Understanding the Molecular Aspects of Tetrahydrocannabinol and Cannabidiol as Antioxidants
Abstract
:1. Introduction
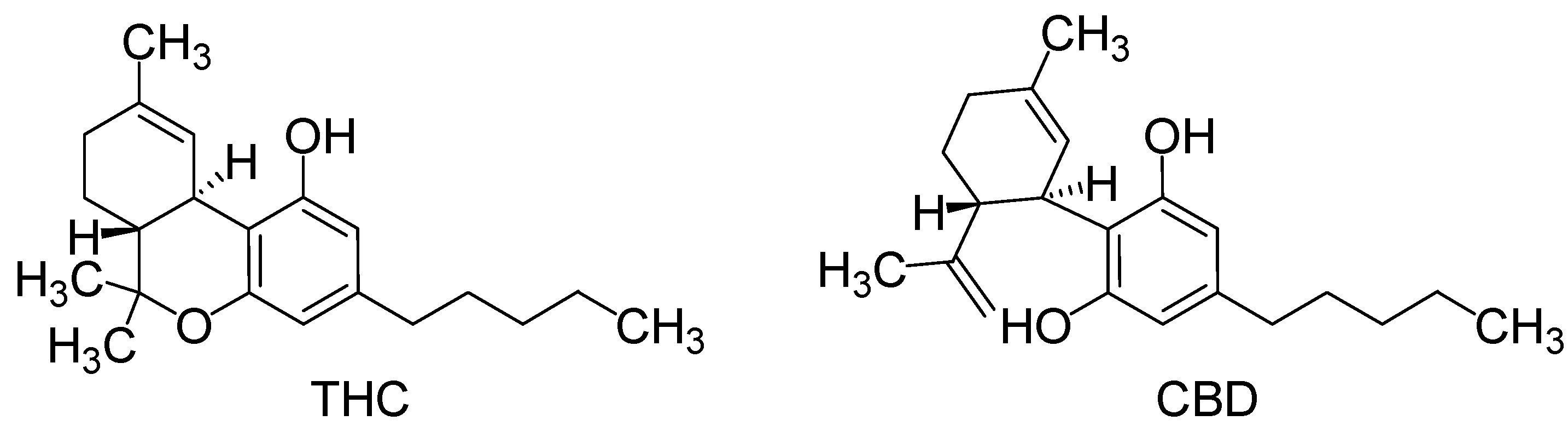
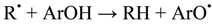
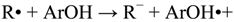
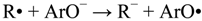
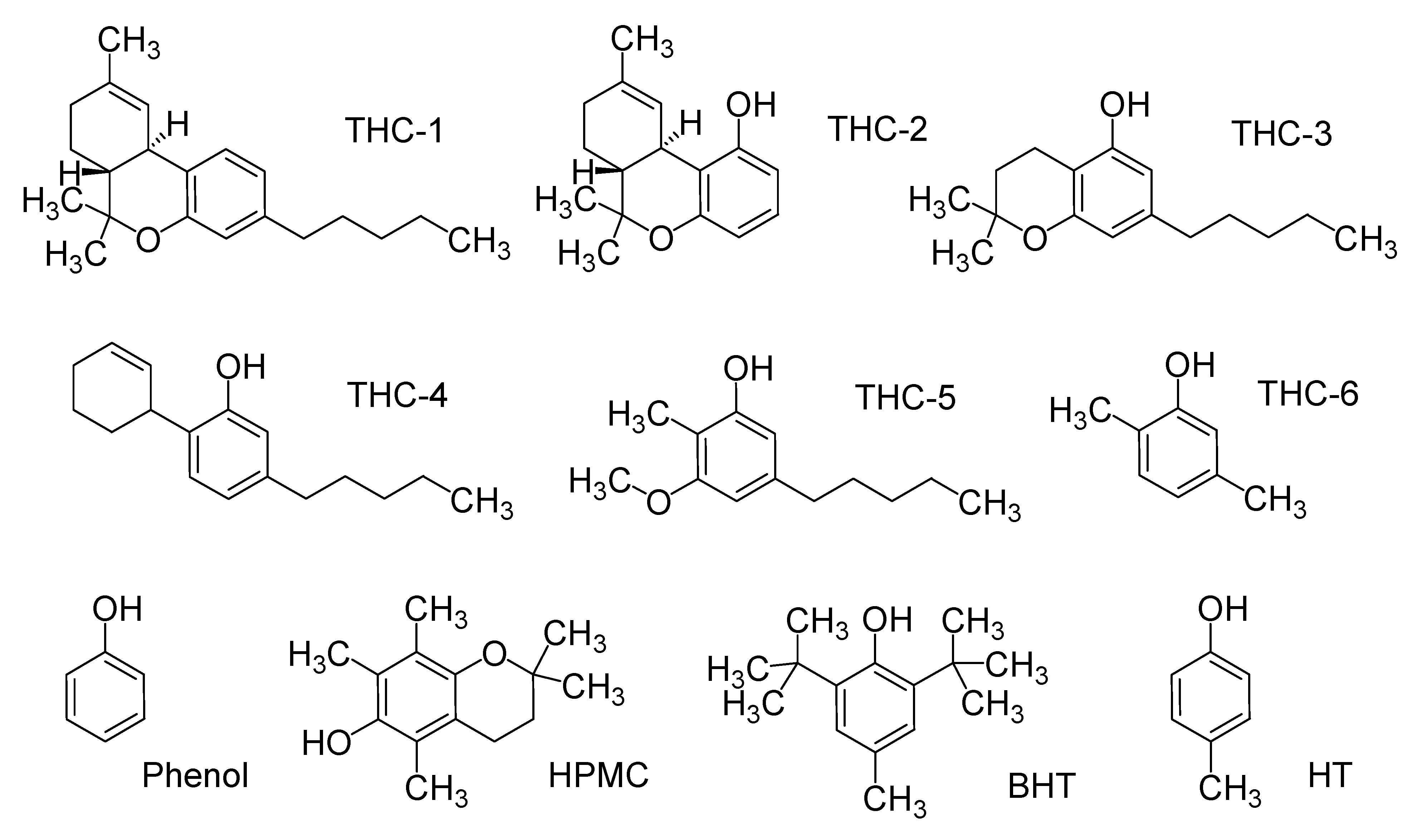
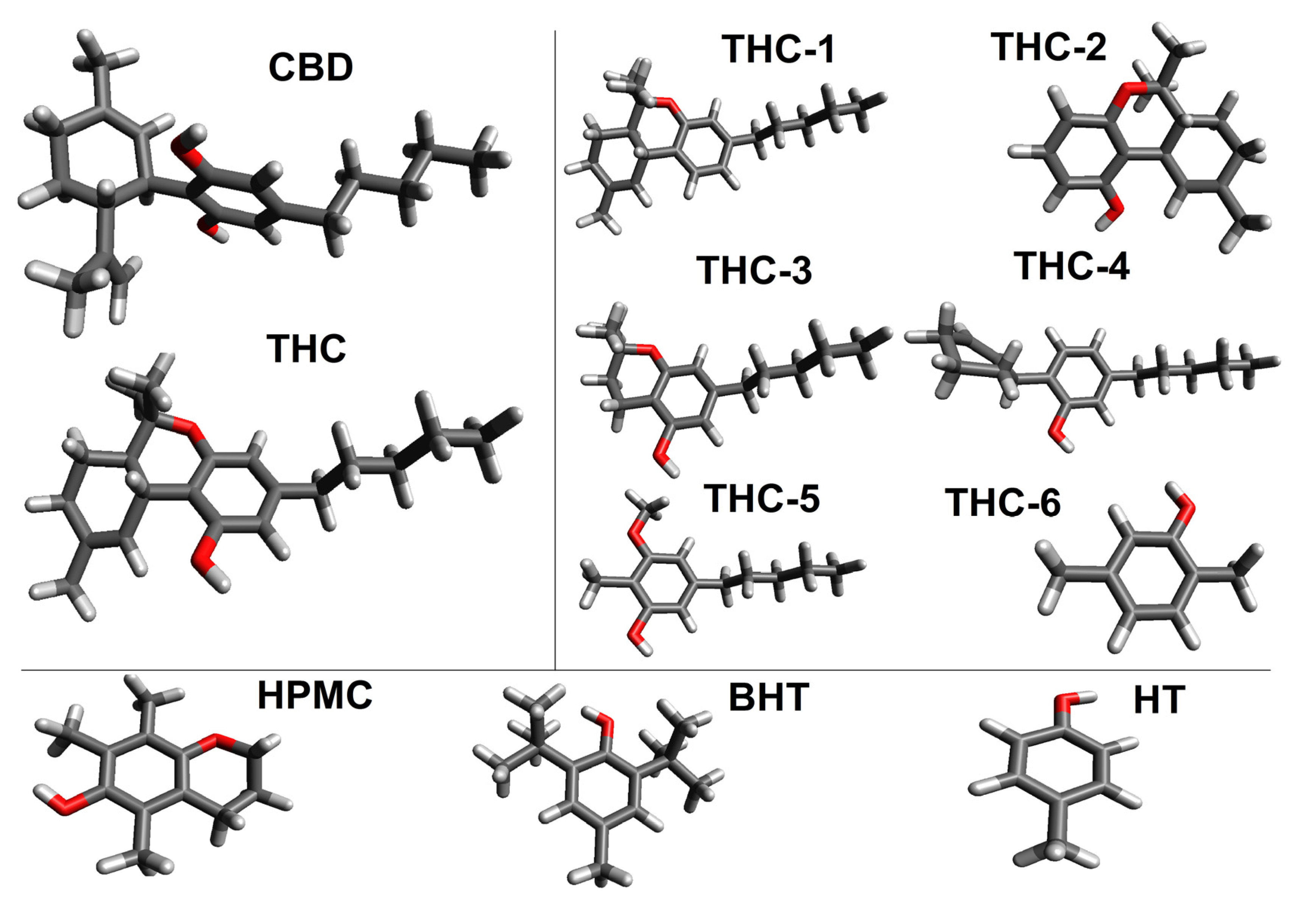
2. Results and Discussion
| Molecules | HOMO | LUMO | IP | BDE |
|---|---|---|---|---|
| CBD | −6.18 | −0.38 | 142.33 | 85.63 |
| THC | −6.03 | −0.36 | 138.88 | 84.49 |
| THC-1 | −6.04 | −0.51 | 138.86 | - |
| THC-2 | −6.11 | −0.44 | 140.23 | 85.11 |
| THC-3 | −6.03 | −0.31 | 138.92 | 84.94 |
| THC-4 | −6.19 | −0.51 | 142.13 | 84.86 |
| THC-5 | −6.16 | −0.31 | 140.63 | 85.68 |
| THC-6 | −6.24 | −0.52 | 142.06 | 84.81 |
| Phenol | −6.29 | −0.42 | 148.82 | 87.93 |
| HPMC | −5.56 | −0.39 | 125.77 | 76.01 |
| BHT | −5.97 | −0.36 | 136.71 | 78.48 |
| HT | −6.25 | −0.64 | 142.54 | 84.86 |
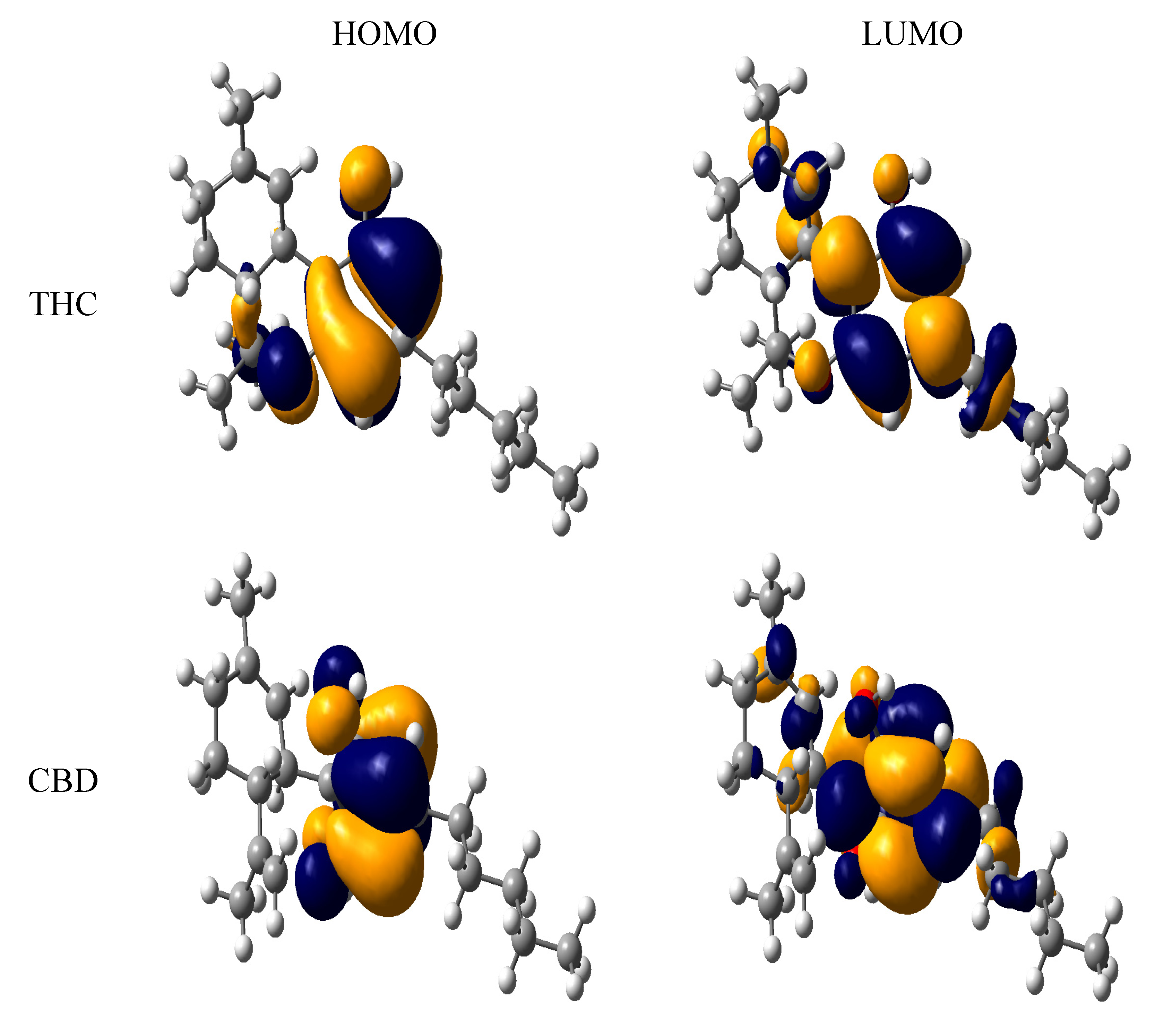
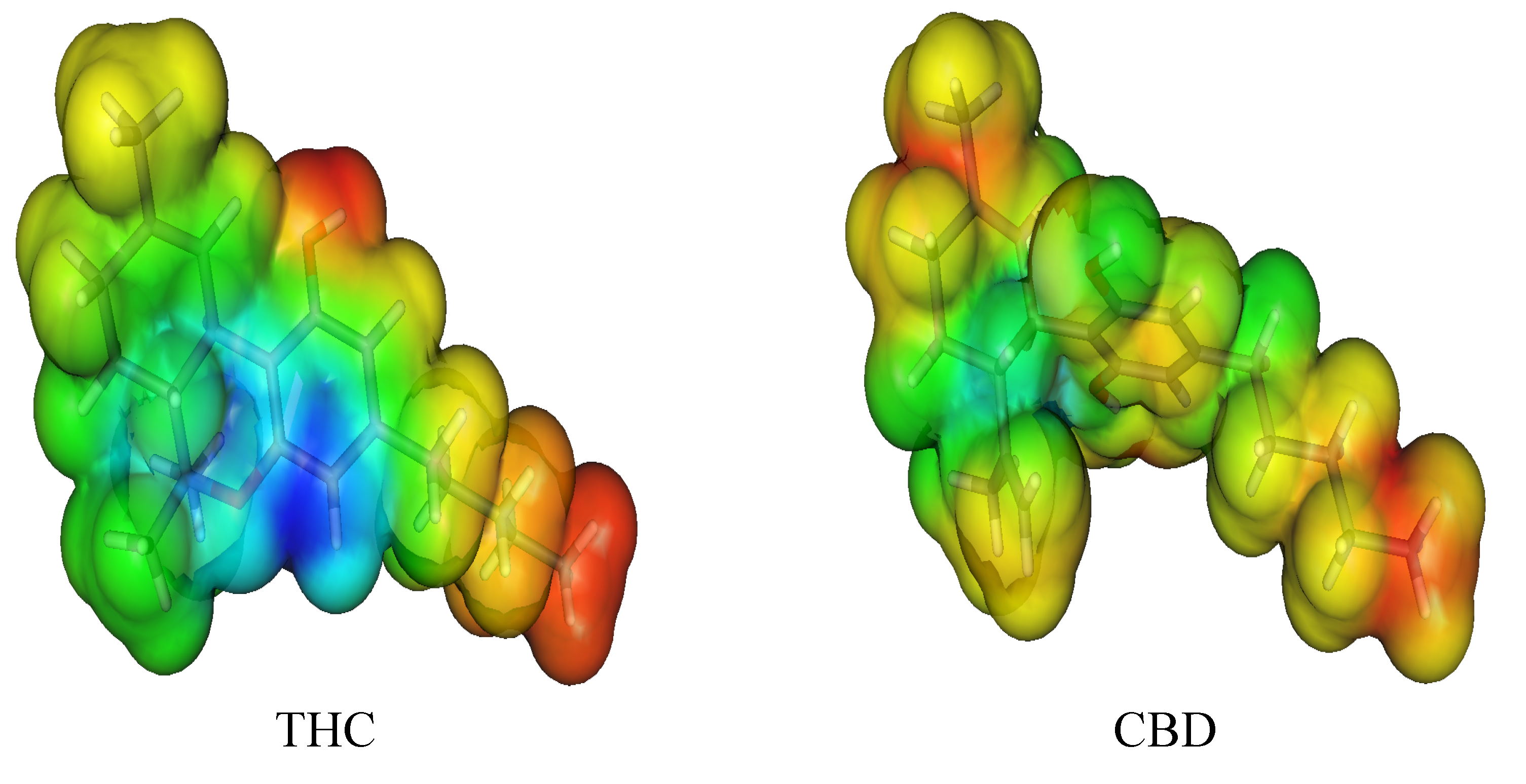
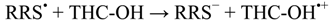
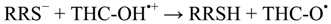
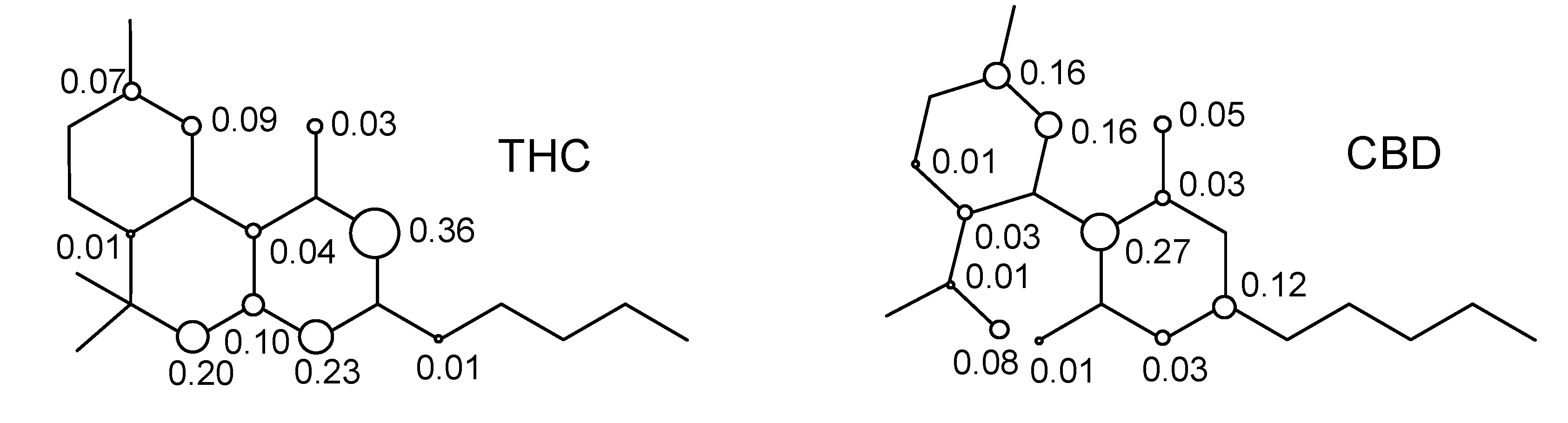
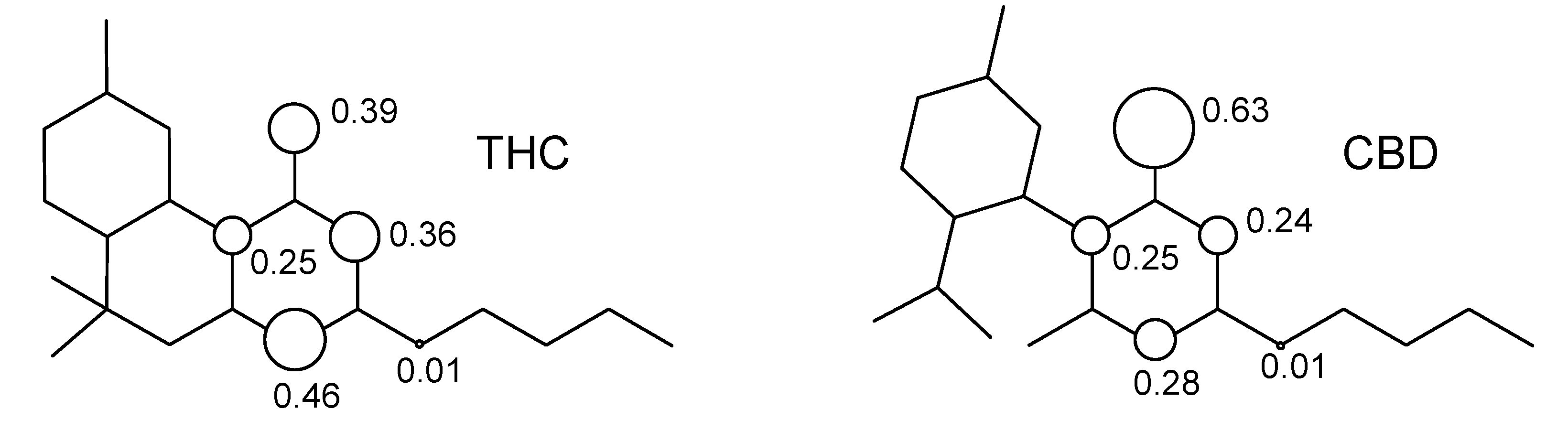
3. Computational Methodology
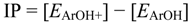
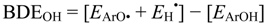
