Application of 2,3-Naphthalenediamine in Labeling Natural Carbohydrates for Capillary Electrophoresis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Compositional Analysis of Ganoderma lucidum Polysaccharide
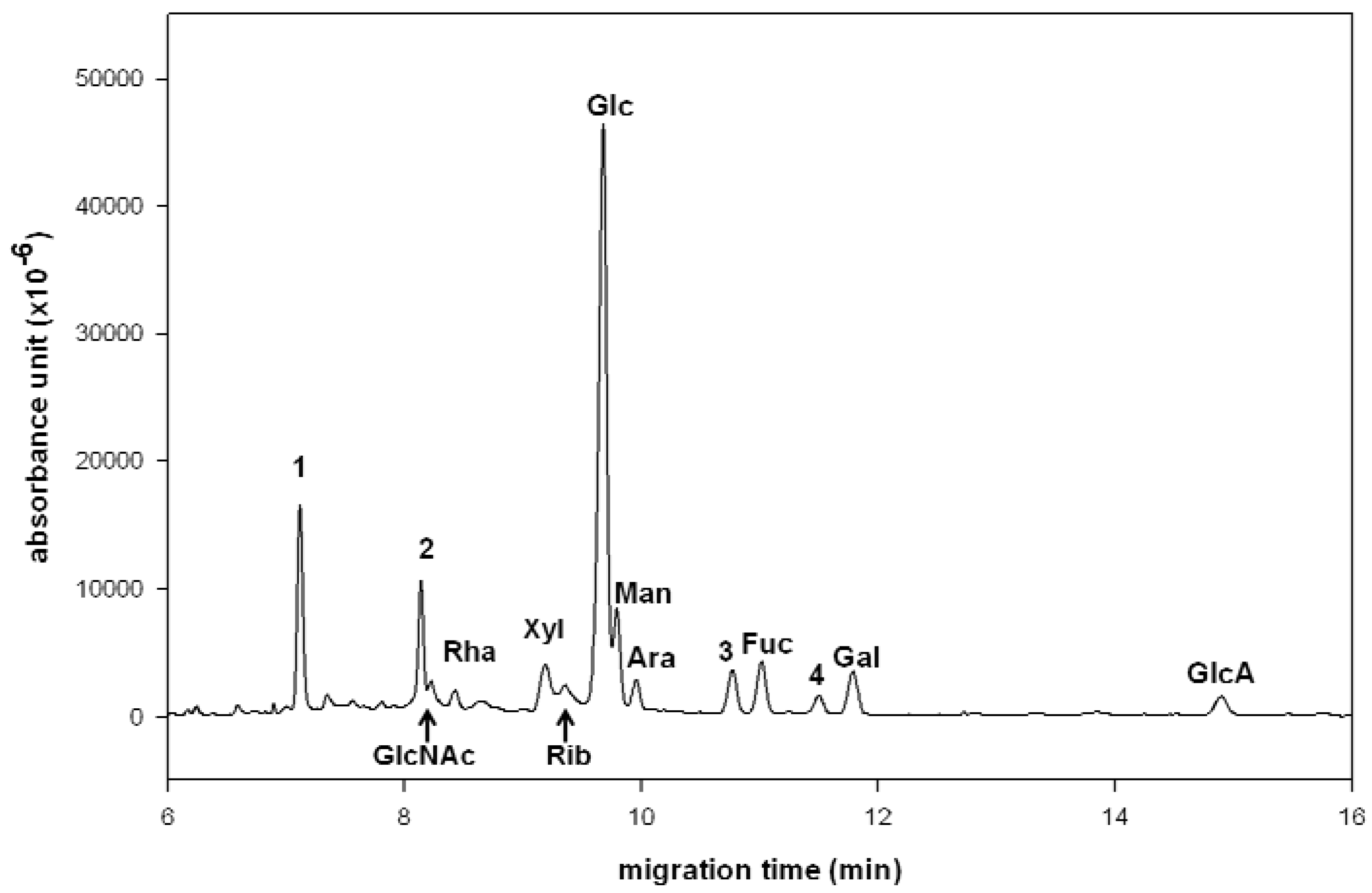
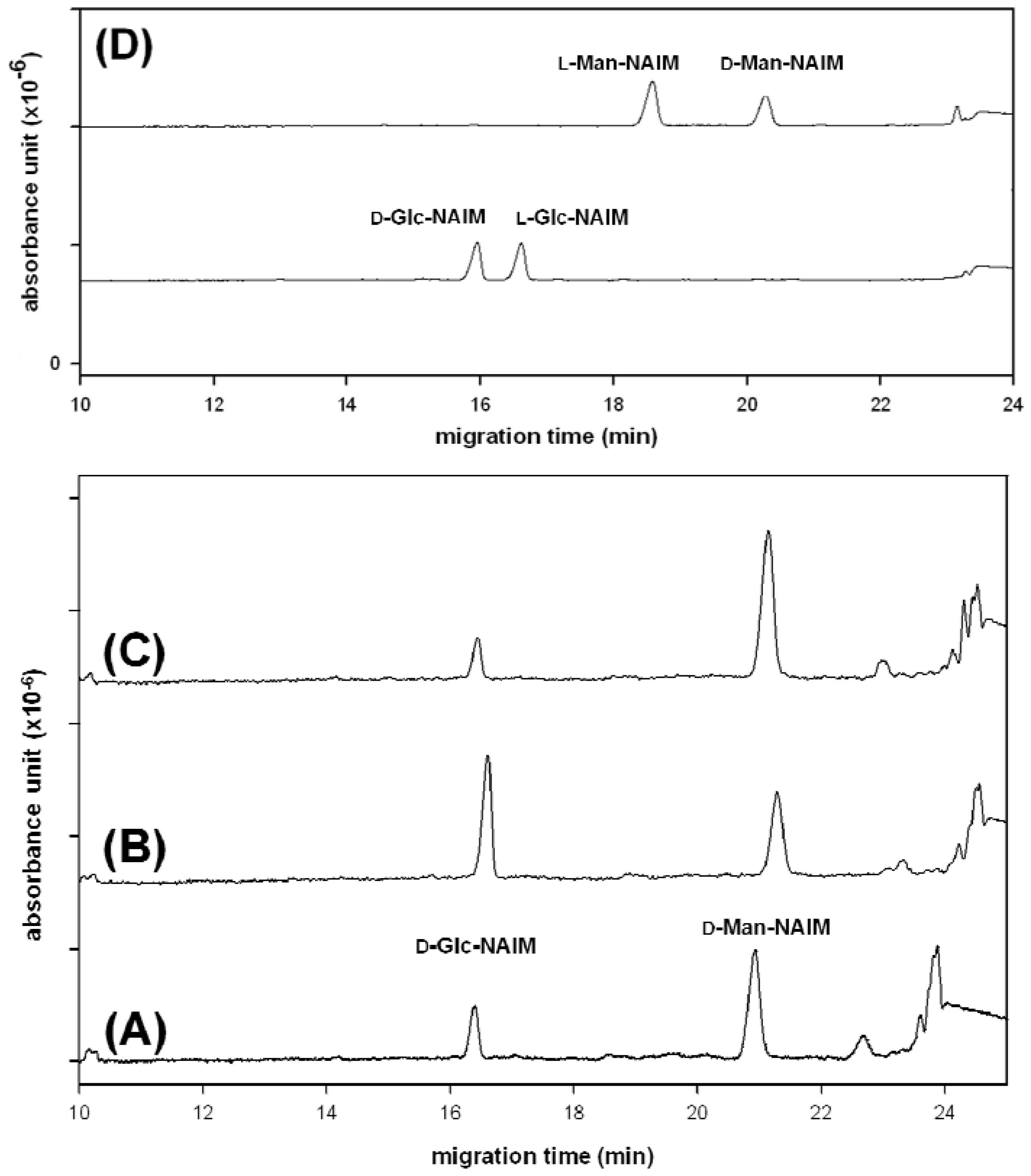
