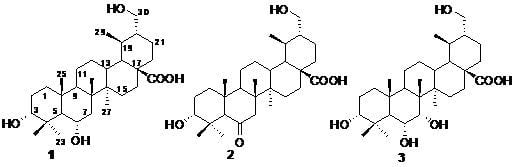
Three New Ursane-Type Triterpenoids from the Stems of Saprosma merrillii
Abstract
:1. Introduction
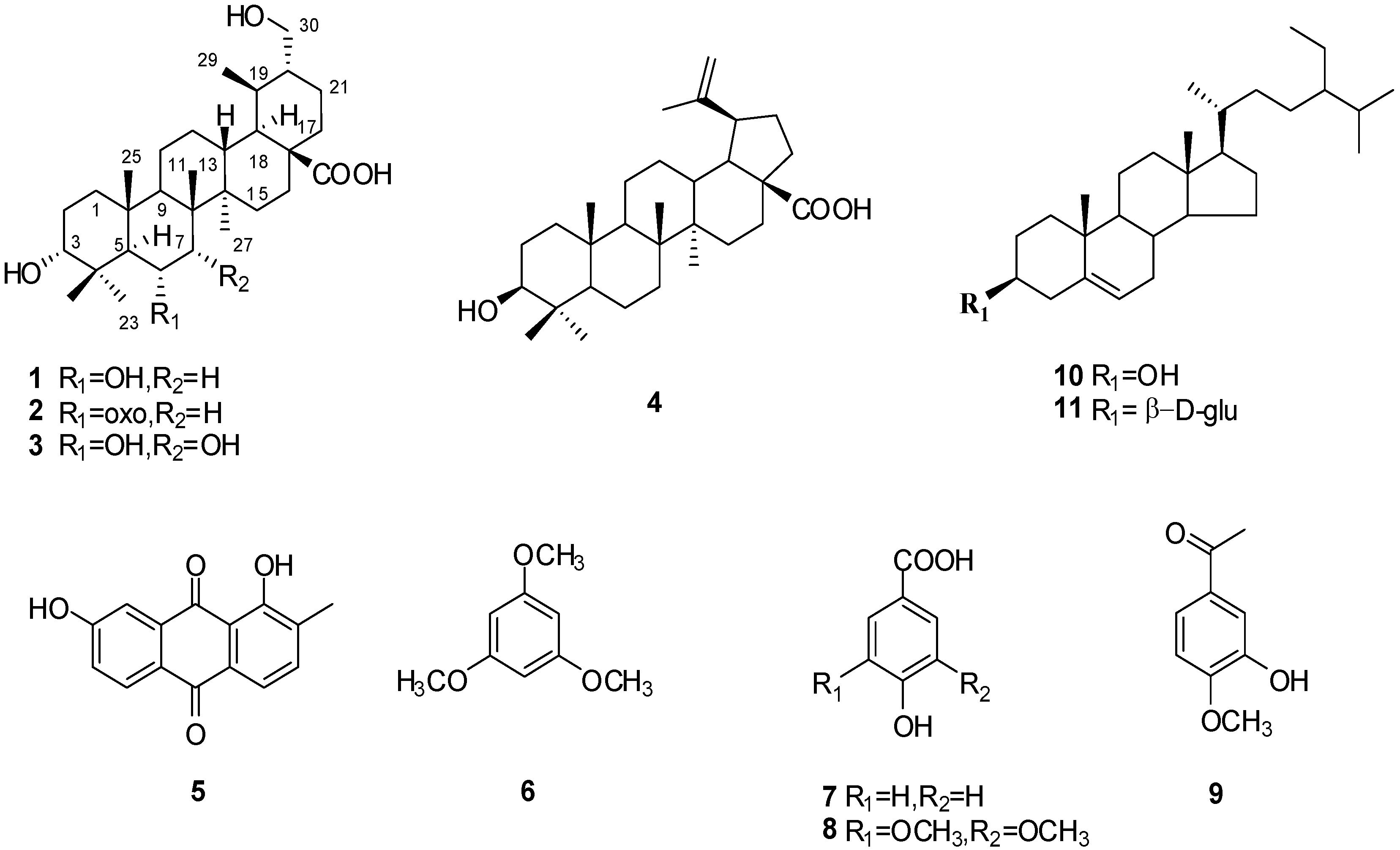
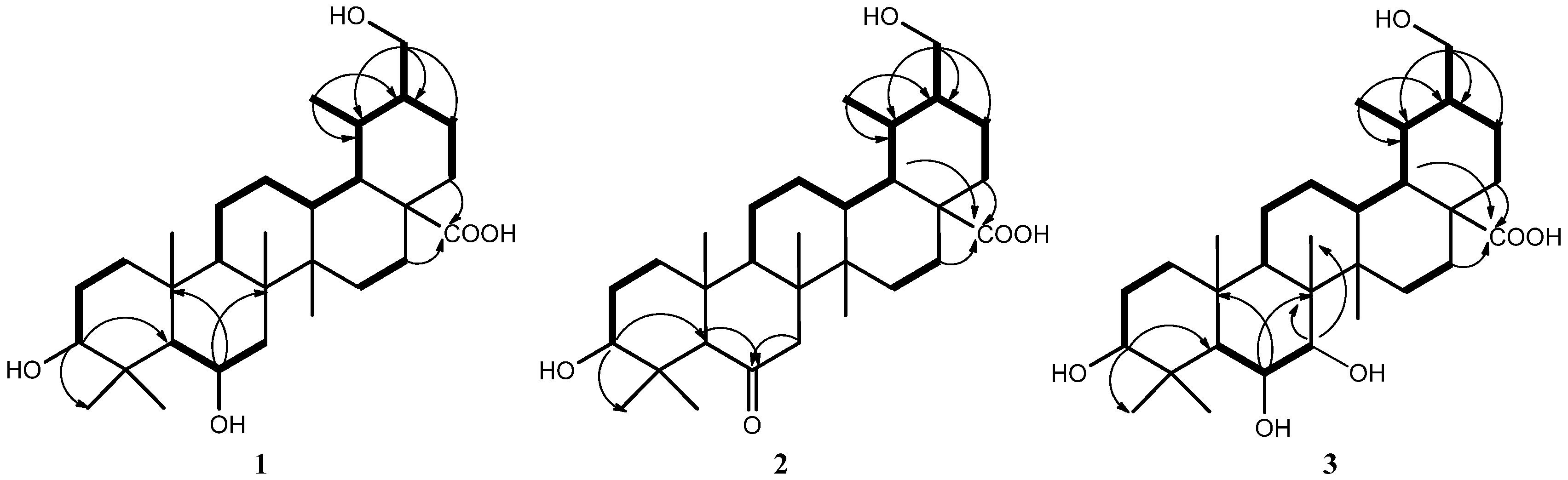
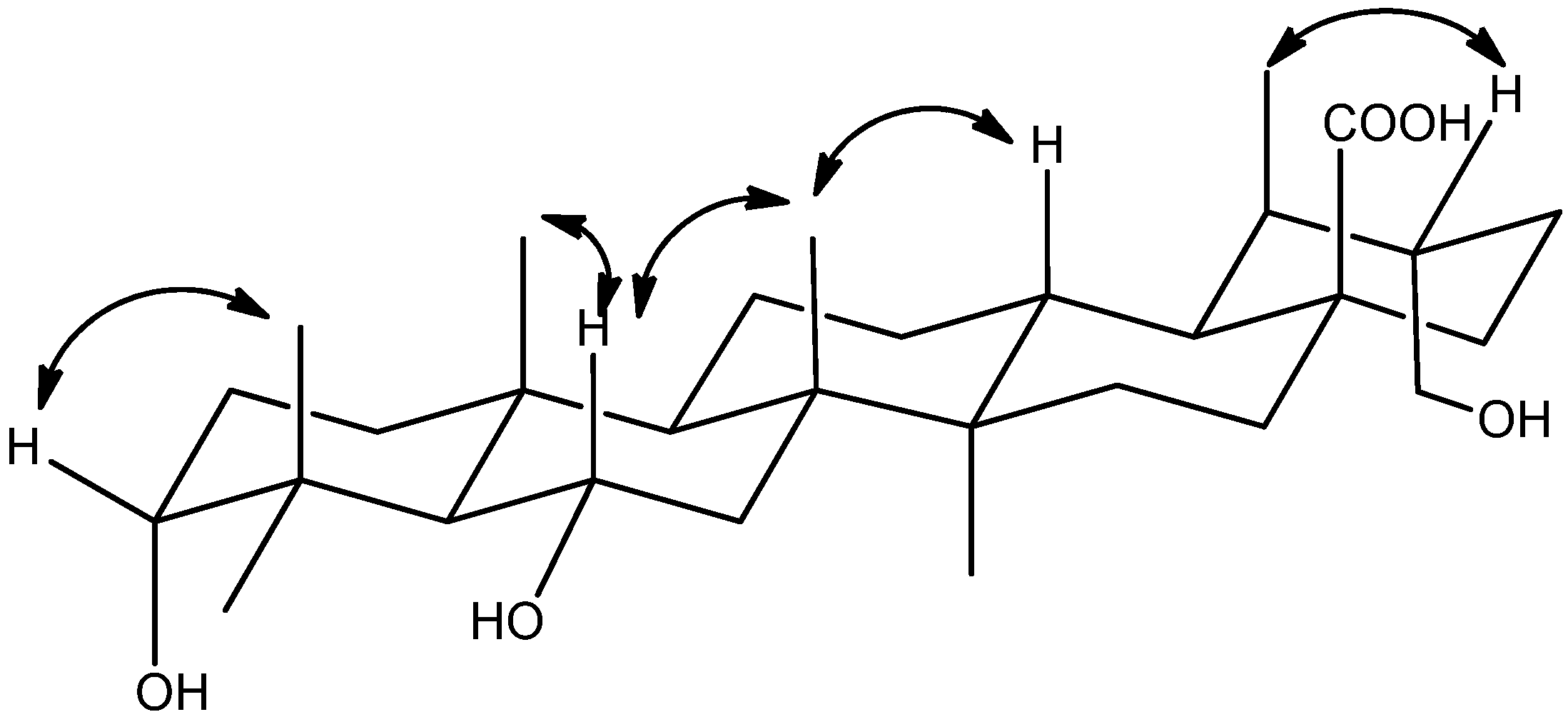
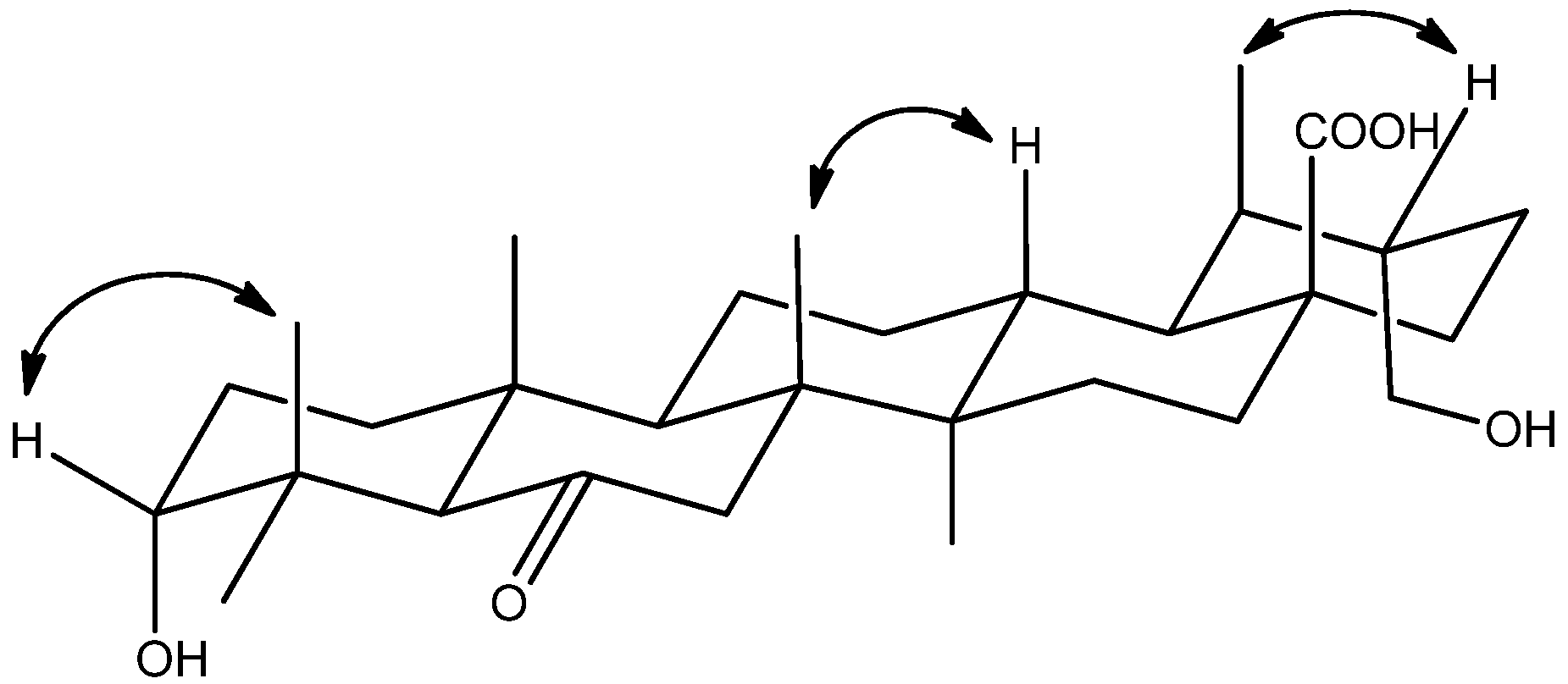
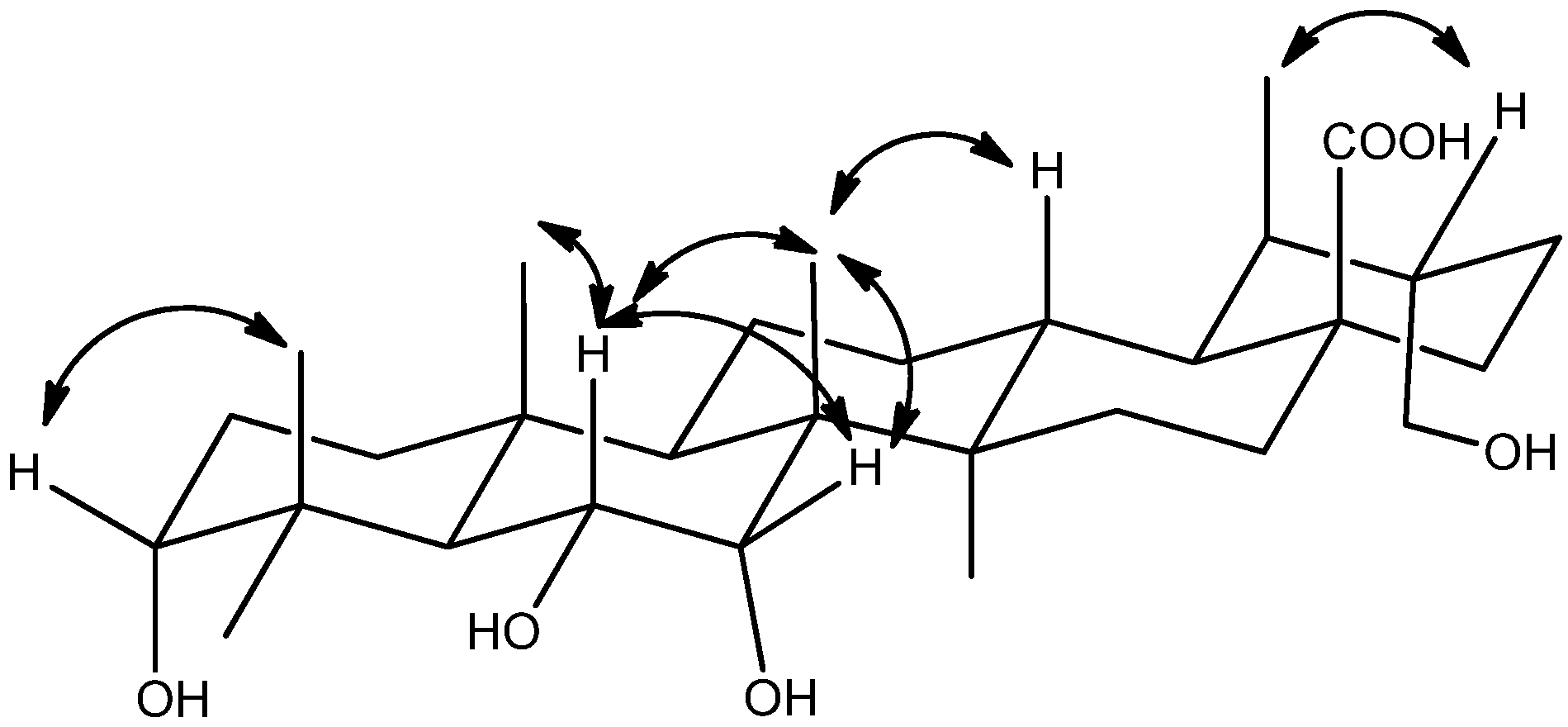
2. Results and Discussion
| NO. | 1 a | 2 b | 3 b | |||
|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | |
| 1 | 38.3 t | 1.54 (1H, m, H-1β), 1.63 (1H, m, H-1α) | 34.5 t | 1.47 (1H, m, H-1β), 1.65 (1H, m, H-1α) | 34.2 t | 1.64 (1H, m, H-1β), 1.83 (1H, m, H-1α) |
| 2 | 26.4 t | 1.51 (1H, m, H-1α), 2.05 (1H, m, H-1β) | 26.0 t | 1.56 (1H, m, H-1α), 1.92 (1H, m, H-1β) | 26.6 t | 1.50 (1H, m, H-1α), 2.04 (1H, m, H-1β) |