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Di Marzo, V.; Bifulco, M.; de Petrocellis, L. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discov. 2004, 3, 771–784. [Google Scholar] [CrossRef]
- Hohmann, A.G.; Suplita, R.L. II Endocannabinoid mechanisms of pain modulation. AAPS J. 2006, 8, E693–E708. [Google Scholar] [CrossRef]
- Walker, J.M.; Huang, S.M. Endocannabinoids in pain modulation. Prostaglands Leukot. Essent. Fatty Acids 2002, 66, 235–242. [Google Scholar] [CrossRef]
- Fox, A.; Bevan, S. Therapeutic potential of cannabinoid receptor agonists as analgesic agents. Expert Opin. Invest. Drugs 2005, 14, 695–703. [Google Scholar] [CrossRef]
- Meng, I.D.; Manning, B.H.; Martin, W.J.; Fields, H.L. An analgesia circuit activated by cannabinoids. Nature 1998, 395, 381–383. [Google Scholar] [CrossRef]
- Fox, A.; Kesingland, A.; Gentry, C.; McNair, K.; Patel, S.; Urban, L.; James, I. The role of central and peripheral Cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain 2001, 92, 91–100. [Google Scholar] [CrossRef]
- Russo, E.B.; Guy, G.W.; Robson, P.J. Cannabis, Pain, and Sleep: Lessons from Therapeutic Clinical Trials of Sativex(R), a Cannabis-Based Medicine. Chem. Biodivers. 2007, 4, 1729–1743. [Google Scholar] [CrossRef]
- Pertwee, R.G. The pharmacology of cannabinoid receptors and their ligands: An overview. Int. J. Obesity 2006, 30, S13–S18. [Google Scholar] [CrossRef]
- Hampson, A.J.; Grimaldi, M.; Axelrod, J.; Wink, D. Cannabidiol and (−)-8,9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. USA 1998, 95, 8268–8273. [Google Scholar] [CrossRef]
- Steffens, S.; Veillard, N.R.; Arnaud, C.; Pelli, G.; Burger, F.; Staub, C.; Karsak, M.; Zimmer, A.; Frossard, J.L.; Mach, F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature 2005, 434, 782–786. [Google Scholar]
- Ramírez, B.G.; Blázquez, C.; Gómez del Pulgar, T.; Guzmán, M.; de Ceballos, M.L. Prevention of Alzheimer’s disease pathology by cannabinoids: Neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005, 25, 1904–1913. [Google Scholar] [CrossRef]
- Eubanks, L.M.; Rogers, C.J.; Beuscher, A.E., 4th; Koob, G.F.; Olson, A.J.; Dickerson, T.J.; Janda, K.D. A molecular link between the active component of marijuana and Alzheimer’s disease pathology. Mol. Pharmacol. 2006, 3, 773–777. [Google Scholar] [CrossRef]
- Munson, A.E.; Harris, L.S.; Friedman, M.A.; Dewey, W.L.; Carchman, R.A. Antineoplastic activity of cannabinoids. J. Nat. Cancer Inst. 1975, 55, 597–602. [Google Scholar]
- Preet, A.; Ganju, R.K.; Groopman, J.E. D9-Tetrahydrocannabinol inhibits epithelial growth factor-induced lung cancer cell migration in vitro as well as its growth and metastasis in vivo. Oncogene 2007, 27, 339–346. [Google Scholar]
- Guzmán, M.; Duarte, M.J.; Blázquez, C.; Ravina, J.; Rosa, M.C.; Galve-Roperh, I.; Sánchez, C.; Velasco, G.; González-Faria, L. A pilot clinical study of 9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br. J. Cancer 2006, 95, 204–209. [Google Scholar] [CrossRef]
- Grant, I.; Gonzalez, R.; Carey, C.L.; Natarajan, L.; Wolfson, T. Non-acute (residual) neurocognitive effects of cannabis use: A meta-analytic study. J. Int. Neuropsychol. Soc. 2003, 9, 679–689. [Google Scholar]
- Jiang, W.; Zhang, Y.; Xiao, L.; Van Cleemput, J.; Ji, S.P.; Bai, G.; Zhang, X. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J. Clin. Invest. 2005, 115, 3104–3116. [Google Scholar] [CrossRef]
- Sarne, Y.; Mechoulam, R. Cannabinoids: Between neuroprotection and neurotoxicity. Curr. Drug Targets CNS Neurol. Disord. 2005, 4, 677–684. [Google Scholar] [CrossRef]
- Correa, F.; Mestre, L.; Molina-Holgado, E.; Arevalo-Martin, A.; Docagne, F.; Romero, E.; Molina-Holgado, F.; Borrell, J.; Guaza, C. The role of cannabinoid system on immune modulation: Therapeutic implications on CNS inflammation. Mini Rev. Med. Chem. 2005, 5, 671–675. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Romero, J.; Velasco, G.; Tolón, R.M.; Ramos, J.A.; Guzmán, M. Cannabinoid CB2 receptor: a new target for controlling neural cell survival? Trends Pharmacol Sci. 2007, 28, 39–45. [Google Scholar] [CrossRef]
