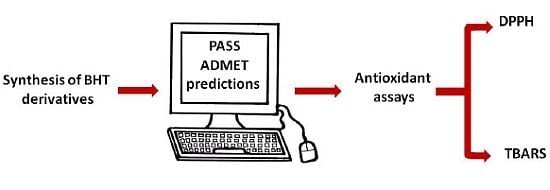
Butylated Hydroxytoluene Analogs: Synthesis and Evaluation of Their Multipotent Antioxidant Activities
Abstract
:1. Introduction


2. Results and Discussion
2.1. Chemistry
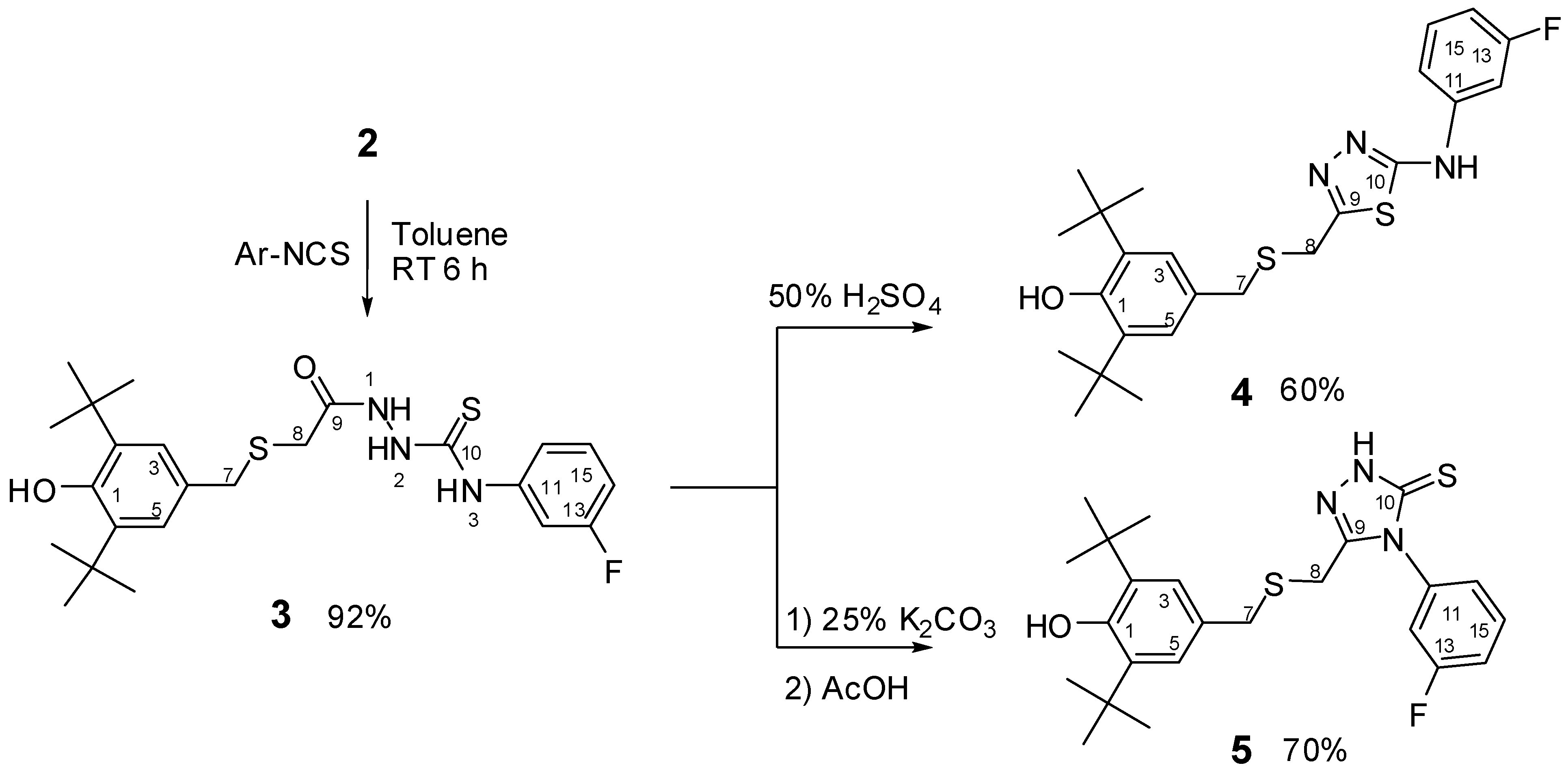
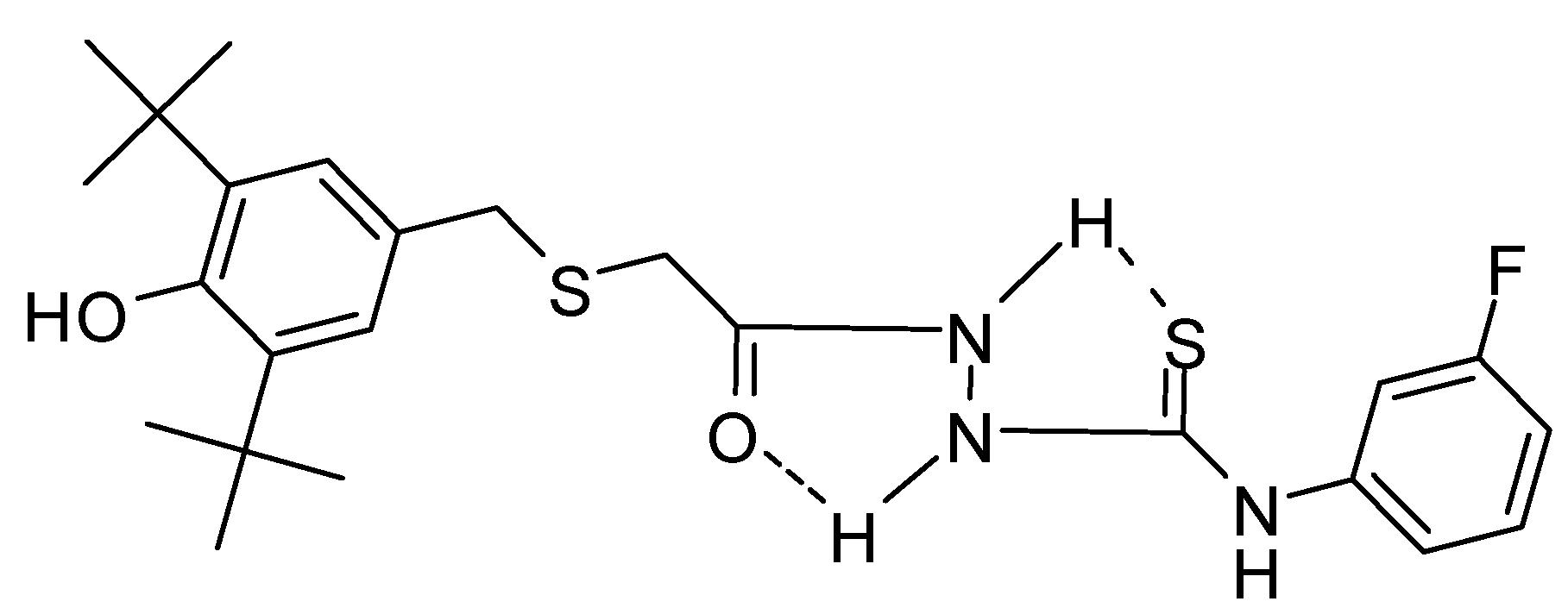
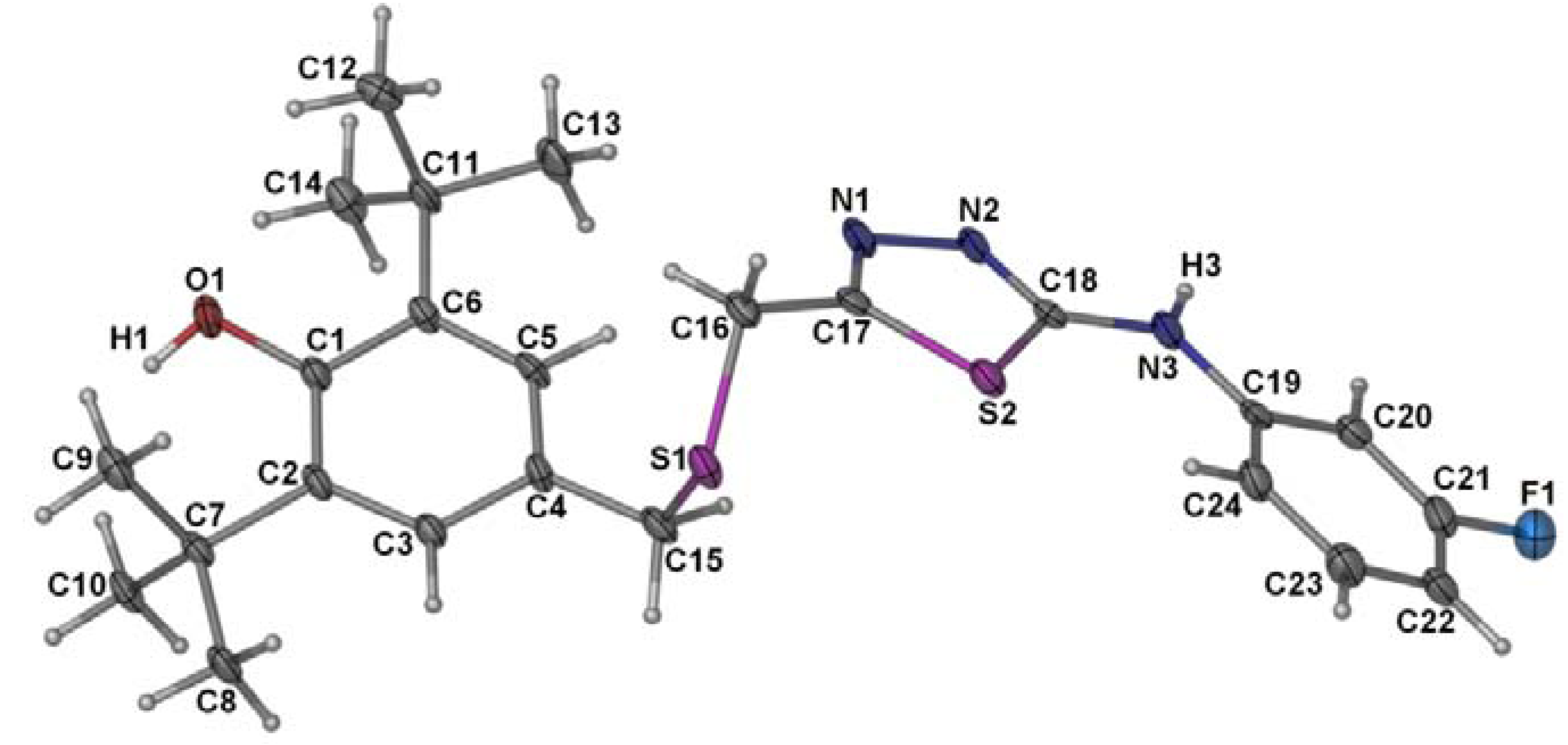
2.2. Single Crystal X-ray Crystallography of Compounds 3 and 4

| 3 | 4 | ||
|---|---|---|---|
| Bond lengths | |||
| S(2)-C(18) | 1.6714(15) | S(2)-C(17) | 1.728(6) |
| O(2)-C(17) | 1.2417(18) | S(2)-C(18) | 1.749(6) |
| N(1)-C(17) | 1.3201(18) | O(1)-C(1) | 1.376(6) |
| N(1)-N(2) | 1.3757(17) | N(1)-C(17) | 1.293(7) |
| N(2)-C(18) | 1.3490(18) | N(1)-N(2) | 1.395(6) |
| N(3)-C(18) | 1.3563(19) | N(2)-C(18) | 1.296(6) |
| N(3)-C(19) | 1.4108(18) | N(3)-C(18) | 1.365(7) |
| Bond angles | |||
| C(16)-S(1)-C(15) | 100.77(7) | C(16)-S(1)-C(15) | 100.4(3) |
| C(18)-N(2)-N(1) | 120.45(13) | C(18)-N(3)-C(19) | 130.8(5) |
| N(2)-C(18)-S(2) | 121.18(11) | C(4)-C(15)-S(1) | 113.1(4) |
| N(3)-C(18)-S(2) | 128.05(11) | C(17)-C(16)-S(1) | 112.1(4) |
| D-H···A | H···A [Å] | D···A [Å] | D-H···A [°] |
|---|---|---|---|
| 3 | |||
| N(3)-H(3N)...O(2) #1 | 2.040 (15) | 2.8570 (16) | 158.1 (17) |
| N(2)-H(2N)...O(2) #1 | 2.019 (16) | 2.7927 (17) | 150.4 (17) |
| 4 | |||
| N(3)-H(3N)...N(2) #2 | 2.05 (2) | 2.910 (7) | 169 (5) |
| 3 | 4 | |
|---|---|---|
| Empirical formula | C24 H32 F N3 O2 S2 | C24 H30 F N3 O S2 |
| Formula weight | 477.65 | 459.63 |
| Crystal system | Triclinic | Monoclinic |
| Space group | P-1 | C 2/c |
| Unit cell dimensions | ||
| a [Å] | 9.3441 (5) | 28.911 (11) |
| b [Å] | 11.1548 (6) | 5.731 (2) |
| c [Å] | 11.7683 (6) | 28.430 (11) |
| α [°] | 91.189 (2) | |
| β [°] | 93.388 (2) | 99.330 (5) |
| γ [°] | 93.224 (2) | |
| Volume [Å3] | 1,222.19 (11) | 4,648 (3) |
| Z | 2 | 8 |
| Independent reflections | 5,587 [Rint = 0.0428] | 4,202 [Rint = 0.2119] |
| Observed reflections [I > 2σ(I)] | 4,988 | 1,848 |
| Final R indices [I > 2σ(I)] | R1 = 0.0405, wR2 = 0.1135 | R1 = 0.0690, wR2 = 0.1340 |
| R indices (all data) | R1 = 0.0448, wR2 =0.1160 | R1 = 0.1792, wR2 = 0.1820 |
2.3. Computational Evaluation of Biological Activity
| Mode of biological activity | 1 | 3 | 4 | 5 | BHT | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | pa | Pi | |
| Lipid peroxidase inhibitor | 0.652 | 0.006 | 0.436 | 0.027 | 0.485 | 0.019 | 0.639 | 0.007 | 0.843 | 0.003 |
