71, 50–54. [Google Scholar] [CrossRef]
- Berrougui, H.; Cordero, M.; Khalil, A.; Hamamouchia, M.; Ettiab, A.; Marhuenda, E.; Herrara, M. Vasorelaxant effects of harmine and harmaline extracted from Peganum harmala L. seeds in isolated rat aorta. Pharmacol. Res. 2006, 54, 150–157. [Google Scholar] [CrossRef]
- Sodaeizadeh, H.; Rafieiolhossaini, M.; Havlik, J.; Damme, P.V. Allelopathic activity of different plant parts of Peganum harmala L. and identification of their growth inhibitors substances. Plant Growth Regul. 2009, 59, 227–236. [Google Scholar] [CrossRef]
- Inderjit; Nilsen, E.T. Bioassays and field studies for allelopathy in terrestrial plants: Progress and problems. Crit. Rev. Plant Sci. 2003, 22, 221–238. [Google Scholar] [CrossRef]
- Callaway, R.M.; Aschehoug, E.T. Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science 2000, 290, 521–523. [Google Scholar] [CrossRef]
- Shao, H.; Huang, X.; Wei, X.; Zhang, C. Phytotoxic effects and a phytotoxin from the invasive plant Xanthium italicum Moretti. Molecules 2012, 17, 4037–4046. [Google Scholar] [CrossRef]
- Shanab, S.M.M.; Shalaby, E.A.; Lightfoot, D.A.; El-Shemy, H.A. Allelopathic effects of water hyacinth (Eichhornia crassipes). 2010; 5, e13200. [Google Scholar] [CrossRef]
- Blair, A.C.; Nissen, S.J.; Brunk, G.R.; Hufbauer, R.A. A lack of evidence for an ecological role of the putative allelochemical (+/−)-catechin in spotted knapweed invasion success. J. Chem. Ecol. 2006, 32, 2327–2331. [Google Scholar] [CrossRef]
- Gibson, D.M.; Krasnoff, S.B.; Biazzo, J.; Milbrath, L. Phytotoxicity of antofine from invasive swallow-worts. J. Chem. Ecol. 2011, 37, 871–879. [Google Scholar] [CrossRef]
- Ens, E.J.; French, K.; Bremner, J.B. Evidence for allelopathy as a mechanism of community composition change by an invasive exotic shrub, Chrysanthemoides monilifera spp. rotundata. Plant Soil 2009, 316, 125–137. [Google Scholar] [CrossRef]
- Bhowmik, P.C.; Inderjit. Challenges and opportunities in implementing allelopathy for natural weed management. Crop. Prot. 2003, 22, 661–671. [Google Scholar] [CrossRef]
- Liu, J.; Hu, H.; Zhao, G. Effects of alkaloid extract from Peganum multisectum on growth and some physiological characteristics of Zea mays seedlings [in Chinese]. Acta Prataculturae Sin. 2007, 1, 75–80. [Google Scholar]
- Liu, J.; Hu, H.; Wang, X. Study on Allelopathy of aqueous extract from Peganum multisectum (Maxim.) Bobr. on perennial ryegrass (Lolium perenne L.) and its physiological biochemical manifestation. [in Chinese]. Acta Agrestia Sin. 2008, 16, 374–379. [Google Scholar]
- Khan, A.M.; Qureshi, R.A.; Ullah, F.; Gilani, S.A. Phytotoxic effects of selected medicinal plants collected from Margalla Hills, Islamabad Pakistan. J. Med. Plants Res. 2011, 5, 4671–4675. [Google Scholar]
- Sodaeizadeh, H.; Rafieiolhossaini, M.; van Damme, P. Herbicidal activity of a medicinal plant, Peganum harmala L., and decomposition dynamics of its phytotoxins in the soil. Ind. Crops Prod. 2010, 31, 385–394. [Google Scholar] [CrossRef]
- Chon, S.U.; Jang, H.G.; Kim, D.K.; Kim, Y.M.; Boo, H.O.; Kim, Y.J. Allelopathic potential in lettuce (Lactuca sativa L.) plants. Sci. Hortic. 2005, 106, 309–317. [Google Scholar]
- Wang, X.; Geng, Y.; Wang, D.J.; Shi, X.G.; Liu, J.H. Separation and purification of harmine and harmaline from Peganum harmala using pH-zone-refining counter-current chromatography. J. Sep. Sci. 2008, 31, 3543–3547. [Google Scholar] [CrossRef]
- Macías, F.A.; Lacret, R.; Varela, R.M.; Nogueiras, C.; Molinillo, J.M.G. Isolation and phytotoxicity of terpenes from Tectona grandis. J. Chem. Ecol. 2010, 36, 396–404. [Google Scholar] [CrossRef]
