Improvement of Ylang-Ylang Essential Oil Characterization by GC×GC-TOFMS
Abstract
:1. Introduction
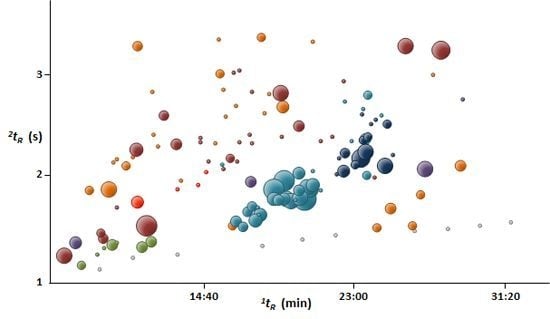
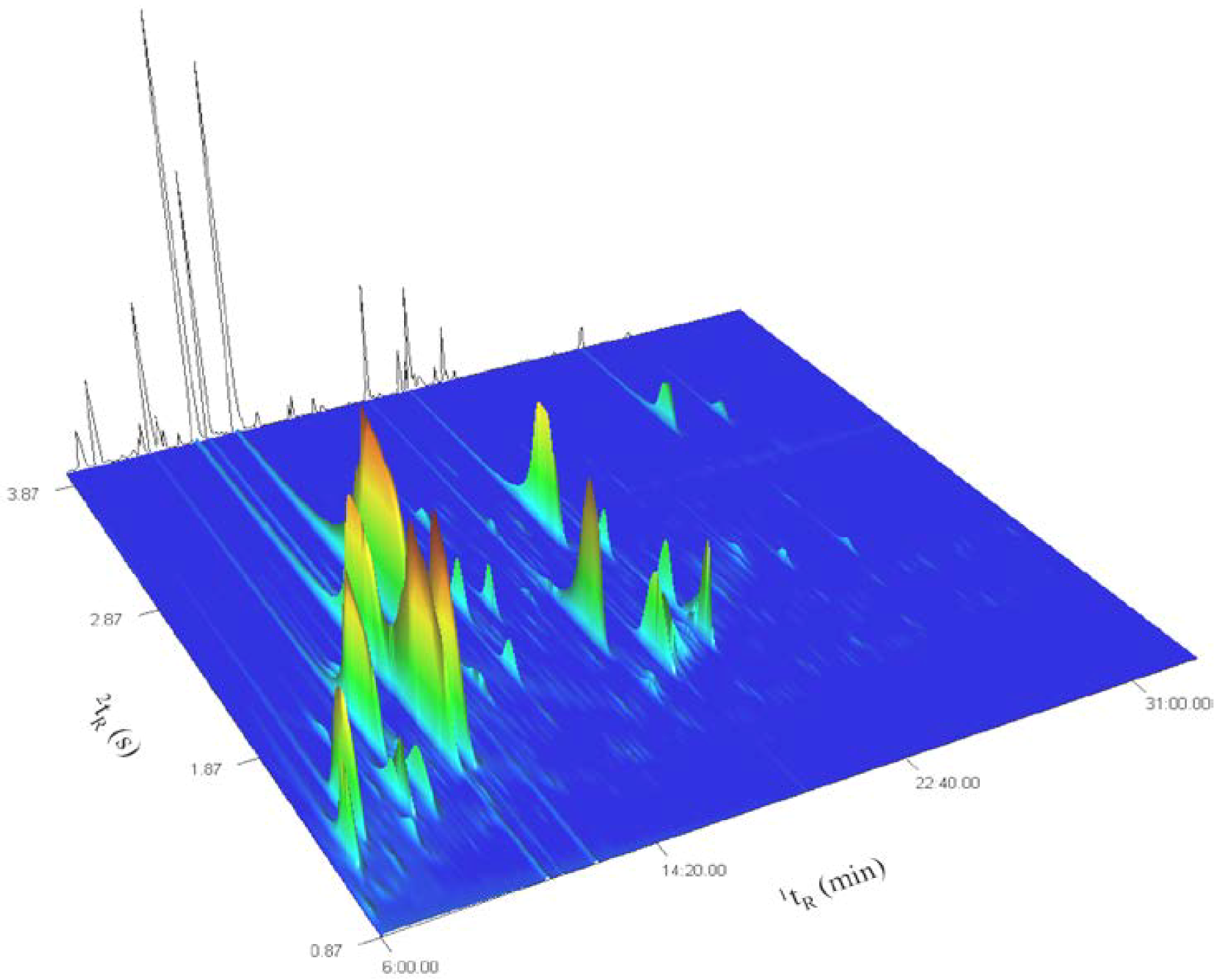
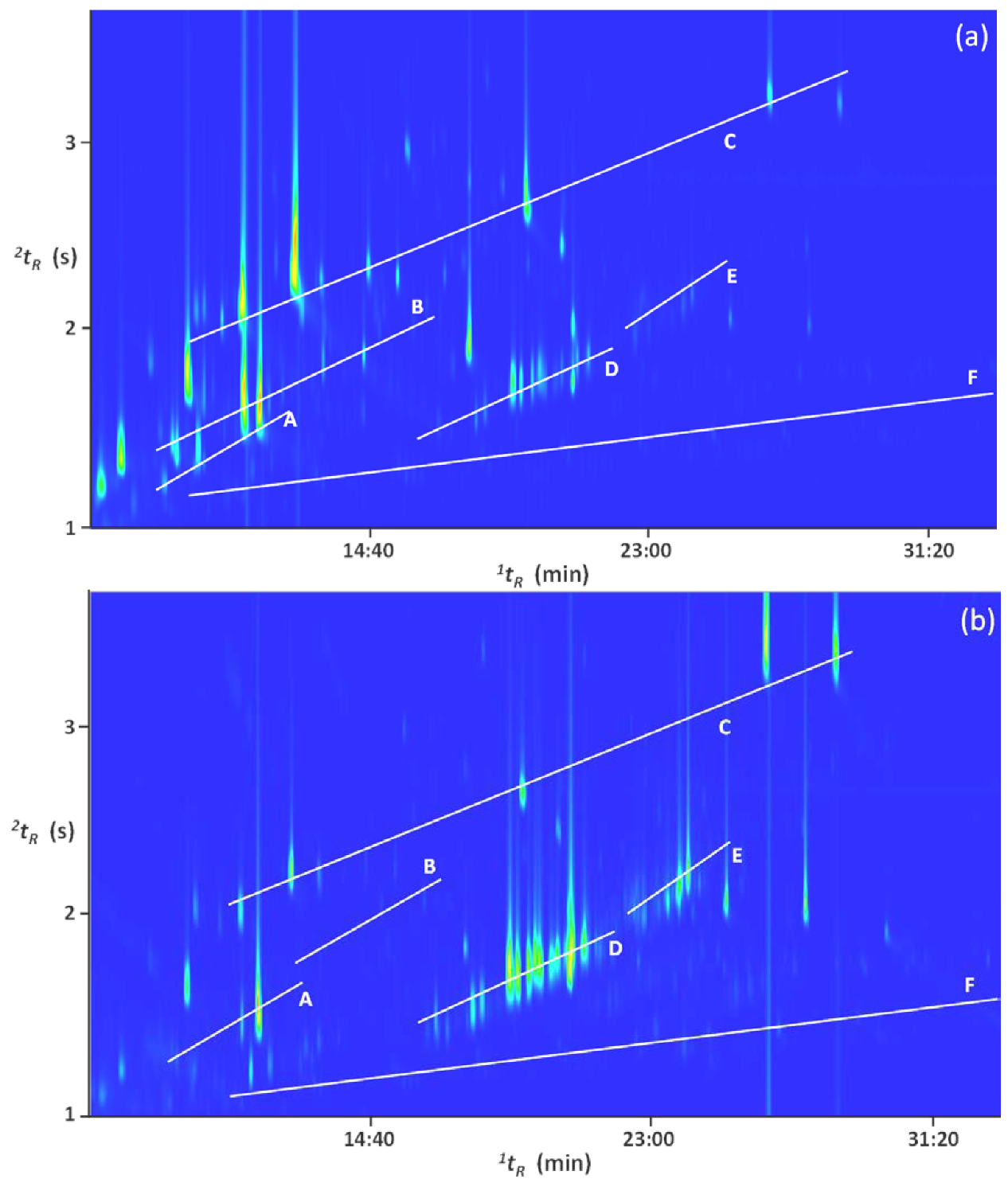
2. Results and Discussion

| No. | Name | IT | 1tR (min:s), 2tR (s) | Formula | Relative abundance % | |
|---|---|---|---|---|---|---|
| Fraction 1 | Fraction 4 | |||||
| 1 | 3-hexen-1-ol # | 867 | 6:16, 1.28 | C6H12O | tr | - |
| 2 | 3-methyl-3-buten-1-ol acetate | 885 | 6:36, 1.26 | C7H12O2 | 2.16 | 0.22 |
| 3 | heptanal | 907 | 7:00, 1.29 | C7H14O | 0.05 † | - |
| 4 | 3-methyl-2-butenyl acetate | 923 | 7:16, 1.38 | C7H12O2 | 4.24 | 0.32 |
| 5 | α-pinene | 941 | 7:36, 1.17 | C10H16 | 0.1 | 0.04 |
| 6 | benzaldehyde | 967 | 8:04, 1.87 | C7H6O | 0.21 | 0.05 |
| 7 | sabinene # | 978 | 8:16, 1.27 | C10H16 | tr | - |
| 8 | 6-methyl-5-hepten-2-one | 986 | 8:24, 1.52 | C8H14O | 0.07 | 0.03 † |
| 9 | β-myrcene | 993 | 8:32, 1.27 | C10H16 | 0.29 | tr |
| 10 | decane * | 1000 | 8:40, 1.13 | C10H22 | tr | 0.03 |
| 11 | ( 3Z)-3-hexenyl acetate | 1003 | 8:44, 1.47 | C8H14O2 | 0.5 | 0.03 |
| 12 | n-hexyl acetate | 1009 | 8:52, 1.42 | C8H16O2 | 0.97 | 0.07 |
| 13 | α-phellandrene * | 1012 | 8:56, 1.33 | C10H16 | tr | tr |
| 14 | p-cresyl methyl ether | 1025 | 9:12, 1.88 | C8H10O | 9.7 | 1.63 |
| 15 | β-limonene* | 1034 | 9:24, 1.36 | C10H16 | 0.13 | 0.15 |
| 16 | 1,8-cineole | 1037 | 9:28, 1.45 | C10H18O | 1.11 | 0.22 |
| 17 | benzyl alcohol | 1037 | 9:28, 2.13 | C7H8O | 0.53 | tr |
| 18 | β-ocimene * | 1046 | 9:40, 1.38 | C10H16 | 0.07 | tr |
| 19 | ester (MW 174) | 1046 | 9:40, 1.71 | C8H14O4 | 0.28 | tr |
| 20 | phenyl acetaldehyde | 1046 | 9:40, 2.16 | C8H8O | 0.17 | tr † |
| 21 | p-cresol | 1070 | 10:12, 2.10 | C7H8O | 0.19 | 0.05 |
| 22 | cis-linalool oxide (furanoid) | 1073 | 10:16, 1.53 | C10H18O2 | 0.02 | - |
| 23 | trans-linalool oxide (furanoid) | 1088 | 10:36, 1.59 | C10H18O2 | tr † | - |