| Antioxidant | 0.712 | 0.004 | 0.385 | 0.035 | 0.420 | 0.028 | 0.529 | 0.015 | 0.845 | 0.003 |
| Free radical scavenger | 0.807 | 0.004 | 0.585 | 0.025 | 0.554 | 0.031 | 0.506 | 0.042 | 0.797 | 0.004 |
| Antiinflammatory | 0.659 | 0.017 | 0.375 | 0.136 | 0.649 | 0.018 | 0.479 | 0.065 | 0.804 | 0.005 |
2.4. Molecular Properties and Drug-Likeness
2.4.1. Calculation of Drug-Likeness Properties
2.4.2. Lipophilicity
| Compound | Violation of Rule of 5 (≤1) | HBA (≤10) | HBD (≤5) | Log P (≤5) | MW (≤500) | NROTB (≤10) | %ABS | PSA A2 ≤90 |
|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 4 | 2 | 4.41 | 310.452 | 6 | 88.66 | 58.93 |
| 3 | 1 | 4 | 4 | 6.43 | 477.658 | 10 | 82.59 | 76.54 |
| 4 | 1 | 5 | 2 | 6.94 | 459.643 | 8 | 89.63 | 56.14 |
| 5 (thione) | 1 | 4 | 2 | 6.84 | 459.643 | 7 | 92.33 | 48.30 |
| 5 (thiol) | 5 | 2 | 6.96 | 459.643 | 7 | 92.21 | 48.68 | |
| BHT | 0 | 1 | 1 | 4.87 | 220.350 | 2 | 101.81 | 20.81 |
| Vitamin E * | - | - | - | 10.44 | 430.71 | - | 98.73 | 29.74 |
| Vitamin C * | - | - | - | −1.70 | 176.12 | - | 71.23 | 109.49 |
2.4.3. Violations of Lipinski’s Rule of Five
2.5. In Vitro Antioxidant Activities
2.5.1. In Vitro DPPH Free Radical Scavenging Activity
| Compounds | IC50 a Values (µM/mL) ± S.E.M b and Max. inhibition % ± S.E.M | |
|---|---|---|
| DPPH Radical Scavenging | Lipid Peroxidation Inhibition | |
| 1 | 96.73 ± 1.87 (51.25 ± 0.82) | 38.84 ± 1.54 (73.99 ± 1.30) |
| 3 | 68.03 ± 1.27 (65.21 ± 0.55) | 56.00 ± 5.05 (74.64 ± 1.68) |
| 4 | > 100 c (26.09 ± 0.33) | 33.20 ± 2.91 (84.99 ± 1.37) |
| 5 | 85.30 ± 1.16 (65.26 ± 0.38) | 16.07 ± 3.51 (83.99 ± 1.65) |
| BHT | >100 c (25.23 ± 0.17) | 36.67 ± 1.78 (79.45 ± 1.27) |
| Ascorbic acid | 67.77 ± 0.17 (71.93 ± 1.61) | - |
| α-TOH | - | 5.63 ± 1.09 (84.69 ± 1.23) |


2.5.2. In Vitro Lipid Peroxidation Activity
3. Experimental
3.1. General



3.2. X-ray Crystallography
3.3. Antioxidant Assays
3.3.1. DPPH Free Radical Scavenging Assay

3.3.2. Lipid Peroxidation Inhibition Assay

4. Conclusions
Acknowledgments
References and Notes
- Schöneich, C. Reactive oxygen species and biological aging: A mechanistic approach. Exp. Gerontol. 1999, 34, 19–34. [Google Scholar] [CrossRef]
- Sahinoglu, T.; Stevens, C.R.; Bhatt, B.; Blake, D.R. The role of reactive oxygen species in inflammatory disease: Evaluation of methodology. Methods 1996, 9, 628–634. [Google Scholar] [CrossRef]