| 3 | 78.5 d | 3.26 (1H, t, 2.4) | 75.7 d | 3.19 (1H, br s) | 77.0 d | 3.28 (1H, t, 2.6) |
| 4 | 39.4 s | 37.2 s | 39.1 s | |||
| 5 | 50.2 d | 1.31 (1H, br.s) | 60.0 d | 2.77 (1H, s) | 48.7 d | 1.41 (1H, br.s) |
| 6 | 69.3 d | 4.34 (1H, m) | 213.7 s | 73.9 d | 4.11 (m) | |
| 7 | 42.9 t | 1.50 (1H, m, H-1α), 1.63 (1H, m, H-1β) | 52.8 t | 1.80 (1H, m, H-1α), 2.57 (1H, d, 11.6 H-1β) | 74.6 d | 3.62 (1H, dd, 4.4, 4.8) |
| 8 | 41.3 s | 48.2 s | 47.0 s | |||
| 9 | 49.8 d | 1.63 (1H, m) | 49.3 d | 1.73 (1H, m) | 49.2 d | 1.70 (1H, m) |
| 10 | 38.0 s | 44.3 s | 37.8 s | |||
| 11 | 22.1 t | 1.43 (1H, m, H-1α), 1.61 (1H, m, H-1β) | 22.1 t | 1.41 (1H, m, H-1α), 1.66 (1H, m, H-1β) | 21.7 t | 1.39 (1H, m, H-1α), 1.64 (1H, m, H-1β) |