| 24 | 2-methoxyphenol | 1089 | 10:36, 2.18 | C7H8O2 | 0.7 | tr |
| 25 | terpinolene # | 1097 | 10:48, 1.78 | C10H16 | 0.07 | - |
| 26 | methyl benzoate | 1098 | 10:48, 2.25 | C8H8O2 | 6.05 | 0.69 |
| 27 | undecane * | 1100 | 10:36, 1.24 | C11H24 | - | tr |
| 28 | linalool | 1100 | 10:52, 1.76 | C10H18O | 8.95 | 0.34 |
| 29 | levoglucosenone * | 1101 | 10:52, 3.22 | C6H6O3 | tr | 0.07 |
| 30 | monoterpene (MW 136) * | 1112 | 11:08, 1.34 | C10H16 | 0.05 | 0.42 |
| 31 | methyl 3-methylbutanoate * | 1117 | 11:16, 1.58 | C6H12O2 | tr | - |
| 32 | methyl caprylate * | 1123 | 11:24, 1.54 | C9H18O2 | 0.43 | 7.24 |
| 33 | α-pyronene * | 1126 | 11:28, 1.52 | C10H16 | 0.04 | - |
| 34 | plinol A * | 1132 | 11:36, 1.67 | C10H18O | 0.14 | - |
| 35 | monoterpene (MW 136) * | 1134 | 11:40, 1.39 | C10H16 | 0.03 | 0.17 |
| 36 | phenylacetonitrile | 1138 | 11:44, 2.79 | C8H7N | 0.02 | tr |
| 37 | veratrole | 1141 | 11:48, 2.39 | C8H10O2 | 0.07 | tr † |
| 38 | plinol D * | 1149 | 12:00, 1.73 | C10H18O | 0.05 | - |
| 39 | 1-phenyl-2-propen-1-ol * | 1152 | 12:04, 2.28 | C9H10O | tr | tr |
| 40 | benzyl acetate | 1167 | 12:24, 2.57 | C9H10O2 | 27.48 | 0.07 |
| 41 | ethyl benzoate | 1175 | 12:36, 2.15 | C9H10O2 | 0.42 | - |
| 42 | 2-methoxy-4-methylphenol | 1192 | 13:00, 2.29 | C8H10O2 | 0.03 | tr |
| 43 | methyl salicylate | 1198 | 13:08, 2.30 | C8H8O3 | 0.32 | 0.15 |
| 44 | dodecane * | 1200 | 13:12, 1.27 | C12H26 | tr | tr |
| 45 | α-terpineol | 1200 | 13:12, 1.88 | C10H18O | 0.26 | tr |
| 46 | methyl chavicol | 1201 | 13:12, 2.10 | C10H12O | 0.06 | - |
| 47 | 1-methoxy-4-propylbenzene * | 1209 | 13:24, 1.96 | C10H14O | tr | tr |
| 48 | nerol # | 1225 | 13:48, 1.82 | C10H18O | tr | - |
| 49 | linalyl acetate # | 1249 | 14:24, 1.63 | C12H20O2 | 0.03 | - |
| 50 | geraniol | 1249 | 14:24, 1.92 | C10H18O | 0.36 | tr |
| 51 | 1-phenylallyl acetate * | 1255 | 14:32, 2.32 | C11H12O2 | tr | tr |
