Two Cerebrosides Isolated from the Seeds of Sterculia lychnophora and Their Neuroprotective Effect
Abstract
:1. Introduction
2. Results and Discussion
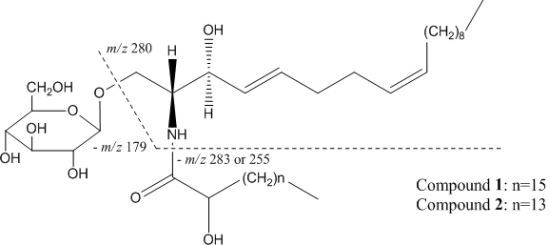
2.1. Structure Identification of Compounds
2.2. Neuroprotective Effect Against SH-SY5Y Cell Damage
3. Experimental
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectral Data of Compounds
3.5. Preparation of Solutions
3.6. Cell Maintenance
3.7. MTT Assay
3.8. Data Analysis
4. Conclusions
Acknowledgments
References
- National Pharmacopoeia Committee of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2000; Volume 1, pp. 216–217.
- Chinese Academy of Medical Sciences Institute of Medicine. Chinese Traditional Medicine Records; People’s Medical Publishing House: Beijing, China, 1984; Volume 3, pp. 538–541. [Google Scholar]
- Chen, J.M.; Li, W.K.; Liu, H.L.; Shen, Y.X.; Yu, M.Q. Chemical studies on Sterculia lychnophora. J. Chin. Med. Mat. 1995, 18, 567–569. [Google Scholar]
- Chen, J.M.; Cao, P.R.; Song, H.T. Study on purification and characterization of Sterculia polysaccharide PP III. Chin. J. Chin. Mater. Med. 1996, 21, 39–41. [Google Scholar]
- Du, L.J.; Sun, S.M.; Yu, L.; Chen, J.M.; Wu, J.H. Pharmacodynamics Semen Sterculia Lychnophora from home and abroad on anti-inflammatory and small intestine’s peristalsis in mice. J. Chin. Med. Mat. 1995, 18, 409–410. [Google Scholar]
- Qu, B.Q.; Ma, T.X. Preliminary report on the cathartic effect of Sterculia lychnophora. Natl. Med. J. China 1954, 5, 337–340. [Google Scholar]
- Zhou, C.J.; Lin, J.; Xu, L.M.; Chen, Q.; Zhang, L. Pharmacological study of Fufang Pangdahai. J. Fujian Coll. Tradit. Chin. Med. 1994, 4, 30–33. [Google Scholar]
- Feng, D.R. Sterculia scaphigera (Pangtaihai or Tungtahai), a Chinese laxative drug. J. Chin. Med. 1942, 61, 9–11. [Google Scholar]
- Jung, J.H.; Lee, C.O.; Kim, Y.C. New bioactive cerebrosides from Arisaema amurense. J. Nat. Prod. 1996, 59, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, H.; Kawashima, K.; Sakagami, M.; Kawanishi, H.; Shimomura, M.; Ohashi, K.; Kitagawa, I. Sphingolipids and glycerolipids. I. Chemical structures and ionophoretic activities of soya-cerebrosides I and II from soybean. Chem. Pharm. Bull. 1990, 38, 2933–2938. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.H.; Ojika, M.; Sakagami, Y. Termitomycesphins A–D, Novel neuritogenic cerebrosides from the edible Chinese mushroom Termitomyces albuminosus. Tetrahedron 2000, 56, 5835–5841. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1-O-β-D-glucopyranosyl-(2S,3R,4E,8Z)-2-[(2-hydroxyoctadecanoyl)amido]-4,8-octadecadiene-1,3-diol and soya-cerebroside I are available from the authors. |


© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, R.-F.; Wu, X.-W.; Geng, D. Two Cerebrosides Isolated from the Seeds of Sterculia lychnophora and Their Neuroprotective Effect. Molecules 2013, 18, 1181-1187. https://doi.org/10.3390/molecules18011181
Wang R-F, Wu X-W, Geng D. Two Cerebrosides Isolated from the Seeds of Sterculia lychnophora and Their Neuroprotective Effect. Molecules. 2013; 18(1):1181-1187. https://doi.org/10.3390/molecules18011181
Chicago/Turabian StyleWang, Ru-Feng, Xiu-Wen Wu, and Di Geng. 2013. "Two Cerebrosides Isolated from the Seeds of Sterculia lychnophora and Their Neuroprotective Effect" Molecules 18, no. 1: 1181-1187. https://doi.org/10.3390/molecules18011181