| 12 | 33.2 t | 1.37 (1H, m, H-1β) 2.22 (1H, dt, 12.6, 2.8 H-1α) | 32.6 t | 1.48 (1H, m, H-1β), 2.24 (1H, m, H-1α) | 33.1 t | 1.35 (1H, m, H-1β), 2.20 (1H, dt, 12.8, 3.6 H-1α) |
| 13 | 38.8 d | 2.41 (1H, dt, 12.2, 3.6) | 39.1 d | 2.37 (1H, m) | 38.9 d | 2.41 (1H, dt, 12.8, 3.6) |
| 14 | 43.9 s | 43.8 s | 44.8 s | |||
| 15 | 31.0 t | 1.17 (1H, m, H-1β), 1.56 (1H, m, H-1α) | 30.4 t | 1.02 (1H, m, H-1β), 1.48 (1H, m, H-1α) | 36.6 t | 1.16 (1H, m, H-1β), 1.34 (1H, m, H-1α) |
| 16 | 28.6 t | 1.34 (1H, m, H-1α), 1.66 (1H, m, H-1β) | 27.6 t | 1.43 (1H, m, H-1α), 1.69 (1H, m, H-1β) | 28.5 t | 1.28 (1H, m, H-1α), 1.66 (1H, m, H-1β) |
| 17 | 57.9 s | 57.0 s | 57.0 s | |||
| 18 | 51.9 d | 1.54 (1H, m) | 51.0 d | 2.08 (1H, t, 4.0) | 51.6 d | 1.40 (1H, m) |