| 52 | 2-phenylethyl acetate | 1255 | 14:32, 2.36 | C10H12O2 | 0.57 | tr |
| 53 | 4-methoxy benzaldehyde * | 1261 | 14:40, 2.90 | C8H8O2 | tr | - |
| 54 | geranial | 1268 | 14:52, 2.04 | C10H16O | 0.03 | tr † |
| 55 | diethyl 1,5-pentanedioate * | 1274 | 15:00, 2.14 | C9H16O4 | tr | tr |
| 56 | trans-anethol | 1290 | 15:24, 2.31 | C10H12O | 0.36 | tr |
| 57 | 1 H-indole * | 1299 | 15:36, 3.28 | C8H7N | tr | tr |
| 58 | 2-phenylnitroethane | 1301 | 15:40, 2.96 | C8H9NO2 | 0.25 | 0.03 |
| 59 | vinyl butyrate * | 1309 | 15:52, 2.07 | C6H10O2 | tr | tr |
| 60 | cinnamyl alcohol | 1309 | 15:52, 2.81 | C9H10O | tr | tr † |
| 61 | p-vinylguaiacol | 1314 | 16:00, 2.56 | C9H10O2 | tr † | tr |
| 62 | sesquiterpene (MW 204) * | 1318 | 15:48, 2.11 | C15H24 | - | tr |
| 63 | diethyl ( 2E)-3-methyl-2-pentanedioate * | 1325 | 16:16, 2.17 | C10H16O4 | tr | 0.02 |
| 64 | 2,5-dimethyl-3-methylene-1,5-heptadiene * | 1330 | 16:24, 1.54 | C10H16 | tr | 0.04 |
| 65 | methyl 2-methoxybenzoate # | 1334 | 16:28, 2.97 | C9H10O3 | tr | tr |
| 66 | bicycloelemene | 1338 | 16:36, 1.58 | C15H24 | 0.03 | 0.29 |
| 67 | 5-indanol | 1339 | 16:36, 2.66 | C9H10O | tr | tr |
| 68 | ester (MW 190) * | 1341 | 16:40, 2.14 | C12H14O2 | tr | tr |
| 69 | benzyl butyrate | 1347 | 16:48, 2.32 | C11H14O2 | 0.06 | tr † |
| 70 | methyl 2-aminobenzoate * | 1347 | 16:48, 2.99 | C8H9NO2 | tr | tr |
| 71 | α-cubebene* | 1354 | 17:00, 1.53 | C15H24 | tr | 0.09 |
| 72 | eugenol | 1355 | 17:00, 2.47 | C10H12O2 | tr | - |
| 73 | benzenepropanol, acetate * | 1371 | 17:24, 2.39 | C11H14O2 | 0.03 | tr |
| 74 | neryl acetate | 1373 | 17:28, 1.95 | C12H20O2 | 2.74 | 0.21 |
| 75 | geranyl acetate | 1376 | 17:32, 2.00 | C12H20O2 | 2 | - |
| 76 | methyl 4-methoxybenzoate | 1377 | 17:32, 2.79 | C9H10O3 | 0.08 | tr |
| 77 | α-ylangene | 1378 | 17:16, 1.67 | C15H24 | - | 0.06 |
| 78 | butyl benzoate | 1376 | 17:32, 2.31 | C11H14O2 | 0.04 | tr † |