- Schöneich, C. Methionine oxidation by reactive oxygen species: Reaction mechanisms and relevance to alzheimer’s disease. Biochim. Biophys. Acta 2005, 1703, 111–119. [Google Scholar] [CrossRef]
- Oka, M.; Tachibana, M.; Noda, K.; Inoue, N.; Tanaka, M.; Kuwabara, K. Relevance of anti-reactive oxygen species activity to anti-inflammatory activity of components of eviprostat, a phytotherapeutic agent for benign prostatic hyperplasia. Phytomedicine 2007, 14, 465–472. [Google Scholar] [CrossRef]
- Kirkinezos, I.G.; Moraes, C.T. Reactive oxygen species and mitochondrial diseases. Semin. Cell Dev. Biol. 2001, 12, 449–457. [Google Scholar]
- de Maria, N.; Colantonl, A.; Fagiuoli, S.; Liu, G.-J.; Rogers, B.K.; Farinati, F.; van Thiel, D.H.; Floyd, R.A. Association between reactive oxygen species and disease activity in chronic hepatitis C. Free Radic. Biol. Med. 1996, 21, 291–295. [Google Scholar] [CrossRef]
- Smith, C.; Zhang, Y.; Koboldt, C.; Muhammad, J.; Zweifel, B.; Shaffer, A.; Talley, J.; Masferrer, J.; Seibert, K.; Isakson, P. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc. Natl. Acad. Sci. USA 1998, 95, 13313–13318. [Google Scholar]
- Shahidi, F.; Naczk, M.; Griffiths, W. Food Phenolics: Sources, Chemistry, Effects, Applications; Technomic Publishing: Lancaster, PA, USA, 1995. [Google Scholar]
- Bandyopadhyay, M.; Chakraborty, R.; Raychaudhuri, U. A process for preparing a natural antioxidant enriched dairy product (sandesh). LWT-Food Sci. Technol. 2007, 40, 842–851. [Google Scholar]
- Dacre, J. The metabolism of 3: 5-di-tert-butyl-4-hydroxytoluene and 3: 5-di-tert-butyl-4-hydroxybenzoic acid in the rabbit. Biochem. J. 1961, 78, 758–766. [Google Scholar]
- Stecher, P. Butylated hydroxytoluene. In The Merck Index, 8th; O’Neil, M.J., Ed.; Merck Research Laboratories: Rahway, NJ, USA, 1968; p. 179. [Google Scholar]
- Lazer, E.S.; Wong, H.C.; Possanza, G.J.; Graham, A.G.; Farina, P.R. Antiinflammatory 2,6-di-tert-butyl-4-(2-arylethenyl)phenols. J. Med. Chem. 1989, 32, 100–104. [Google Scholar] [CrossRef]
- Song, Y.; Connor, D.; Sercel, A.; Sorenson, R.; Doubleday, R.; Unangst, P.; Roth, B.; Beylin, V.; Gilbertsen, R.; Chan, K. Synthesis, structure-activity relationships, and in vivo evaluations of substituted di-tert-butylphenols as a novel class of potent, selective, and orally active cyclooxygenase-2 inhibitors. 2. 1,3,4- and 1,2,4-Thiadiazole series. J. Med. Chem. 1999, 42, 1161–1169. [Google Scholar]