| 19 | 39.5 d | 1.83 (1H, m) | 38.8 d | 1.86 (1H, m) | 39.3 d | 1.87 (1H, m) |
| 20 | 44.6 d | 2.31 (1H, tt, 10.8, 3.0) | 44.2 d | 2.29 (1H, m) | 44.4 d | 2.30 (1H, m) |
| 21 | 24.7 t | 1.31 (1H, m, H-1α), 1.51 (1H, m, H-1β), | 24.4 t | 1.39 (1H, m, H-1α), 1.55 (1H, m, H-1β) | 24.4 t | 1.32 (1H, m, H-1α), 1.54 (1H, m, H-1β) |
| 22 | 36.8 t | 1.30 (1H, m, H-1α), 1.37 (1H, m, H-1β) | 37.7 t | 1.34 (1H, m, H-1α), 1.37 (1H, m, H-1β) | 37.9 t | 1.32 (1H, m, H-1α), 1.35 (1H, m, H-1β) |
| 23 | 28.9 q | 0.98 (3H, s) | 27.4 q | 0.94 (3H, s) | 28.7 q | 0.98 (3H, s) |
| 24 | 24.8 q | 1.20 (3H, s) | 22.6 q | 1.19 (3H, s) | 25.0 q | 1.22 (3H, s) |
| 25 | 15.4 q | 0.99 (3H, s) | 17.4 q | 0.86 (3H, s) | 15.6 q | 1.06 (3H, s) |