| 79 | α-copaene | 1384 | 17:44, 1.59 | C15H24 | 0.11 | 0.76 |
| 80 | sesquiterpene (MW 204) * | 1389 | 17:32, 1.72 | C15H24 | - | 0.07 |
| 81 | β-bourbonene | 1392 | 17:56, 1.64 | C15H24 | tr † | tr |
| 82 | β-cubebene | 1395 | 18:00, 1.64 | C15H24 | 0.13 | 0.56 |
| 83 | vanillin | 1399 | 18:04, 3.30 | C8H8O3 | tr | 0.05 |
| 84 | tetradecane * | 1400 | 18:08, 1.35 | C14H30 | tr | tr |
| 85 | sesquiterpene (MW 204) * | 1403 | 17:52, 1.71 | C15H24 | - | tr |
| 86 | 2-methoxy-4-(1-propenyl)-phenol * | 1407 | 18:16, 2.59 | C10H12O2 | tr | tr |
| 87 | p-anisyl acetate | 1418 | 18:32, 2.77 | C10H12O3 | 0.04 | tr |
| 88 | β-ylangene * | 1429 | 18:48, 1.79 | C15H24 | 1.71 | 0.73 |
| 89 | β-copaene | 1440 | 19:04, 1.78 | C15H24 | 0.72 | 0.12 |
| 90 | sesquiterpene (MW 204) * | 1442 | 18:48, 1.88 | C15H24 | - | 7.48 |
| 91 | cinnamyl acetate | 1447 | 19:12, 2.78 | C11H12O2 | 0.9 | 1.59 |
| 92 | 3-methyl-3-butenyl benzoate * | 1449 | 19:16, 2.37 | C12H14O2 | 0.03 | tr |
| 93 | isoeugenol | 1452 | 19:20, 2.65 | C10H12O2 | 0.63 | 0.38 |
| 94 | β-caryophyllene | 1455 | 19:24, 1.79 | C15H24 | 0.37 | 0.3 |
| 95 | aromandendrene* | 1460 | 19:32, 1.79 | C15H24 | 0.06 | 1.53 |
| 96 | α-humulene | 1467 | 19:24, 1.96 | C15H24 | - | 6.2 |
| 97 | isogermacrene-D | 1472 | 19:48, 1.77 | C15H24 | 0.03 | 1.83 |
| 98 | α-ionene* | 1483 | 20:04, 1.78 | C13H18 | tr † | tr |
| 99 | germacrene-D | 1495 | 20:20, 1.80 | C11H22O | tr | 2.76 |
| 100 | 3-methyl-2-butenyl benzoate | 1492 | 20:16, 2.47 | C12H14O2 | 0.39 | 0.21 |
| 101 | pentadecane* | 1500 | 20:28, 1.41 | C15H32 | tr | tr |
| 102 | ( Z,E)-α-farnesene* | 1503 | 20:16, 1.87 | C15H24 | - | 0.19 |
| 103 | α-muurolene | 1503 | 20:16, 2.03 | C15H24 | - | 0.31 |
| 104 | ( E,E)-α-farnesene | 1506 | 20:36, 1.79 | C15H24 | 1.62 | 10.1 |
| 105 | β-curcumenene | 1512 | 20:44, 1.90 | C15H24 | 0.39 | 2.73 |
| 106 | γ-cadinene | 1521 | 20:56, 1.88 | C15H24 | tr | 2.14 |
| 107 | δ-cadinene | 1527 | 21:04, 1.92 | C15H24 | 0.28 | 0.61 |
| 108 | guaiacyl acetone * | 1528 | 21:04, 3.26 | C10H12O3 | tr | tr |
| 109 | zonarene | 1547 | 21:32, 1.87 | C15H24 | tr † | tr |
| 110 | sesquiterpene (MW 202) * | 1540 | 21:04, 2.05 | C15H22 | - | tr |
| 111 | benzyl 4-methylpentanoate * | 1548 | 21:32, 2.33 | C13H18O2 | tr | tr |
| 112 | elemol | 1556 | 21:44, 1.99 | C15H26O | tr | 0.02 |
| 113 | cis-3-hexenyl benzoate | 1577 | 22:12, 2.37 | C13H16O2 | tr | tr |
| 114 | germacren D-4-ol * | 1589 | 22:28, 2.02 | C15H26O | 0.06 | tr |
| 115 | caryophyllene oxide | 1595 | 22:36, 2.17 | C15H24O | 0.06 | tr |
| 116 | hexadecane * | 1600 | 22:24, 1.51 | C16H34 | tr | tr |
| 117 | guaiol | 1607 | 22:52, 2.05 | C15H26O | tr | 0.46 |
| 118 | isoeugenol acetate | 1607 | 22:52, 2.89 | C12H14O3 | tr | tr |
| 119 | oxygenated sesquiterpene (MW 220) * | 1625 | 22:56, 2.22 | C15H24O | - | 0.06 |
| 120 | sesquiterpene (MW 206) * | 1625 | 22:56, 2.70 | C15H26 | - | tr |
| 121 | copaborneol | 1637 | 23:32, 2.11 | C15H26O | tr | 0.03 |
| 122 | sesquiterpene (MW 202) * | 1643 | 23:20, 2.33 | C15H22 | - | tr |
| 123 | τ-muurolol | 1652 | 23:52, 2.17 | C15H26O | 0.06 | 4.43 |
| 124 | α-cadinol | 1664 | 24:08, 2.23 | C15H26O | 0.07 | 1.52 |
| 125 | oxygenated sesquiterpene (MW 222) * | 1671 | 23:56, 2.58 | C15H26O | - | tr |
| 126 | bulnesol * | 1673 | 24:00, 2.34 | C15H26O | tr | 0.05 |
| 127 | sesquiterpene (MW 200) * | 1674 | 24:00, 2.63 | C15H20 | - | tr |
| 128 | farnesene* | 1679 | 24:08, 2.35 | C15H24 | - | 0.11 |
| 129 | sesquiterpene (MW 204) * | 1682 | 24:12, 2.01 | C15H24 | - | 0.02 |
| 130 | ( 2Z,6E)-farnesol * | 1686 | 24:16, 2.37 | C15H26O | - | 0.02 |
| 131 | sesquiterpene (MW 204)* | 1686 | 24:16, 2.76 | C15H24 | - | 0.02 |
| 132 | cetene* | 1694 | 24:48, 1.52 | C16H32 | tr | 0.04 |
| 133 | oxygenated sesquiterpene (MW 222)* | 1695 | 24:28, 2.49 | C15H26O | - | tr |
| 134 | ester (MW 196)* | 1704 | 24:40, 1.99 | C12H20O2 | - | tr |
| 135 | globulol* | 1708 | 24:44, 2.53 | C15H26O | - | tr |
| 136 | ( 2Z,6Z)-farnesol* | 1717 | 25:16, 2.10 | C15H26O | 0.09 | 1.43 |
| 137 | sesquiterpene (MW 206)* | 1724 | 25:04, 2.57 | C15H26 | - | tr |
| 138 | ledane* | 1732 | 25:36, 1.70 | C15H26 | tr | 0.15 |
| 139 | ( 2E,6E)-farnesal* | 1739 | 25:44, 2.20 | C15H24O | tr | tr |
| 140 | (2 E,6E)-farnesol | 1741 | 25:24, 2.49 | C15H26O | - | 0.03 |
| 141 | benzyl benzoate | 1776 | 26:28, 3.22 | C14H12O2 | 0.97 | 1.24 |
| 142 | cis-2-methyl-7-octadecene* | 1794 | 26:52, 1.54 | C19H38 | tr | 0.05 |
| 143 | octadecane* | 1800 | 27:00, 1.49 | C18H38 | tr | tr |
| 144 | octadecanal* | 1818 | 27:20, 1.83 | C18H36O | tr | 0.02 |
| 145 | ( 2E,6E)-farnesyl acetate | 1832 | 27:36, 2.07 | C17H28O2 | 0.05 | 2.05 |
| 146 | cis-Z-α-bisabolene epoxide* | 1875 | 28:04, 2.95 | C15H24O | - | tr |
| 147 | benzyl salicylate | 1881 | 28:32, 3.18 | C14H12O3 | 0.21 | 4.18 |
| 148 | nonadecane * | 1900 | 28:56, 1.51 | C19H40 | tr | tr |
| 149 | hexadecanoic acid * | 1958 | 29:40, 2.10 | C16H32O2 | - | 0.27 |
| 150 | geranyl benzoate | 1965 | 29:48, 2.72 | C17H22O2 | - | tr |
| 151 | eicosane # | 2000 | 30:48, 1.54 | C20H42 | tr | tr |
| 152 | heneicosane * | 2100 | 32:36, 1.57 | C21H44 | tr | tr |
| 153 | benzyl cinnamate | 2102 | 32:16, 3.80 | C16H14O2 | - | tr |
| 154 | docosane * | 2200 | 34:20, 1.59 | C22H46 | tr | tr |
| 155 | tricosane | 2300 | 35:56, 1.62 | C23H48 | tr | tr |
| 156 | tetracosane | 2400 | 37:32, 1.66 | C24H50 | tr | tr |
| 157 | pentacosane * | 2500 | 39:04, 1.70 | C25H52 | tr | tr |
| 158 | hexacosane * | 2600 | 40:32, 1.74 | C26H54 | tr | tr |
| 159 | heptacosane * | 2700 | 41:56, 1.79 | C27H56 | tr | tr |
| 160 | octacosane * | 2800 | 43:16, 1.86 | C28H58 | tr | - |
| 161 | nonacosane * | 2900 | 44:36, 2.02 | C29H60 | tr | - |

3. Experimental