- Marnett, L.J.; Kalgutkar, A.S. Design of selective inhibitors of cyclooxygenase-2 as nonulcerogenic anti-inflammatory agents. Curr. Opin. Chem. Biol. 1998, 2, 482–490. [Google Scholar] [CrossRef]
- Inagaki, M.; Tsuri, T.; Jyoyama, H.; Ono, T.; Yamada, K.; Kobayashi, M.; Hori, Y.; Arimura, A.; Yasui, K.; Ohno, K. Novel antiarthritic agents with 1,2-isothiazolidine-1,1-dioxide ([gamma]-sultam) skeleton: Cytokine suppressive dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase. J. Med. Chem. 2000, 43, 2040–2048. [Google Scholar] [CrossRef]
- Unangst, P.C.; Shrum, G.P.; Connor, D.T.; Dyer, R.D.; Schrier, D.J. Novel 1,2,4-oxadiazoles and 1,2,4-thiadiazoles as dual 5-lipoxygenase and cyclooxygenase inhibitors. J. Med. Chem. 1992, 35, 3691–3698. [Google Scholar] [CrossRef]
- Unangst, P.C.; Connor, D.T.; Cetenko, W.A.; Sorenson, R.J.; Kostlan, C.R.; Sircar, J.C.; Wright, C.D.; Schrier, D.J.; Dyer, R.D. Synthesis and biological evaluation of 5-[[3,5-bis(1,1-dimethylethyl)-4-hydroxyphenyl]methylene]oxazoles, -thiazoles, and -imidazoles: Novel dual 5-lipoxygenase and cyclooxygenase inhibitors with antiinflammatory activity. J. Med. Chem. 1994, 37, 322–328. [Google Scholar] [CrossRef]
- Mullican, M.D.; Wilson, M.W.; Conner, D.T.; Kostlan, C.R.; Schrier, D.J.; Dyer, R.D. Design of 5-(3,5-di-tert-butyl-4-hydroxyphenyl)-1,3,4-thiadiazoles, -1,3,4-oxadiazoles, and -1,2,4-triazoles as orally active, nonulcerogenicantiinflammatory agents. J. Med. Chem. 1993, 36, 1090–1099. [Google Scholar] [CrossRef]
- Leventis, I.; Andreadou, I.; Papalois, A.; Sfiniadakis, I.; Gorgoulis, V.; Korkolis, D.; Hadjipavlou-Litina, D.; Kourounakis, P.; Fotiadis, C. A novel antioxidant non-steroidal anti-inflammatory agent protects rat liver against ischemia-reperfusion injury. In Vivo 2004, 18, 161–169. [Google Scholar]
- Weber, V.; Rubat, C.; Duroux, E.; Lartigue, C.; Madesclaire, M.; Coudert, P. New 3- and 4-hydroxyfuranones as anti-oxidants and anti-inflammatory agents. Bioorg. Med. Chem. 2005, 13, 4552–4564. [Google Scholar] [CrossRef]
- Amorati, R.; Lucarini, M.; Mugnaini, V.; Pedulli, G. Antioxidant activity of o-bisphenols: The role of intramolecular hydrogen bonding. J. Org. Chem. 2003, 68, 5198–5204. [Google Scholar]