2.2. Compositional Analysis of Dendrobium huoshanense Polysaccharide
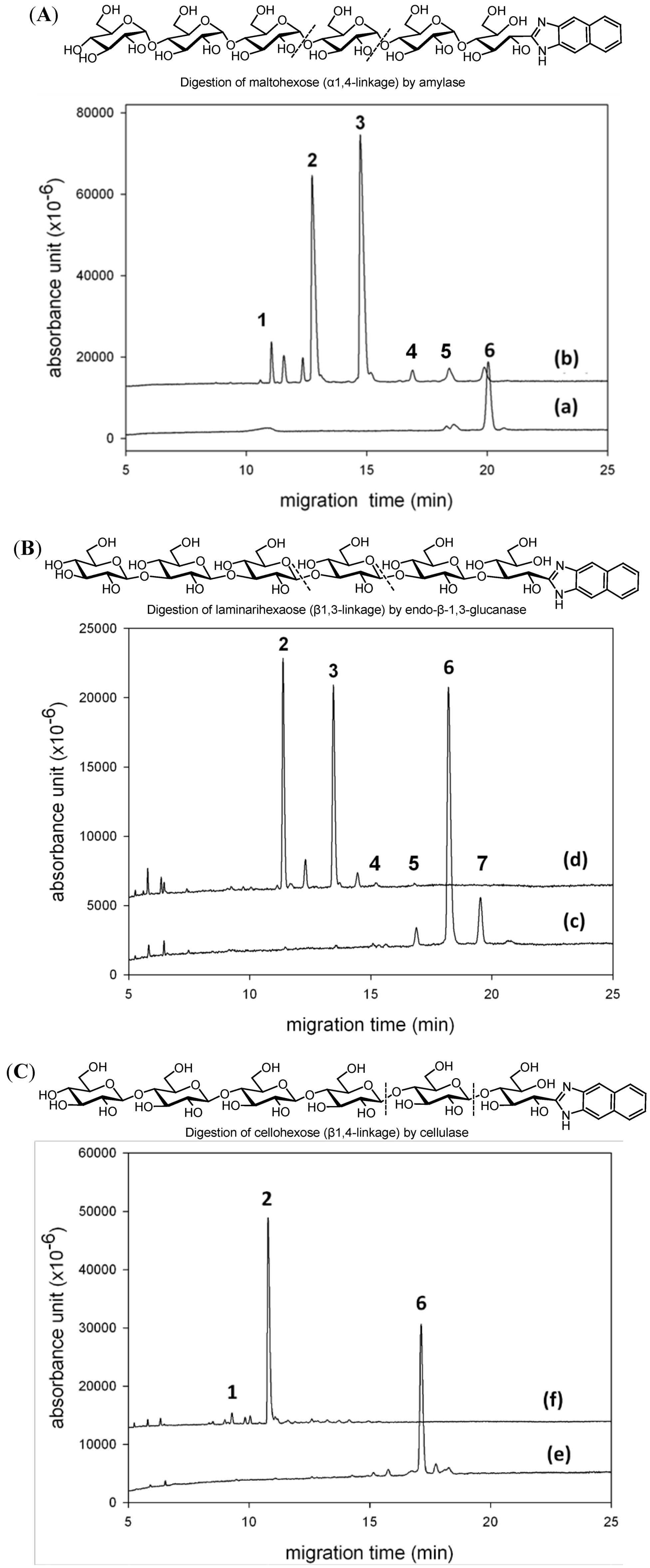
2.3. CE Analysis for Enzymatic Digestion of Oligosaccharides
2.4. CE Analysis of Heparin Disaccharides with NAIM Labeling
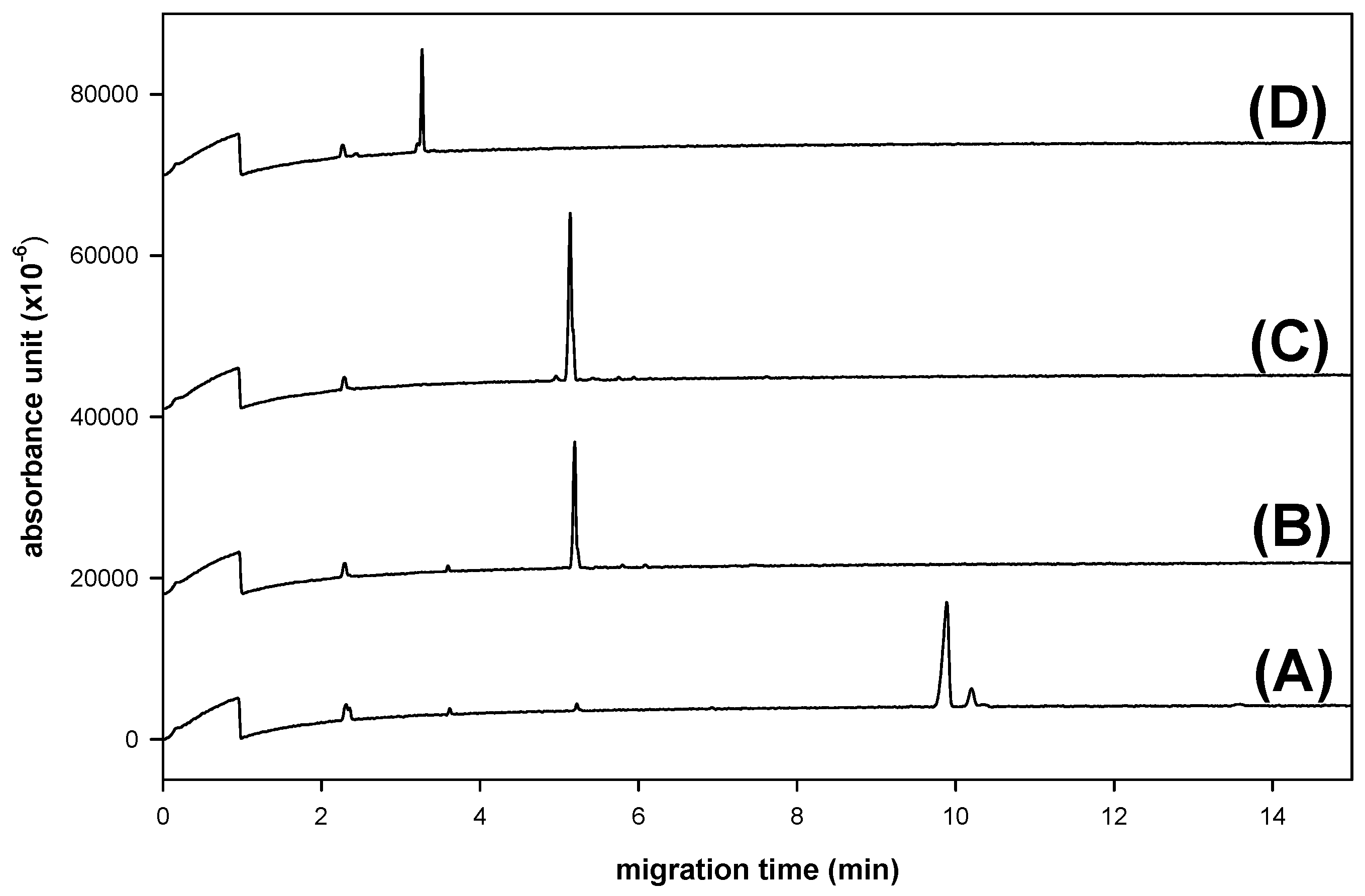
3. Experimental
3.1. Chemicals and Materials
3.2. Synthesis of Saccharide–Naphthimidazole Derivatives
3.3. Instrument and Experimental Conditions for Capillary Electrophoresis
3.4. Hydrolysis of Polysaccharides from Medicinal Herbs
3.5. Validation of Experimental Data
4. Conclusions
Acknowledgements
- Sample Availability: Samples of aldo–NAIMs are available from the authors.
References
- EI Rassi, Z. Recent developments in capillary electrophoresis and capillary electrochromatography of carbohydrate species. Electrophoresis 1999, 20, 3134–3144. [Google Scholar] [CrossRef]
- Paulus, A.; