Microwave Assisted Synthesis of Some New Thiazolopyrimidine, Thiazolodipyrimidine and Thiazolopyrimidothiazolopyrimidine Derivatives with Potential Antioxidant and Antimicrobial Activity
Abstract
:1. Introduction
2. Results and Discussion
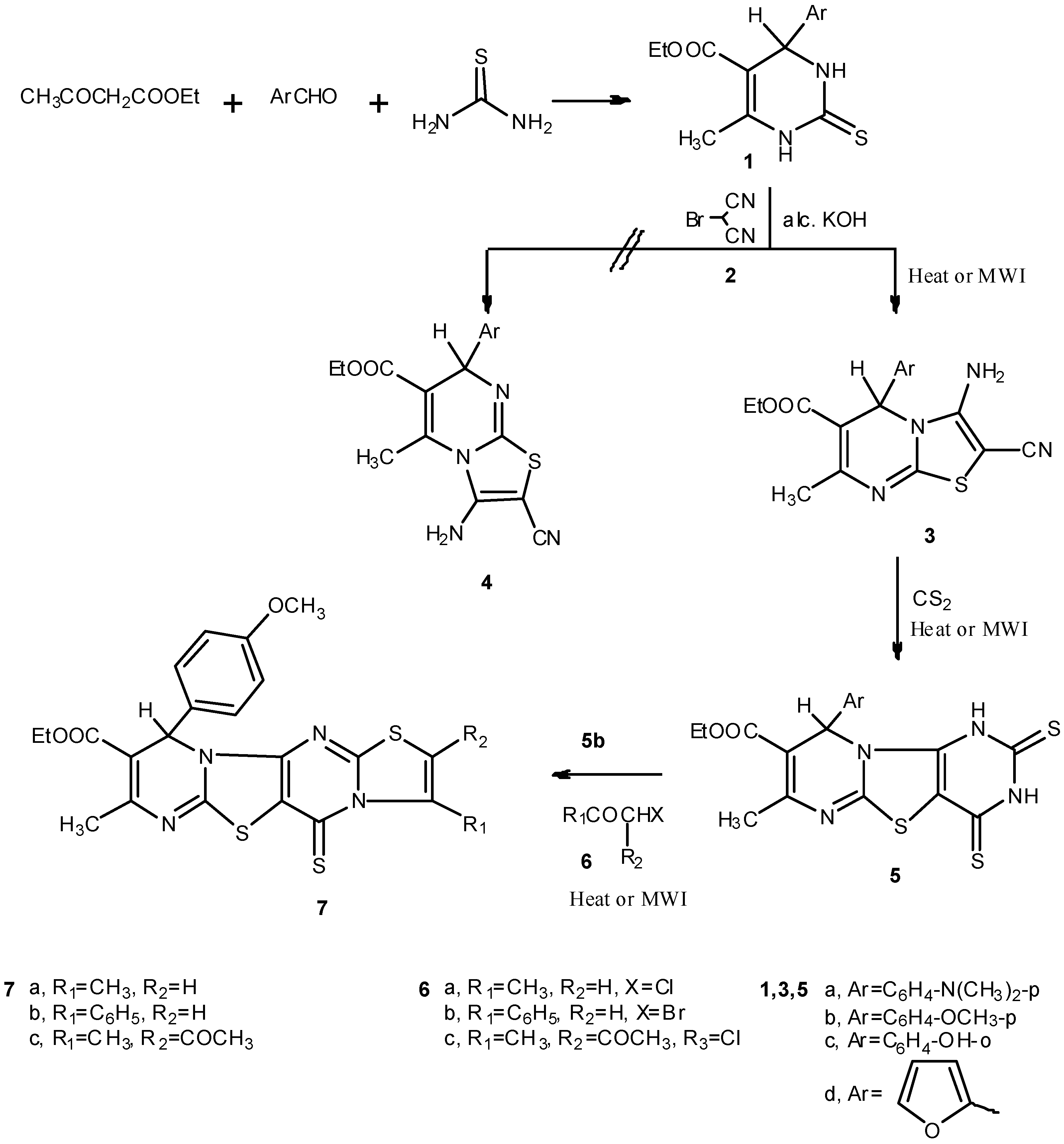
2.1. Chemistry
| Compound no. | Reaction Yield % | Reaction Time | ||
|---|---|---|---|---|
| Microwave | Conventional Method | Microwave | Conventional Method | |
| 1a | 82 | 55 | 5 min | 3 h |
| 1b | 87 | 58 | 5 min | 3 h |
| 1c | 70 | 42 | 5 min | 3 h |
| 1d | 62 | 35 | 5 min | 3 h |
| 3a | 81 | 56 | 10 min | Overnight |
| 3b | 85 | 48 | 10 min | Overnight |
| 3c | 85 | 53 | 10 min | Overnight |
| 3d | 68 | 40 | 10 min | Overnight |
| 5a | 82 | 50 | 15 min | 8 h |
| 5b | 80 | 53 | 15 min | 8 h |
| 5c | 79 | 44 | 15 min | 8 h |
| 5d | 69 | 37 | 15 min | 8 h |
| 7a | 74 | 43 | 5 min | 3 h |
| 7b | 88 | 52 | 5 min | 3 h |
| 7c | 83 | 58 | 5 min | 3 h |
2.2. Biological Evaluation
2.2.1. Antioxidant Screening
| Compounds | Absorbance of samples (A) | Hemolysis (%) |
|---|---|---|
| Complete hemolysis with distilled water (B) | 0.660 | - |
| Ascorbic acid | 0.026 | 3.93 |
| 1a | 0.082 | 10.33 |
| 1b | 0.075 | 9.12 |
| 1c | 0.090 | 13.01 |
| 1d | 0.092 | 14.12 |
| 3a | 0.035 | 5.22 |
| 3b | 0.031 | 4.68 |
| 3c | 0.045 | 6.92 |
| 3d | 0.051 | 8.02 |
| 5a | 0.115 | 21.60 |
| 5b | 0.112 | 19.25 |
| 5c | 0.132 | 24.07 |
| 5d | 0.130 | 23.12 |
| 7a | 0.042 | 6.36 |
| 7b | 0.045 | 6.81 |
| 7c | 0.043 | 6.51 |
| Compounds | Absorbance of sample | Inhibition (%) |
|---|---|---|
| ABTS control | 0.54 | 0 |
| Ascorbic acid | 0.06 | 88.8 |
| 1a | 0.20 | 63.0 |
| 1b | 0.23 | 57.4 |
| 1c | 0.29 | 46.3 |