- Moore, G.; Swingle, K. 2,6-di-tert-butyl-4-(2-thenoyl)phenol(r-830): A novel nonsteroidal anti-inflammatory agent with antioxidant properties. Inflamm. Res. 1982, 12, 674–683. [Google Scholar]
- Grosso, P.; Vogl, O. Functional polymers. Polym. Bull. 1985, 14, 245–250. [Google Scholar]
- Zhang, H. Structure-activity relationships and rational design strategies for radical-scavenging antioxidants. Curr. Comput. Aided Drug Des. 2005, 1, 257–273. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Yang, D.-P.; Tang, G.-Y. Multipotent antioxidants: From screening to design. Drug Discov. Today 2006, 11, 749–754. [Google Scholar] [CrossRef]
- Kato, T.; Ozaki, T.; Tamura, K.; Suzuki, Y.; Akima, M.; Ohi, N. Novel calcium antagonists with both calcium overload inhibition and antioxidant activity. 1. 2-(3,5-di-tert-butyl-4-hydroxyphenyl)-3-(aminopropyl)thiazolidinones. J. Med. Chem. 1998, 41, 4309–4316. [Google Scholar]
- Dobek, A.; Klayman, D.; Dickson, E., Jr.; Scovill, J.; Oster, C. Thiosemicarbazones of 2-acetylpyridine, 2-acetylquinoline, 1-and 3-acetylisoquinoline and related compounds as inhibitors of clinically significant bacteria in vitro. Arzneimittelforschung 1983, 33, 1583–1591. [Google Scholar]
- Eid, A.I.; Ragab, F.A.; El-Ansary, S.L.; El-Gazayerly, S.M.; Mourad, F.E. Synthesis of new 7-substituted 4-methylcoumarin derivatives of antimicrobial activity. Arch. Pharm. 1994, 327, 211–213. [Google Scholar] [CrossRef]
- Liesen, A.P.; de Aquino, T.M.; Carvalho, C.S.; Lima, V.T.; de Araújo, J.M.; de Lima, J.G.; de Faria, A.R.; de Melo, E.J.T.; Alves, A.J.; Alves, E.W.; et al. Synthesis and evaluation of anti-toxoplasma gondii and antimicrobial activities of thiosemicarbazides, 4-thiazolidinones and 1,3,4-thiadiazoles. Eur. J. Med. Chem. 2010, 45, 3685–3691. [Google Scholar]
- Kus, C.; Ayhan-Kilcigil, G.; Eke, B.; iŞcan, M. Synthesis and antioxidant properties of some novel benzimidazole derivatives on lipid peroxidation in the rat liver. Arch. Pharm. Res. 2004, 27, 156–163. [Google Scholar] [CrossRef]
- Hussain, S.; Sharma, J.; Amir, M. Synthesis and antimicrobial activities of 1,2,4-triazole and 1,3,4-thiadiazole derivatives of 5-amino-2-hydroxybenzoic acid. E-J. Chem. 2008, 5, 963–968. [Google Scholar]
- Vasoya, S.L.; Paghdar, D.J.; Chovatia, P.T.; Joshi, H.S. Synthesis of some new thiosemicarbazide and 1,3,4-thiadiazole heterocycles bearing benzo[b]thiophene nucleus as a potent antitubercular and antimicrobial agents. J. Sci. Islam. Repub. Iran 2005, 16, 33–36. [Google Scholar]