| 26 | 17.4 q | 1.28 (3H, s) | 16.5 q | 0.93 (3H, s) | 11.0 q | 1.21 (3H, s) |
| 27 | 18.0 q | 1.23 (3H, s) | 15.1 q | 1.14 (3H, s) | 17.8 q | 1.22 (3H, s) |
| 28 | 180.2 s | 177.7 s | 177.8 s | |||
| 29 | 18.8 q | 0.96 (3H, d, 6.8) | 18.8 q | 0.93 (3H, d, 7.0) | 18.8 q | 0.94 (3H, d, 6.8) |
| 30 | 64.2 t | 3.33 (1H, m, H-1β), 3.75 (1H, dd, 10.6, 4.4, H-1α) | 63.7 t | 3.37 (1H, dd, 10.0, 8.4, H-1β), 3.76 (1H, dd, 10.0, 4.6, H-1α) | 63.8 t | 3.34 (1H, m, H-1α), 3.76 (1H,10.2, 4.6, H-1β) |
| Compounds | IC50 (μM) | |||
|---|---|---|---|---|
| A549 | MDA-MB-231 | HEPG2 | B16F10 | |
| 1 | N | N | N | 72.72 |
| 2 | 24.66 | N | 127.80 | N |
| 3 | 129.22 | N | N | N |
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Characterization of Compounds 1–3
 −108.33 (c 0.0024, MeOH); IR (KBr) υmax 3432, 2949, 2873, 1704, 1641, 1459, 1383, 1278, 1181, 1040, 990 cm–1; 1H- and 13C-NMR data see Table 1; ESIMS m/z 489.4 [M−H]−, 513.3 [M+Na]+; HRESIMS m/z: 513.3559 [M+Na]+ (calcd for C30H49O5Na, 513.3551).
−108.33 (c 0.0024, MeOH); IR (KBr) υmax 3432, 2949, 2873, 1704, 1641, 1459, 1383, 1278, 1181, 1040, 990 cm–1; 1H- and 13C-NMR data see Table 1; ESIMS m/z 489.4 [M−H]−, 513.3 [M+Na]+; HRESIMS m/z: 513.3559 [M+Na]+ (calcd for C30H49O5Na, 513.3551). −20.73 (c 0.0019, MeOH); IR (KBr) υmax 3440, 2948, 2873, 1704, 1636, 1458, 1387, 1174, 1070, 1032, 986, 928 cm–1; 1H- and 13C-NMR data see Table 1; ESIMS m/z: 487.6 [M−H]−, 511.5 [M+Na]+; HRESIMS m/z: 511.3389 [M+Na]+ (calcd for C30H48O5Na, 511.3394).
−20.73 (c 0.0019, MeOH); IR (KBr) υmax 3440, 2948, 2873, 1704, 1636, 1458, 1387, 1174, 1070, 1032, 986, 928 cm–1; 1H- and 13C-NMR data see Table 1; ESIMS m/z: 487.6 [M−H]−, 511.5 [M+Na]+; HRESIMS m/z: 511.3389 [M+Na]+ (calcd for C30H48O5Na, 511.3394). –37.84 (c 0.0037, MeOH); IR (KBr) υmax 3442, 2951, 2875, 1704, 1647, 1458, 1384, 1229, 1176, 1110, 1035, 920 cm–1; 1H- and 13C-NMR data see Table 1; ESIMS m/z: 505.7 [M−H]−, 529.5 [M+Na]+; HRESIMS m/z: 529.3501 [M+Na]+ (calcd for C30H50O6Na, 529.3499).
–37.84 (c 0.0037, MeOH); IR (KBr) υmax 3442, 2951, 2875, 1704, 1647, 1458, 1384, 1229, 1176, 1110, 1035, 920 cm–1; 1H- and 13C-NMR data see Table 1; ESIMS m/z: 505.7 [M−H]−, 529.5 [M+Na]+; HRESIMS m/z: 529.3501 [M+Na]+ (calcd for C30H50O6Na, 529.3499).3.5. Cytotoxicity Assays
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ling, S.K.; Komorita, A.; Tanaka, T. Sulfur-containing bis-iridoid glucosides and iridoid glucosides from Saprosmas cortechinii. J. Nat. Prod. 2002, 65, 656–660. [Google Scholar] [CrossRef]
- Lu, X.L.; Xin, C.; Liu, X.Y. Iridoid glycosides from Saprosma ternatum. Planta Med. 2010, 76, 1746–1748. [Google Scholar] [CrossRef]
- Singh, D.N.; Verma, N.; Raghuwanshi, S. Antifungal anthraquinones from Saprosma fragrans. Bioorg. Med. Chem. Lett. 2006, 16, 4512–4514. [Google Scholar] [CrossRef]
- Wang, L.; Chen, G.Y.; Han, C.R.; Yuan, Y.; Yang, B.; Zhang, Y.; Wang, J.; Zhong, X.Q.; Huang, X. Two novel alkaloids from the Stem of Saprosma hainanense and their cytotoxic activities in vitro. Chem. Pharm. Bull. 2011, 59, 338–340. [Google Scholar] [CrossRef]
- Dai, C.Y.; Yang, B.; Zhang, D.S.; Chen, G.Y.; Han, C.R. Antitumor activities of extracts from Trigonostemon xyphophylloides and Saprosma merrillii. J. Hainan Normal Univ. (Nat. Sci.) 2012, 25, 184–187. [Google Scholar]
- Wang, Q.; Lin, H.W.; Shen, Y.; Jin, C.Y. An ke su capsule of antitumor effect. Zhong Yao Cai 2000, 23, 634–636. [Google Scholar]
- Ling, S.K.; Komorita, A.; Tanaka, T. Iridoids and anthraquinones from the Malaysian medicinal plant, Saprosma scortechinii (Rubiaceae). Chem. Pharm. Bull. 2002, 50, 1035–1040. [Google Scholar] [CrossRef]
- Singh, D.N.; Verma, N.; Kulshreshtha, D.K. Sulfur-containing bis-iridoid glucoside from Saprosma fragrans. Ind. J. Heter. Chem. 2011, 21, 5–8. [Google Scholar]
- Singh, D.N.; Verma, N. Iridoid glucosides and anthraquinone from the aerial parts of Saprosma fragrans. J. Ind. Chem. Soc. 2012, 89, 429–431. [Google Scholar]
- Huang, P.L.; Wang, L.W.; Lin, C.N. New triterpenoids of Mallotus repandus. J. Nat. Prod. 1990, 62, 891–892. [Google Scholar] [CrossRef]
- Sun, H.X.; Zhang, J.X.; Ye, Y.P.; Shen, Y.A. Cytotoxic pentacyclic triterpenoids from the rhizome of Astilbe chinensis. Helv. Chim. Acta 2003, 86, 2414–2423. [Google Scholar] [CrossRef]
- Song, Q.Y.; Qi, W.Y.; Li, Z.M.; Zhao, J.; Chen, J.J.; Gao, K. Antifungal activities of triterpenoids from the roots of Astilbe myriantha Diels. Phytochemistry 2011, 128, 495–499. [Google Scholar]