| 1d | 0.28 | 48.1 |
| 3a | 0.10 | 81.5 |
| 3b | 0.12 | 77.7 |
| 3c | 0.15 | 72.2 |
| 3d | 0.13 | 75.9 |
| 5a | 0.45 | 16.6 |
| 5b | 0.42 | 22.2 |
| 5c | 0.48 | 11.1 |
| 5d | 0.43 | 20.3 |
| 7a | 0.17 | 68.5 |
| 7b | 0.19 | 64.8 |
| 7c | 0.16 | 70.3 |
| Compound | Absorbance of Samples |
|---|---|
| Ascorbic acid | 0.020 |
| 3a | 0.026 |
| 3b | 0.029 |
| 3c | 0.037 |
| 3d | 0.033 |
2.2.2. Antimicrobial Evaluation
| Compd. No. | Inhibition zone (mm) | ||||||
|---|---|---|---|---|---|---|---|
| Gram-negative | Gram-positive | Fungi Yeast | |||||
| E. coli | P. putida | B. subtilis | S. lactis | A. niger | P. sp. | C. albicans | |
| 1a | 12 | 8 | 6 | 8 | 5 | 5 | 0 |
| 1b | 14 | 9 | 6 | 7 | 4 | 2 | 0 |
| 1c | 6 | 3 | 0 | 0 | 2 | 2 | 0 |
| 1d | 3 | 2 | 0 | 0 | 0 | 0 | 0 |
| 3a | 15 | 11 | 9 | 6 | 7 | 5 | 0 |
| 3b | 12 | 7 | 7 | 5 | 7 | 5 | 0 |
| 3c | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3d | 2 | 2 | 0 | 0 | 0 | 0 | 0 |
| 5a | 10 | 7 | 8 | 6 | 4 | 3 | |
| 5b | 11 | 8 | 9 | 6 | 5 | 2 | |
| 5c | 4 | 2 | 0 | 0 | 0 | 0 | 0 |
| 5d | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7a | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7b | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7c | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chloram-phenicol® | 22 | 21 | 18 | 19 | 20 | 12 | 0 |
| Ampicillin® | 24 | 20 | 19 | 22 | 24 | 14 | 14 |
3. Experimental
3.1. General
3.1.1. Ethyl 4-aryl-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylates 1a–d
3.1.2. Ethyl 3-amino-5-aryl-2-cyano-7-methyl-5H-thiazolo[3,2-a]pyrimidine-6-carboxylates (3a–d)
3.1.3. Ethyl 9-aryl-7-methyl-2,4-dithioxo-2,3,4,9-tetrahydro-1H-thiazolo[3,2-a:4,5-d']dipyrimidine-8-carboxylates 5a–d
3.1.4. Ethyl 8-methyl-10-(4-methoxyphenyl)-3-substituted-5-thioxo-2-(un)substituted-10H-thiazolo-[3'',2'':1',2']pyrimido[4',5':4,5]thiazolo[3,2-a]pyrimidine-9-carboxylates 7a–c
3.2. Antioxidant Screening
3.2.1. Assay for Erythrocyte Hemolysis
3.2.2. Antioxidant Activity Screening Assay—ABTS Method

3.2.3. Bleomycin-Dependent DNA Damage
3.3. Antimicrobial Screening
4. Conclusions
Acknowledgements
References
- Geist, J.G.; Lauw, S.; Illarinova, V.; Fischer, M.; Gwawert, T.; Rohdich, F.; Eisenreich, W.; Kaiser, J.; Groll, M.; Scheurer, C.; et al. Thiazolopyrimidine inhibitors of 2-methylerythritol 2,4-cyclodiphosphate synthase (IspF) from Mycobacterium tuberculosis and Plasmodium falciparum. ChemMedChem 2010, 5, 1092–1101. [Google Scholar] [CrossRef]
- Amr, A.-E.-G.; Maigali, S.S.; Abdulla, M.M. Synthesis, and analgesic and antiparkinsonian activities of thiopyrimidine, pyrane, pyrazoline, and thiazolopyrimidine derivatives from 2-chloro-6-ethoxy-4-acetylpyridine. Monatsh. Chem. 2008, 139, 1409–1415. [Google Scholar] [CrossRef]
- Branstetter, B.J.; Breitenbucher, J.G.; Lebsack, A.D.; Xiao, W. Thiaolopyrimidine Modulators of TRPV1. U.S. Patent WO 005,303, 2008. [Google Scholar]
- Flefel, E.E.; Salama, M.A.; El-Shahat, M.; El-Hashash, M.A.; El-Farargy, A.F. A novel synthesis of some new pyrimidine and thiazolopyrimidine derivatives for anticancer evaluation. Phosphorus Sulfur Silicon Relat. Elem. 2007, 182, 1739–1756. [Google Scholar] [CrossRef]
- Hammam, A.G.; Sharaf, M.A.; Abdel Hafez, N.A. Synthesis and anti-cancer activity of pyridine and thiazolopyrimidine derivatives using ethylpiperidone as a synthon. Indian J. Chem. 2001 40B, 213–221.