- Rzeski, W.; Matysiak, J.; Kandefer-Szerszen, M. Anticancer, neuroprotective activities and computational studies of 2-amino-1,3,4-thiadiazole based compound. Bioorg. Med. Chem. 2007, 15, 3201–3207. [Google Scholar] [CrossRef]
- Abdel-Rahman, T.M. Synthesis, reactions, and anticancer activity of some 1,3,4-thiadiazole/thiadiazine derivatives of carbazole. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 1737–1754. [Google Scholar] [CrossRef]
- Khan, I.; Ali, S.; Hameed, S.; Rama, N.H.; Hussain, M.T.; Wadood, A.; Uddin, R.; Ul-Haq, Z.; Khan, A.; Ali, S.; et al. Synthesis, antioxidant activities and urease inhibition of some new 1,2,4-triazole and 1,3,4-thiadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 5200–5207. [Google Scholar]
- Palaska, E.; Sahin, G.; Kelicen, P.; Durlu, N.T.; Altinok, G. Synthesis and anti-inflammatory activity of 1-acylthiosemicarbazides, 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazole-3-thiones. Il Farmaco 2002, 57, 101–107. [Google Scholar] [CrossRef]
- Navidpour, L.; Shafaroodi, H.; Abdi, K.; Amini, M.; Ghahremani, M.H.; Dehpour, A.R.; Shafiee, A. Design, synthesis, and biological evaluation of substituted 3-alkylthio-4,5-diaryl-4h-1,2,4-triazoles as selective COX-2 inhibitors. Bioorg. Med. Chem. 2006, 14, 2507–2517. [Google Scholar]
- Wujec, M.; Pitucha, M.; Dobosz, M.; Kosikowska, U.; Malm, A. Synthesis and potential antimycotic activity of 4-substituted-3-(thiophene-2-yl-methyl)-delta2-1,2,4-triazoline-5-thiones. Acta Pharm. 2004, 54, 251–260. [Google Scholar]
- Kajiyama, T.; Ohkatsu, Y. Effect of meta-substituents of phenolic antioxidants—Proposal of secondary substituent effect. Polym. Degrad. Stab. 2002, 75, 535–542. [Google Scholar] [CrossRef]
- Hossain, M.; Shaha, S.; Aziz, F. Antioxidant potential study of some synthesized N-heterocycles. Bangladesh Med. Res. Counc. Bull. 2009, 35, 49–52. [Google Scholar]
- Ferreira, I.; Queiroz, M.; Vilas-Boas, M.; Estevinho, L. Evaluation of the antioxidant properties of diarylamines in the benzo [b] thiophene series by free radical scavenging activity and reducing power. Bioorg. Med. Chem. Lett. 2006, 16, 1384–1387. [Google Scholar]
- Accelrys Homepage. Available online: http://accelrys.com/ (accessed on 12 January 2012).