- Calabria, L.M.; Piacente, S.; Kapusta, I.; Dharmawardhane, S.F.; Segarra, F.M.; Pessiki, P.J.; Mabry, T.J. Triterpene saponins from Silphium radula. Phytochemistry 2008, 69, 961–972. [Google Scholar] [CrossRef]
- Song, Y.L.; Wang, Y.H.; Lu, Q.; Qiao, H.J.; Cheng, Y.X. Triterpenoids from the edible leaves of Photinia serrulata. Helv. Chim. Acta 2008, 91, 665–672. [Google Scholar] [CrossRef]
- Ghan, W.R.; Sheppard, V.; Medford, K.A.; Tinto, W.F.; Reynolds, W.F.; McLea, S. Triterpenes from Miconia stenostachya. J. Nat. Prod. 1992, 55, 963–966. [Google Scholar] [CrossRef]
- Cory, A.H.; Owen, T.C.; Barltrop, J.A.; Cory, J.G. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991, 3, 207–212. [Google Scholar]
- Cai, X.H.; Luo, X.D.; Zhou, J.; Hao, X.J. Quinones from Chirita eburnea. J. Nat. Prod. 2005, 68, 797–799. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Z.W.; Gao, T.X.; Fu, H.; Lin, W. Chemical constituents from the mangrove plant Ceriops tagal. Acta Pharm. Sin. 2005, 40, 935–939. [Google Scholar]
- Liu, Y.B.; Cheng, X.R.; Qin, J.J.; Yan, S.K.; Jin, H.Z.; Zhang, W.D. Chemical Constituents of Toona ciliata var. pubescens. Chin. J. Nat. Med. 2011, 9, 115–119. [Google Scholar]
- Bai, H.; Dou, D.Q.; Pei, Y.P.; Chen, Y.J.; Wu, L.J. Chemical constituents of cultivated Glycyrrhiza uralensis. Chin. Tradit. Herbal Drug. 2005, 36, 652–654. [Google Scholar]
- Zhang, Y.L.; Gan, M.L.; Li, S.; Wang, S.J.; Zhu, C.G.; Hu, J.F.; Chen, N.H.; Shi, J.G. Chemical constituents of stems and branches of Adina polycephala. Zhongguo Zhong Yao Za Zhi 2010, 35, 1261–1271. [Google Scholar]
- Xu, J.J.; Tan, N.H.; Zeng, G.Z.; Han, H.J.; Huang, H.Q.; Ji, C.J.; Zhu, M.J.; Zhang, Y.M. Studies on chemical constituents in fruit of Alpinia oxyphylla. Zhongguo Zhong Yao Za Zhi 2009, 34, 990–993. [Google Scholar]
- Sample Availability: Samples of the compounds 1–11 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, D.; Chen, W.; Chen, W.; Song, X.; Han, C.; Wang, Y.; Chen, G. Three New Ursane-Type Triterpenoids from the Stems of Saprosma merrillii. Molecules 2013, 18, 14496-14504. https://doi.org/10.3390/molecules181214496
Zhang D, Chen W, Chen W, Song X, Han C, Wang Y, Chen G. Three New Ursane-Type Triterpenoids from the Stems of Saprosma merrillii. Molecules. 2013; 18(12):14496-14504. https://doi.org/10.3390/molecules181214496
Chicago/Turabian StyleZhang, Dashuai, Wenhao Chen, Wenxing Chen, Xiaoping Song, Changri Han, Yan Wang, and Guangying Chen. 2013. "Three New Ursane-Type Triterpenoids from the Stems of Saprosma merrillii" Molecules 18, no. 12: 14496-14504. https://doi.org/10.3390/molecules181214496
APA StyleZhang, D., Chen, W., Chen, W., Song, X., Han, C., Wang, Y., & Chen, G. (2013). Three New Ursane-Type Triterpenoids from the Stems of Saprosma merrillii. Molecules, 18(12), 14496-14504. https://doi.org/10.3390/molecules181214496