3.1. Plant Material and Essential Oil Distillation
3.2. GC×GC-TOFMS Parameters
3.3. Data Processing
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the essential oil fraction are available from the authors.
References
- Benini, C.; Danflous, J.P.; Wathelet, J.P.; du Jardin, P.; Fauconnier, M.L. Ylang-ylang [Cananga odorata (Lam.) Hook. f. & Thomson]: An unknown essential oil plant in an endangered sector. Biotechnologie Agronomie Société et Environnement 2010, 14, 693–705. [Google Scholar]
- Benini, C.; Ringuet, M.; Wathelet, J.-P.; Lognay, G.; du Jardin, P.; Fauconnier, M.-L. Variations in the essential oils from ylang-ylang (Cananga odorata [Lam.] Hook f. & Thomson forma genuina) in the Western Indian Ocean islands. Flavour Fragr. J. 2012, 27, 356–366. [Google Scholar] [CrossRef]
- Burdock, G.A.; Carabin, L.G. Safety assessment of ylang-ylang oil as a food ingredient. Food Chem. Toxicol. 2008, 46, 433–445. [Google Scholar] [CrossRef]
- Gaydou, E.M.; Randriamiharisoa, R.; Bianchini, J.P. Composition of the essential oil of Ylang-Ylang (Cananga odorata Hook Fil. & Thomson forma genuina) from Madagascar. J. Agric. Food Chem. 1986, 34, 481–487. [Google Scholar]
- Craig Elevitch. Cananga odorata (ylang-ylang). Species profiles for Pacific island agroforestry. Available online: http://www.traditionaltree.org/ (accessed on 3 April 2012).
- FAOSTAT. Exports: Commodities by countries. Available online: http://faostat.fao.org/site/342/defaut.aspx/ (accessed on 13 January 2013).
- Stashenko, E.E.; Torres, W.; Morales, J.R.M. A study of the compositional variation of the essential oil of ylang-ylang (Cananga odorata Hook Fil. & Thomson, forma genuina) during flower development. J. High Resolut. Chromatogr. 1995, 18, 101–104. [Google Scholar] [CrossRef]
- AFNOR. French Standard. In Oil of Ylang-ylang [Cananga odorata (Lam.) Hook. f. & Thomson forma genuina]; AFNOR: Paris, France, 2005.