- Said, M.; Abouzid, K.; Mouneer, A.; Ahmedy, A.; Osman, A.-M. Synthesis and biological evaluation of new thiazolopyrimidines. Arch. Pharm. Res. 2004, 27, 471–477. [Google Scholar]
- Linder, W.; Brandes, W. Pesticidal Thiazolopyrimidine Derivatives. U.S. Patent 367,820,1991.
- Duval, R.; Kolb, S.; Braud, E.; Genest, D.; Garbay, C. Rapid discovery of triazolobenzylidene-thiazolopyrimidines (TBTP) as CDC25 phosphatase inhibitors by parallel click chemistry and in situ screening. J. Comb. Chem. 2009, 11, 947–950. [Google Scholar] [CrossRef]
- Kolb, S.; Mondésert, O.; Goddard, M.L.; Jullien, D.; Villoutreix, B.O.; Ducommun, B.; Garbay, C.; Braud, E. Development of novel thiazolopyrimidines as CDC25B phosphatase inhibitors. ChemMedChem 2009, 4, 633–648. [Google Scholar] [CrossRef]
- Zhi, H.; Chen, L.; Zhang, L.; Liu, S.; Wan, D.C.C.; Lin, H.; Hu, C. Design, synthesis, and biological evaluation of 5H-thiazolo[3,2-a]pyrimidine derivatives as a new type of acetylcholinesterase inhibitors. ARKIVOC 2008, xiii, 266–277. [Google Scholar]
- Rashad, A.E.; Shamroukh, A.H.; Abdel-Megeid, R.E.; El-Sayed, W.A. Synthesis, reactions and antimicrobial evaluation of some polycondensed thieno-pyrimidine derivatives. Synth. Commun. 2010, 40, 1149–1160. [Google Scholar] [CrossRef]
- El-Emary, T.I.; Abdel-Mohsen, S.A. Synthesis and antimicrobial activity of some new 1,3-diphenylpyrazoles bearing pyrimidine, Pyrimidinethione, thiazolopyrimidine, triazolopyrimidine, thio- and alkylthiotriazolopyrimidinone moieties at the 4-position. Phosphorus Sulfur 2006, 181, 2459–2474. [Google Scholar] [CrossRef]
- Maddila, S.; Damu, G.L.V.; Oseghe, E.O.; Abafe, O.A.; Venakata, R.C.; Lavanya, P. Synthesis and biological studies of novel biphenyl-3,5-dihydro-2H-thiazolo-pyrimidines derivatives. J. Korean Chem. Soc. 2012, 56, 334–340. [Google Scholar] [CrossRef]
- Sosnowski, M.; Skulski, L. Microwave-accelerated iodination of some aromatic amines, using urea-hydrogen peroxide addition compound (UHP) as the oxidant. Molecules 2002, 7, 867–870. [Google Scholar] [CrossRef]
- Gregg, B.; Golden, K.; Quinn, J. Indium(III) trifluoromethanesulfonate as an efficient catalyst for the deprotection of acetals and ketals. J. Org. Chem. 2007, 72, 5890–5893. [Google Scholar]
- Lerebours, R.; Wolf, C. Palladium(II)-catalyzed conjugate addition of arylsiloxanes in water. Org. Lett. 2007, 9, 2737–2740. [Google Scholar] [CrossRef]
- Marion, N.; Gealageas, R.; Nolan, S. [(NHC)AuI]-catalyzed rearrangement of allylic acetates. Org. Lett. 2007, 9, 2653–2656. [Google Scholar] [CrossRef]
- Biginelli, P. Synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Gazz. Chem. Ital. 1893, 23, 360–372. [Google Scholar]
- Kappe, C.O. 100 Years of the Biginelli dihydropyridine synthesis. Tetrahedron 1993, 49, 6937–6963. [Google Scholar] [CrossRef]
- Akbas, E.; Aslanoglu, F. Studies on reactions of pyrimidine compounds. Microwave-assisted synthesis of 1,2,3,4-tetrahydro-2-thioxopyrimidine derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 82–89. [Google Scholar]