- Phillips, E.; Wasson, R.C. Phenols as corrosion inhibitors and antioxidants. EP330613A2, 30 August 1989. [Google Scholar]
- Macleay, R.E.; Myers, T.N. Multipurpose polymer bound stabilizers. EP303986A2, 7 July 1993. [Google Scholar]
- Akinchan, N.T.; West, D.X.; Yang, Y.; Salberg, M.M.; Klein, T.L. Magnetic and spectroscopic properties of copper(II) complexes with 1-salicoyl-4-phenylthiosemicarbazide. Transit. Met. Chem. 1995, 20, 481–484. [Google Scholar] [CrossRef]
- Angelusiu, M.V.; Almajan, G.L.; Rosu, T.; Negoiu, M.; Almajan, E.-R.; Roy, J. Copper(II) and uranyl(II) complexes with acylthiosemicarbazide: Synthesis, characterization, antibacterial activity and effects on the growth of promyelocytic leukemia cells HL-60. Eur. J. Med. Chem. 2009, 44, 3323–3329. [Google Scholar]
- Jampílek, J.; Doležal, M.; Kuneš, J.; Raich, I.; Liška, F. 4-substituted aryl bromides coupling with 4-methoxybenzene-1-thiol by means of copper catalysts. Chem. Papers 2005, 59, 178–181. [Google Scholar]
- PharmaExpert. Available online: http://www.pharmaexpert.ru/passonline/ (accessed on 4 March 2012).
- Parasuraman, S. Prediction of activity spectra for substances. J. Pharmacol. Pharmacother. 2011, 2, 52–53. [Google Scholar] [CrossRef]
- Stepanchikova, A.V.; Lagunin, A.A.; Filimonov, D.A.; Poroikov, V.V. Prediction of biological activity spectra for substances: Evaluation on the diverse sets of drug-like structures. Curr. Med. Chem. 2003, 10, 225–233. [Google Scholar]
- Anzali, S.; Barnickel, G.; Cezanne, B.; Krug, M.; Filimonov, D.; Poroikov, V. Discriminating between drugs and nondrugs by prediction of activity spectra for substances (pass). J. Med. Chem. 2001, 44, 2432–2437. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Tafazoli, S.; Wright, J.S.; O’Brien, P.J. Prooxidant and antioxidant activity of vitamin e analogues and troglitazone. Chem. Res. Toxicol. 2005, 18, 1567–1574. [Google Scholar] [CrossRef]
- Massaeli, H.; Sobrattee, S.; Pierce, G.N. The importance of lipid solubility in antioxidants and free radical generating systems for determining lipoprotein peroxidation. Free Radic. Biol. Med. 1999, 26, 1524–1530. [Google Scholar] [CrossRef]
- Bakht, M.A.; Yar, M.S.; Abdel-Hamid, S.G.; Al Qasoumi, S.I.; Samad, A. Molecular properties prediction, synthesis and antimicrobial activity of some newer oxadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 5862–5869. [Google Scholar] [CrossRef]
- Ingold, K.; Burton, G. Vitamin E: Or why we don’t go rancid. J. Chin. Chem. Soc. 1992, 39, 199–204. [Google Scholar]
- Mishra, H.; Singh, N.; Lahiri, T.; Misra, K. A comparative study on the molecular descriptors for predicting drug-likeness of small molecules. Bioinformation 2009, 3, 384–388. [Google Scholar]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Refsgaard, H.H.F.; Jensen, B.F.; Brockhoff, P.B.; Guldbrandt, M.; Christensen, M.S. In silico prediction of membrane permeability from calculated molecular parameters. J. Med. Chem. 2005, 48, 805–811. [Google Scholar] [CrossRef]
- Wang, R.; Fu, Y.; Lai, L. A new atom-additive method for calculating partition coefficients. J. Chem. Inf. Comput. Sci. 1997, 37, 615–621. [Google Scholar]
- Eklund, P.C.; Langvik, O.K.; Warna, J.P.; Salmi, T.O.; Willfor, S.M.; Sjoholm, R.E. Chemical studies on antioxidant mechanisms and free radical scavenging properties of lignans. Org. Biomol. Chem. 2005, 3, 3336–3347. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. Dpph antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Frankel, E. Lipid Oxidation, 2 ed; Oily Press Lipid Library Bridgewater: Bridgewater, UK, 2005; Volume 10, pp. 1–470. [Google Scholar]
- Nawar, W. Lipids, Chapter 5. In Food Chemistry, 3rd; Fennema, O.R., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1996; pp. 254–299. [Google Scholar]
- Kus, C.; Ayhan-KIlcIgil, G.; Özbey, S.; Kaynak, F.; Kaya, M.; Çoban, T.; Can-Eke, B. Synthesis and antioxidant properties of novel N-methyl-1,3,4-thiadiazol-2-amine and 4-methyl-2h-1,2,4-triazole-3(4h)-thione derivatives of benzimidazole class. Bioorg. Med. Chem. 2008, 16, 4294–4303. [Google Scholar]