- Benini, C.; Mahy, G.; Bizoux, J.-P.; Wathelet, J.-P.; du Jardin, P.; Brostaux, Y.; Fauconnier, M.-L. Comparative chemical and molecular variability of Cananga odorata (Lam.) Hook. f. & Thomson forma genuina (Ylang-Ylang) in the western indian ocean islands: Implication for valorization. Chem. Biodivers. 2012, 9, 1389–1402. [Google Scholar] [CrossRef]
- Adahchour, M.; Beens, J.; Vreuls, R.J.J.; Brinkman, U.A.T. Recent developments in comprehensive two-dimensional gas chromatography (GCxGC) IV. Further applications, conclusions and perspectives. Trends Anal. Chem. 2006, 25, 821–840. [Google Scholar] [CrossRef]
- Górecki, T.; Harynuk, J.; Panić, O. The evolution of comprehensive two-dimensional gas chromatography (GCxGC). J. Sep. Sci. 2004, 27, 359–379. [Google Scholar] [CrossRef]
- Cordero, C.; Rubiolo, P.; Sgorbini, B.; Galli, M.; Bicchi, C. Comprehensive two-dimensional gas chromatography in the analysis of volatile samples of natural origin: A multidisciplinary approach to evaluate the influence of second dimension column coated with mixed stationary phases on system orthogonality. J. Chromatogr. A 2006, 1132, 268–279. [Google Scholar] [CrossRef]
- Shellie, R.; Marriott, P. Opportunities for ultra-high resolution analysis of essential oils using comprehensive two-dimensional gas chromatography: A review. Flavour Fragr. J. 2003, 18, 179–191. [Google Scholar] [CrossRef]
- Tranchida, P.Q.; Shellie, R.A.; Purcaro, G.; Conte, L.S.; Dugo, P.; Dugo, G.; Mondello, L. Analysis of fresh and aged tea tree essential oils by using GC×GC-qMS. J. Chromatogr. Sci. 2010, 48, 262–266. [Google Scholar]
- Shellie, R.; Mondello, L.; Marriott, P.; Dugo, G. Characterisation of lavender essential oils by using gas chromatography-mass spectrometry with correlation of linear retention indices and comparison with comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2002, 970, 225–234. [Google Scholar] [CrossRef]
- Phillips, J.B.; Beens, J. Comprehensive two-dimensional gas chromatography: A hyphenated method with strong coupling between the two dimensions. J. Chromatogr. A 1999, 856, 331–347. [Google Scholar] [CrossRef]
- Adahchour, M.; Beens, J.; Vreuls, R.J.J.; Brinkman, U.A.T. Recent developments in comprehensivetwo-dimensional gas chromatography (GCxGC) II. Modulation and detection. Trends Anal. Chem. 2006, 25, 540–553. [Google Scholar] [CrossRef]
- Hilton, D.C.; Jones, R.S.; Sjödin, A. A method for rapid, non-targeted screening for environmental contaminants in household dust. J. Chromatogr. A 2010, 1217, 6851–6856. [Google Scholar] [CrossRef]
- Brokl, M.; Bishop, L.; Wright, C.G.; Liu, C.; McAdam, K.; Focant, J.-F. Analysis of mainstream tobacco smoke particulate phase using comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. J. Sep. Sci. 2013. [Google Scholar] [CrossRef]
- Brasseur, C.; Dekeirsschieter, J.; Schotsmans, E.M.J.; de Koning, S.; Wilson, A.S.; Haubruge, E.; Focant, J.-F. Comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry for the forensic study of cadaveric volatile organic compounds released in soil by buried decaying pig carcasses. J. Chromatogr. A 2012, 1255, 163–170. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Brokl, M.; Fauconnier, M.-L.; Benini, C.; Lognay, G.; Jardin, P.D.; Focant, J.-F. Improvement of Ylang-Ylang Essential Oil Characterization by GC×GC-TOFMS. Molecules 2013, 18, 1783-1797. https://doi.org/10.3390/molecules18021783
Brokl M, Fauconnier M-L, Benini C, Lognay G, Jardin PD, Focant J-F. Improvement of Ylang-Ylang Essential Oil Characterization by GC×GC-TOFMS. Molecules. 2013; 18(2):1783-1797. https://doi.org/10.3390/molecules18021783
Chicago/Turabian StyleBrokl, Michał, Marie-Laure Fauconnier, Céline Benini, Georges Lognay, Patrick Du Jardin, and Jean-François Focant. 2013. "Improvement of Ylang-Ylang Essential Oil Characterization by GC×GC-TOFMS" Molecules 18, no. 2: 1783-1797. https://doi.org/10.3390/molecules18021783
APA StyleBrokl, M., Fauconnier, M.-L., Benini, C., Lognay, G., Jardin, P. D., & Focant, J.-F. (2013). Improvement of Ylang-Ylang Essential Oil Characterization by GC×GC-TOFMS. Molecules, 18(2), 1783-1797. https://doi.org/10.3390/molecules18021783