- Pertwee, R.G. Cannabinoid Receptor Ligands. Available online: http://www.tocris.com/pdfs/pdf_downloads/Cannabinoid_Receptor_Ligands_Review.pdf (accessed on 4 October 2013).
- Huestis, M.A. Pharmacokinetics and Metabolism of the Plant Cannabinoids, Δ9-Tetrahydrocannibinol, Cannabidiol and Cannabinoids. Cannabinoids. Handb. Exp. Pharmacol. 2005, 168, 657–90. [Google Scholar]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Galano, A.; Macías-Ruvalcaba, N.A.; Campos, O.N.M.; Pedraza-Chaverri. , J. Mechanism of the OH Radical Scavenging Activity of Nordihydroguaiaretic Acid: A Combined Theoretical and Experimental Study. J. Phys. Chem. B 2010, 114, 6625–6635. [Google Scholar] [CrossRef]
- Galano, A. On the direct scavenging activity of melatonin towards hydroxyl and a series of peroxyl radicals. Phys. Chem. Chem. Phys. 2011, 13, 7147–7157. [Google Scholar]
- Iuga, C.; Alvarez-Idaboy, J.R.; Russo, N. Antioxidant Activity of trans-Resveratrol toward Hydroxyl and Hydroperoxyl Radicals: A Quantum Chemical and Computational Kinetics Study. J. Org. Chem. 2012, 77, 3868–3877. [Google Scholar]
- Chiodo, S.G.; Leopoldini, M.; Russo, N.; Toscano, M. The inactivation of lipid peroxide radical by quercetin. A theoretical insight. Phys. Chem. Chem. Phys. 2010, 12, 7662–7670. [Google Scholar] [CrossRef]
- Leopoldini, M.; Chiodo, S.G.; Russo, N.; Toscano, M. Detailed Investigation of the OH Radical Quenching by Natural Antioxidant Caffeic Acid Studied by Quantum Mechanical Models. J. Chem. Theory Comput. 2011, 7, 4218–4233. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant Properties of Phenolic Compounds: H-Atom versus Electron Transfer Mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. Gas and Liquid Phase Acidity of Natural Antioxidants. J. Agric. Food Chem. 2006, 54, 3078–3085. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. A comparative study of the antioxidant power of flavonoid catechin and its planar analogue. J. Agric. Food Chem. 2007, 55, 7944–7949. [Google Scholar] [CrossRef]
- Hamelink, C.; Hampson, A.; Wink, D.A.; Eiden, L.E.; Eskay, R.L. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J. Pharmacol. Exp. Ther. 2005, 314, 780–788. [Google Scholar] [CrossRef]
- Queiroz, A.N.; Gomes, B.A.Q.; Moraes, W.M., Jr; Borges, R.S. A theoretical antioxidant pharmacophore for resveratrol. Eur. J. Med. Chem. 2009, 44, 1644–1649. [Google Scholar]
- Honório, K.M.; da Silva, A.B.F. An AM1 study on the electron-donating and electron-accepting character of biomolecules. Int. J. Quant. Chem. 2003, 95, 126–132. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A.; Stratmann, R.E.; Burant, J.C.; et al. Gaussian 09, Revision C.02; Gaussian, Inc: Wallingford, CT, USA, 2004. [Google Scholar]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods I. Method. J. Comput. Chem. 1989, 10, 209–220. [Google Scholar] [CrossRef]
- Kohn, W.; Becke, A.D.; Parr, R.G. Density functional theory of electronic structure. J. Phys. Chem. 1996, 100, 12974–12980. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Hehre, W.J.; Radom, L.; Schleyer, P.V.R.; Pople, J.A. Ab Initio Molecular Orbital Theory; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Scalmani, G.; Frisch, M.J. A continuous surface charge formalism for the polarizable continuum model of solvation. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef]
- Borges, R.S.; Queiroz, A.N.; Mendes, A.P.S.; Araújo, S.C.; França, L.C.S.; Franco, E.C.S.; Leal, W.G.; da Silva, A.B.F. Density Functional Theory (DFT) Study of Edaravone Derivatives as Antioxidants. Int. J. Mol. Sci. 2012, 13, 7594–7606. [Google Scholar] [CrossRef]
- Mikulski, D.; Górniak, R.; Molski, M. A theoretical study of the structure-radical scavenging activity of trans-resveratrol analogues and cis-resveratrol in gas phase and water environment. Eur. J. Med. Chem. 2010, 45, 1015–1027. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Portmann, S.; Lüthi, H.P. Molekel: an interactive molecular graphics. Chimia 2000, 54, 766–770. [Google Scholar]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Borges, R.S.; Batista, J., Jr.; Viana, R.B.; Baetas, A.C.; Orestes, E.; Andrade, M.A.; Honório, K.M.; Da Silva, A.B.F. Understanding the Molecular Aspects of Tetrahydrocannabinol and Cannabidiol as Antioxidants. Molecules 2013, 18, 12663-12674. https://doi.org/10.3390/molecules181012663
Borges RS, Batista J Jr., Viana RB, Baetas AC, Orestes E, Andrade MA, Honório KM, Da Silva ABF. Understanding the Molecular Aspects of Tetrahydrocannabinol and Cannabidiol as Antioxidants. Molecules. 2013; 18(10):12663-12674. https://doi.org/10.3390/molecules181012663
Chicago/Turabian StyleBorges, Rosivaldo S., João Batista, Jr., Rommel B. Viana, Ana C. Baetas, Ednilsom Orestes, Marcieni A. Andrade, Káthia M. Honório, and Albérico B. F. Da Silva. 2013. "Understanding the Molecular Aspects of Tetrahydrocannabinol and Cannabidiol as Antioxidants" Molecules 18, no. 10: 12663-12674. https://doi.org/10.3390/molecules181012663
APA StyleBorges, R. S., Batista, J., Jr., Viana, R. B., Baetas, A. C., Orestes, E., Andrade, M. A., Honório, K. M., & Da Silva, A. B. F. (2013). Understanding the Molecular Aspects of Tetrahydrocannabinol and Cannabidiol as Antioxidants. Molecules, 18(10), 12663-12674. https://doi.org/10.3390/molecules181012663





