A New Synthetic Route to Original Sulfonamide Derivatives in 2-Trichloromethylquinazoline Series: A Structure-Activity Relationship Study of Antiplasmodial Activity
Abstract
:1. Introduction
2. Results and Discussion
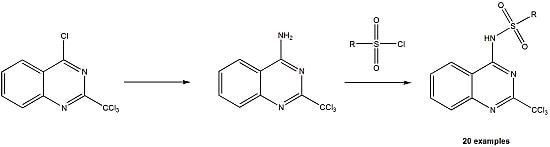
2.1. Synthesis




| Entry | R- | Product | Yield % | CC50 (µM) a | IC50 P fK1 (µM) b |
|---|---|---|---|---|---|
| 1 | Ph- | 8a | 62 | 82.0 | >10 * |
| 2 | 4-Me-Ph- | 8b | 51 | 68.7 | >10 * |
| 3 | 4-MeO-Ph- | 8c | 56 | 102.3 | >10 * |
| 4 | 4-CN-Ph- | 8d | 73 | 73.8 | >10 * |
| 5 | 4-Cl-Ph | 8e | 50 | 71.1 | >10 * |
| 6 | 4-Br-Ph- | 8f | 60 | 57.4 | >10 * |
| 7 | 4-F-Ph- | 8g | 66 | 69.5 | >10 * |
| 8 | 3-F-Ph- | 8h | 53 | 106.4 | >10 * |
| 9 | 2-F-Ph- | 8i | 60 | 136.1 | >10 * |
| 10 | 4-NO2-Ph- | 8j | 91 | 88.3 | >10 * |
| 11 | 3-NO2-Ph- | 8k | 56 | 119.8 | >10 * |
| 12 | 2-NO2-Ph- | 8l | 59 | 126.0 | >10 * |
| 13 | 4-CF3-Ph- | 8m | 88 | 49.0 | >10 * |
| 14 | 3-CF3-Ph- | 8n | 100 | 75.1 | >10 * |
| 15 | 2-CF3-Ph- | 8o | 50 | 73.8 | >10 * |
| 16 | Biphenyl-4-yl | 8p | 23 | 38.2 | >10 * |
| 17 | Napht-2-yl- | 8q | 43 | 48.8 | >10 * |
| 18 | Napht-1-yl- | 8r | 61 | 47.8 | >10 * |
| 19 | Thiophen-2-yl- | 8s | 43 | 121.1 | >10 * |
| 20 | Methyl- | 8t | 18 | 241.2 | >10 * |
| Doxorubicine a | 0.2 | - | |||
| Chloroquine b | 30 | 0.6 | |||
| Doxycycline b | 20 | 6.0 | |||
2.2. Biological Evaluation and Structure-Activity Relationship (SAR) Study
| Entry | Structure | CC50 HepG2 (µM) | IC50 P. falc. (µM) | SI a |
|---|---|---|---|---|
| 1 |  | >125 | 2.5 | >50 |
| 2 |  | 16 | 0.4 | 40 |
| 3 |  | 50 | 1.1 | 45 |
| 4 |  | >25 | 0.9 | >28 |
| 5 |  | 49–136 | >10 | nd |
| Doxorubicine b | 0.2 | - | - | |
| Chloroquine c | 30 | 0.6 | ||
| Doxycycline c | 20 | 6.0 | ||

3. Experimental
3.1. General Procedures
3.2. Preparation of 4-Amino-2-trichloromethylquinazoline (7)
3.3. General Procedure for the Preparation of Compounds 8a to 8t
4. Conclusions
Acknowledgments
References
- Fry, D.W.; Kraker, A.J.; McMichael, A.; Ambroso, L.A.; Nelson, J.M.; Leopold, W.R.; Connors, R.W.; Bridges, A.J. A specific inhibitor of the epidermal growth factor receptor tyrosine kinase. Science 1994, 265, 1093–1095. [Google Scholar]
- Colotta, V.; Catarzi, D.; Varano, F.; Lenzi, O.; Filacchioni, G.; Costagli, C.; Galli, A.; Ghelardini, C.; Galeotti, N.; Gratteri, P.; et al. Structural Investigation of the 7-Chloro-3-hydroxy-1H-quinazoline-2,4-dione. Scaffold to Obtain AMPA and Kainate Receptor Selective Antagonists. Synthesis, Pharmacological, and Molecular Modeling Studies. J. Med. Chem. 2006, 49, 6015–6026. [Google Scholar]
- Malecki, N.; Carato, P.; Rigo, G.; Goossens, J.F.; Houssin, R.; Bailly, C.; Henichart, J.P. Synthesis of condensed quinolines and quinazolines as DNA ligands. Bioorg. Med. Chem. 2004, 12, 641–647. [Google Scholar]
- Doyle, L.A.; Ross, D.D. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 2003, 22, 7340–7358. [Google Scholar] [CrossRef]
- Henderson, E.A.; Bavetsias, V.; Theti, D.S.; Wilson, S.C.; Clauss, R.; Jackman, A.L. Targeting the alpha-folate receptor with cyclopenta[g]quinazoline-based inhibitors of thymidylate synthase. Bioorg. Med. Chem. 2006, 14, 5020–5042. [Google Scholar]
- Foster, A.; Coffrey, H.A.; Morin, M.J.; Rastinejad, F. Pharmacological rescue of mutant p53 conformation and function. Science 1999, 286, 2507–2510. [Google Scholar] [CrossRef]
- Chien, T.-C.; Chen, C.-S.; Yu, F.-H.; Chern, J.-W. Nucleosides XI. Synthesis and antiviral evaluation of 5'-alkylthio-5'-deoxyquinazolinone nucleoside derivatives as S-adenosyl-L-homocysteine analogs. Chem. Pharm. Bull. 2004, 52, 1422–1426. [Google Scholar]
- Herget, T.; Freitag, M.; Morbitzer, M.; Kupfer, R.; Stamminger, T.; Marschall, M. Novel chemical class of pUL97 protein kinase-specific inhibitors with strong anticytomegaloviral activity. Antimicrob. Agents Chemother. 2004, 48, 4154–4162. [Google Scholar]
- Martin, T.A.; Wheeler, A.G.; Majewski, R.F.; Corrigan, J.R. Sulfanilamidoquinazolines. J. Med. Chem. 1964, 7, 812–814. [Google Scholar] [CrossRef]
- Kunes, J.; Bazant, J.; Pour, M.; Waisser, K.; Slosarek, M.; Janota, J. Quinazoline derivatives with antitubercular activity. Farmaco 2000, 55, 725–729. [Google Scholar]
- Waisser, K.; Gregor, J.; Dostal, H.; Kunes, J.; Kubicova, L.; Klimesova, V.; Kaustova, J. Influence of the replacement of the oxo function with the thioxo group on the antimycobacterial activity of 3-aryl-6,8-dichloro-2H-1,3-benzoxazine-2,4(3H)-diones and 3-arylquinazoline-2,4(1H,3H)-diones. Farmaco 2001, 56, 803–807. [Google Scholar]
- Gundla, R.; Kazemi, R.; Sanam, R.; Muttineni, R.; Sarma, J.A.R.P.; Dayam, R.; Neamati, N. Discovery of novel small-molecule inhibitors of human epidermal growth factor receptor-2: Combined ligand and target-based approach. J. Med. Chem. 2008, 51, 3367–3377. [Google Scholar] [CrossRef]
- Rewcastle, G.W.; Palmer, B.D.; Bridges, A.J.; Showalter, H.D.H.; Sun, L.; Nelson, J.; McMichael, A.; Kraker, A.J.; Fry, D.W.; Denny, W.A. Tyrosine kinase inhibitors. 9. Synthesis and evaluation of fused tricyclic quinazoline analogues as ATP site inhibitors of the tyrosine kinase activity of the epidermal growth factor receptor. J. Med. Chem. 1996, 39, 918–928. [Google Scholar] [CrossRef]
- Luth, A.; Lowe, W. Syntheses of 4-(indole-3-yl)quinazolines: A new class of epidermal growth factor receptor tyrosine kinase inhibitors. Eur. J. Med. Chem. 2008, 43, 1478–1488. [Google Scholar] [CrossRef]
- Mendes da Silva, J.F.; Walters, M.; Al-Damluji, S.; Ganellin, C.R. Molecular features of the prazosin molecule required for activation of Transport-P. Bioorg. Med. Chem. 2008, 16, 7254–7263. [Google Scholar]
- Vanelle, P.; de Meo, M.P.; Maldonado, J.; Nouguier, R.; Crozet, M.P.; Laget, M.; Dumenil, G. Genotoxicity in oxazolidine derivatives: Influence of the nitro group. Eur. J. Med. Chem. 1990, 25, 241–250. [Google Scholar] [CrossRef]
- Crozet, M.P.; Giraud, L.; Sabuco, J.F.; Vanelle, P.; Barreau, M. SRN1 reactions of a tetrasubstituted-1,4-benzoquinone. Tetrahedron Lett. 1991, 32, 4125–4128. [Google Scholar]
- Baraldi, P.G.; El-Kashef, H.; Farghaly, A.R.; Vanelle, P.; Fruttarolo, F. Synthesis of new pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines and related heterocycles. Tetrahedron 2004, 60, 5093–5104. [Google Scholar] [CrossRef]
- Boufatah, N.; Gellis, A.; Maldonado, J.; Vanelle, P. Efficient microwave-assisted synthesis of new sulfonylbenzimidazole-4,7-diones: Heterocyclic quinones with potential antitumor activity. Tetrahedron 2004, 60, 9131–9137. [Google Scholar] [CrossRef]
- Verhaeghe, P.; Azas, N.; Gasquet, M.; Hutter, S.; Ducros, C.; Laget, M.; Rault, S.; Rathelot, P.; Vanelle, P. Synthesis and antiplasmodial activity of new 4-aryl-2-trichloromethylquinazolines. Bioorg. Med. Chem. Lett. 2008, 18, 396–401. [Google Scholar] [CrossRef]
- Verhaeghe, P.; Azas, N.; Hutter, S.; Castera-Ducros, C.; Laget, M.; Dumètre, A.; Gasquet, M.; Reboul, J.-P.; Rault, S.; Rathelot, P.; et al. Synthesis and in vitro antiplasmodial evaluation of 4-anilino-2-trichloromethylquinazolines. Bioorg. Med. Chem. 2009, 17, 4313–4322. [Google Scholar] [CrossRef]
- Kabri, Y.; Azas, N.; Dumètre, A.; Hutter, S.; Laget, M.; Verhaeghe, P.; Gellis, A.; Vanelle, P. Original quinazoline derivatives displaying antiplasmodial properties. Eur. J. Med. Chem. 2010, 45, 616–622. [Google Scholar]
- Castera-Ducros, C.; Azas, N.; Verhaeghe, P.; Hutter, S.; Garrigue, P.; Dumètre, A.; Mbatchi, L.; Laget, M.; Remusat, V.; Sifredi, F.; et al. Targeting the human malaria parasite Plasmodium falciparum: In vitro identification of a new antiplasmodial hit in 4-phenoxy-2-trichloromethylquinazoline series. Eur. J. Med. Chem. 2011, 46, 4184–4191. [Google Scholar]
- Verhaeghe, P.; Dumètre, A.; Castera-Ducros, C.; Hutter, S.; Laget, M.; Fersing, C.; Prieri, M.; Yzombard, J.; Sifredi, F.; Rault, S.; et al. 4-Thiophenoxy-2-trichloromethyquinazolines display in vitro selective antiplasmodial activity against the human malaria parasite Plasmodium falciparum. Bioorg. Med. Chem. Lett. 2011, 21, 6003–6006. [Google Scholar]
- Augart, K.-D.; Kresze, G.; Schönberger, N. 1,6-Cyclisierungen von Phenylimino methyliminokumulenen: Bildung von Chinazolinderivaten. Justus Liebigs Ann. Chem. 1973, 1973, 1457–1466. [Google Scholar] [CrossRef]
- Ried, W.; Heine, B.; Merkel, W.; Kothe, N. Neuartige Synthese von 4-Tosylimino-3,4-dihydrochinazolin-Derivaten. Synthesis 1976, 1976, 534–535. [Google Scholar] [CrossRef]
- Nordvall, G.; Yngve, U. Novel Quinazolines as 5-HT6 Modulators II. WO2007108743, 2007. [Google Scholar]
- Yokoyama, K.; Ishikawa, N.; Igarashi, S.; Kawano, N.; Masuda, N.; Hamaguchi, W.; Yamasaki, S.; Koganemaru, Y.; Hattori, K.; Miyazaki, T.; et al. Potent and orally bioavailable CCR4 antagonists: Synthesis and structure-activity relationship study of 2-aminoquinazolines. Bioorgan. Med. Chem. 2009, 17, 64–73. [Google Scholar]
- Verhaeghe, P.; Rathelot, P.; Gellis, A.; Rault, S.; Vanelle, P. Highly efficient microwave assisted α-trichlorination reaction of α-methylated nitrogen containing heterocycles. Tetrahedron 2006, 62, 8173–8176. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Primas, N.; Verhaeghe, P.; Cohen, A.; Kieffer, C.; Dumètre, A.; Hutter, S.; Rault, S.; Rathelot, P.; Azas, N.; Vanelle, P. A New Synthetic Route to Original Sulfonamide Derivatives in 2-Trichloromethylquinazoline Series: A Structure-Activity Relationship Study of Antiplasmodial Activity. Molecules 2012, 17, 8105-8117. https://doi.org/10.3390/molecules17078105
Primas N, Verhaeghe P, Cohen A, Kieffer C, Dumètre A, Hutter S, Rault S, Rathelot P, Azas N, Vanelle P. A New Synthetic Route to Original Sulfonamide Derivatives in 2-Trichloromethylquinazoline Series: A Structure-Activity Relationship Study of Antiplasmodial Activity. Molecules. 2012; 17(7):8105-8117. https://doi.org/10.3390/molecules17078105
Chicago/Turabian StylePrimas, Nicolas, Pierre Verhaeghe, Anita Cohen, Charline Kieffer, Aurélien Dumètre, Sébastien Hutter, Sylvain Rault, Pascal Rathelot, Nadine Azas, and Patrice Vanelle. 2012. "A New Synthetic Route to Original Sulfonamide Derivatives in 2-Trichloromethylquinazoline Series: A Structure-Activity Relationship Study of Antiplasmodial Activity" Molecules 17, no. 7: 8105-8117. https://doi.org/10.3390/molecules17078105
APA StylePrimas, N., Verhaeghe, P., Cohen, A., Kieffer, C., Dumètre, A., Hutter, S., Rault, S., Rathelot, P., Azas, N., & Vanelle, P. (2012). A New Synthetic Route to Original Sulfonamide Derivatives in 2-Trichloromethylquinazoline Series: A Structure-Activity Relationship Study of Antiplasmodial Activity. Molecules, 17(7), 8105-8117. https://doi.org/10.3390/molecules17078105