- Shao, H.; Peng, S.; Wei, X.; Zhang, D.; Zhang, C. Potential allelochemicals from an invasive weed Mikania micrantha H.B.K. J. Chem. Ecol. 2005, 31, 1657–1668. [Google Scholar] [CrossRef]
- Popovici, J.; Bertrand, C.; Jacquemoud, D.; Bellvert, F.; Fernandez, M.P.; Comte, G.; Piola, F. An allelochemical from myrica gale with strong phytotoxic activity against highly invasive fallopia x bohemica taxa. Molecules 2011, 16, 2323–2333. [Google Scholar] [CrossRef]
- Inderjit; Dakshini, K.M.M. On laboratory bioassays in allelopathy. Bot. Rev. 1995, 61, 28–44. [Google Scholar] [CrossRef]
- Inderjit; Weston, L.A. Are laboratory bioassays for allelopathy suitable for prediction of field responses? J. Chem. Ecol. 2000, 26, 2111–2118. [Google Scholar] [CrossRef]
- Sosa, T.; Valares, C.; Alias, J.C.; Lobon, C. Persistence of flavonoids in Cistus ladanifer soils. Plant Soil 2010, 337, 51–63. [Google Scholar] [CrossRef]
- Inderjit; Bhowmik, P.C. Sorption of benzoic acid onto soil colloids and its implications for allelopathy studies. Biol. Fert. Soils 2004, 40, 345–348. [Google Scholar] [CrossRef]
- Jilani, G.; Mahmood, S.; Chaudhry, A.N.; Hassan, I.; Akram, M. Allelochemicals: Sources, toxicity and microbial transformation in soil—A review. Ann. Microbiol. 2008, 58, 351–357. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, L.; Hu, Z. Xeromorphic characters in the vegetative organs of Peganum harmala. Acta Phytoeco. Geobot. Sin. 1992, 16, 243–248. [Google Scholar]
- Asgarpanah, J.; Ramezanloo, F. Chemistry, pharmacology and medicinal properties of Peganum harmala L. Afr. J. Pharm. Pharmacol. 2012, 6, 1573–1580. [Google Scholar]
- Heap, I.M. The occurrence of herbicide-resistant weeds worldwide. Pestic. Sci. 1997, 51, 235–243. [Google Scholar] [CrossRef]
- Vyvyan, J.R. Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 2002, 58, 1631–1646. [Google Scholar] [CrossRef]
- Mitchell, G.; Bartlett, D.W.; Fraser, T.E.; Hawkes, T.R.; Holt, D.C.; Townson, J.K.; Wichert, R.A. Mesotrione: A new selective herbicide for use in maize. Pest Manag. Sci. 2001, 57, 120–128. [Google Scholar] [CrossRef]
- Grayson, B.T.; Williams, K.S.; Freehauf, P.A.; Pease, R.R.; Ziesel, W.T.; Sereno, R.L.; Reinsfelder, R.E. The physical and chemical properties of the herbicide cinmethylin (SD 95481). Pestic. Sci. 1987, 21, 143–153. [Google Scholar] [CrossRef]
- Duke, S.O.; Dayan, F.E.; Romangni, J.G.; Rimando, A.M. Natural products as sources of herbicides: current status and future trends. Weed Res. 2000, 40, 99–111. [Google Scholar] [CrossRef]
- Weston, L.A. Utilization of allelopathy for weed management in agroecosystems. Agron. J. 1996, 88, 860–866. [Google Scholar] [CrossRef]
- Sample Availability: Sample of harmine is available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shao, H.; Huang, X.; Zhang, Y.; Zhang, C. Main Alkaloids of Peganum harmala L. and Their Different Effects on Dicot and Monocot Crops. Molecules 2013, 18, 2623-2634. https://doi.org/10.3390/molecules18032623
Shao H, Huang X, Zhang Y, Zhang C. Main Alkaloids of Peganum harmala L. and Their Different Effects on Dicot and Monocot Crops. Molecules. 2013; 18(3):2623-2634. https://doi.org/10.3390/molecules18032623
Chicago/Turabian StyleShao, Hua, Xiaoli Huang, Yuanming Zhang, and Chi Zhang. 2013. "Main Alkaloids of Peganum harmala L. and Their Different Effects on Dicot and Monocot Crops" Molecules 18, no. 3: 2623-2634. https://doi.org/10.3390/molecules18032623