- Lucarini, M.; Pedrielli, P.; Pedulli, G.F.; Valgimigli, L.; Gigmes, D.; Tordo, P. Bond dissociation energies of the N-H bond and rate constants for the reaction with alkyl, alkoxyl, and peroxyl radicals of phenothiazines and related compounds. J. Am. Chem. Soc. 1999, 121, 11546–11553. [Google Scholar]
- Scott, G. Antioxidants. Bull. Chem. Soc. Jpn. 1988, 61, 165–170. [Google Scholar] [CrossRef]
- Escobar-Valderrama, J.; Garcia-Tapia, J.; Ramirez-Ortiz, J.; Rosales, M.; Toscano, R.; Valdes-Martinez, J. Crystal, molecular and electronic structure of 1-h-3-methyl-4-amine-5-thione-1,2,4-triazol. Can. J. Chem. 1989, 67, 198–201. [Google Scholar] [CrossRef]
- Matsuura, T.; Ohkatsu, Y. Phenolic antioxidants: Effect of O-benzyl substituents. Polym. Degrad. Stab. 2000, 70, 59–63. [Google Scholar] [CrossRef]
- Amorati, R.; Ferroni, F.; Pedulli, G.F.; Valgimigli, L. Modeling the co-antioxidant behavior of monofunctional phenols. Applications to some relevant compounds. J. Org. Chem. 2003, 68, 9654–9658. [Google Scholar]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E. Development and validation of oxygen radical absorbance capacity assay for lipophilic antioxidants using randomly methylated-cyclodextrin as the solubility enhancer. J. Agric. Food Chem. 2002, 50, 1815–1821. [Google Scholar] [CrossRef]
- McClements, D.; Decker, E. Lipid oxidation in oil in water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Alamed, J. Impact of Chemical and Physical Properties on the Ability of Antioxidants to Inhibit Lipid Oxidation in Foods; University of Massachusetts Amherst: Amherst, MA, USA, 2008. [Google Scholar]
- Decker, E.A. Strategies for manipulating the prooxidative/antioxidative balance of foods to maximize oxidative stability. Trends Food Sci. Technol. 1998, 9, 241–248. [Google Scholar] [CrossRef]
- Khan, I.; Ali, S.; Hameed, S.; Rama, N.; Hussain, M.; Wadood, A.; Uddin, R.; Ul-Haq, Z.; Khan, A. Synthesis, antioxidant activities and urease inhibition of some new 1,2,4-triazole and 1,3,4-thiadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 5200–5207. [Google Scholar] [CrossRef]
- Hilton, J. Antioxidants: Function, types and necessity of inclusion in pet foods. Can. Vet. J. 1989, 30, 682–684. [Google Scholar]
- Bruker. APEX2 and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2007. [Google Scholar]
- Sheldrick, G. A short history of shelx. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Barbour, L.J. X-seed—A software tool for supramolecular crystallography. J. Supramol. Chem. 2001, 1, 189–191. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determination by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Daker, M.; Abdullah, N.; Vikineswary, S.; Goh, P.C.; Kuppusamy, U.R. Antioxidant from maize and maize fermented by Marasmiellus sp. As stabiliser of lipid-rich foods. Food Chem. 2008, 107, 1092–1098. [Google Scholar]
- Sample Availability: Samples of the compounds 1–3 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yehye, W.A.; Abdul Rahman, N.; A. Alhadi, A.; Khaledi, H.; Ng, S.W.; Ariffin, A. Butylated Hydroxytoluene Analogs: Synthesis and Evaluation of Their Multipotent Antioxidant Activities. Molecules 2012, 17, 7645-7665. https://doi.org/10.3390/molecules17077645
Yehye WA, Abdul Rahman N, A. Alhadi A, Khaledi H, Ng SW, Ariffin A. Butylated Hydroxytoluene Analogs: Synthesis and Evaluation of Their Multipotent Antioxidant Activities. Molecules. 2012; 17(7):7645-7665. https://doi.org/10.3390/molecules17077645
Chicago/Turabian StyleYehye, Wageeh A., Noorsaadah Abdul Rahman, Abeer A. Alhadi, Hamid Khaledi, Seik Weng Ng, and Azhar Ariffin. 2012. "Butylated Hydroxytoluene Analogs: Synthesis and Evaluation of Their Multipotent Antioxidant Activities" Molecules 17, no. 7: 7645-7665. https://doi.org/10.3390/molecules17077645
APA StyleYehye, W. A., Abdul Rahman, N., A. Alhadi, A., Khaledi, H., Ng, S. W., & Ariffin, A. (2012). Butylated Hydroxytoluene Analogs: Synthesis and Evaluation of Their Multipotent Antioxidant Activities. Molecules, 17(7), 7645-7665. https://doi.org/10.3390/molecules17077645