- Hafez, H.N.; Hussein, H.A.R.; El-Gazzar, A.B.A. Synthesis of substituted thieno-[2,3-d]pyrimidine-2,4-dithiones and their S-glycoside analogues as potential antiviral and antibacterial agents. Eur. J Med. Chem. 2010, 45, 4026–4032. [Google Scholar] [CrossRef]
- Youssef, M.M.; Amin, M.A. Microwave assisted synthesis of some new heterocyclic spiro-derivatives with potential antimicrobial and antioxidant activity. Molecules 2010, 15, 8827–8840. [Google Scholar] [CrossRef]
- Faty, R.M.; Youssef, M.M.; Youssef, A.M.S. Microwave assisted synthesis and unusual coupling of some novel Pyrido[3,2-f][1,4]thiazepines. Molecules 2011, 16, 4549–4559. [Google Scholar]
- Saad, H.A.; Youssef, M.M.; Mosselhi, M.A. Microwave assisted synthesis of some new fused 1,2,4-triazine bearing thiophene moiety of expected pharmacological activity. Molecules 2011, 16, 4937–4957. [Google Scholar]
- Youssef, A.M.S.; Azab, M.E.; Youssef, M.M. Bromination and diazo-coupling of pyridinethiones; microwave assisted synthesis of isothiazolopyridine, pyridothiazine and pyridothiazepines. Molecules 2012, 17, 6930–6943. [Google Scholar]
- Morimoto, Y.; Tanaka, K.; Iwakiri, Y.; Tokuhiro, S.; Fukushima, S.; Takeuchi, Y. Protective effects of some neutral amino acids against hypotonic hemolysis. Biol. Pharm. Bull. 1995, 18, 1417–1422. [Google Scholar] [CrossRef]
- Lissi, E.; Modak, B.; Torres, R.; Escobar, J.; Urzua, A. Total antioxidant potential of resinousexudates from Heliotropium species, and a comparison of ABTS and DPPH methods. Free Radic. Res. 1999, 30, 471–477. [Google Scholar] [CrossRef]
- Aeschlach, R.; Loliger, J.; Scott, B.C.; Murciao, A.; Butler, J.; Halliwell, B.; Aruoma, O. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem. Toxicol. 1994, 32, 31–36. [Google Scholar] [CrossRef]
- Coffen, D.L.; Korzan, D.G. Synthetic quinine analogs. III. Frangomeric and anchimeric processes in the preparation and reactions of α,β-epoxy ketones. J. Org. Chem. 1971, 36, 390–395. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Youssef, M.M.; Amin, M.A. Microwave Assisted Synthesis of Some New Thiazolopyrimidine, Thiazolodipyrimidine and Thiazolopyrimidothiazolopyrimidine Derivatives with Potential Antioxidant and Antimicrobial Activity. Molecules 2012, 17, 9652-9667. https://doi.org/10.3390/molecules17089652
Youssef MM, Amin MA. Microwave Assisted Synthesis of Some New Thiazolopyrimidine, Thiazolodipyrimidine and Thiazolopyrimidothiazolopyrimidine Derivatives with Potential Antioxidant and Antimicrobial Activity. Molecules. 2012; 17(8):9652-9667. https://doi.org/10.3390/molecules17089652
Chicago/Turabian StyleYoussef, Mohamed M., and Mahmoud A. Amin. 2012. "Microwave Assisted Synthesis of Some New Thiazolopyrimidine, Thiazolodipyrimidine and Thiazolopyrimidothiazolopyrimidine Derivatives with Potential Antioxidant and Antimicrobial Activity" Molecules 17, no. 8: 9652-9667. https://doi.org/10.3390/molecules17089652




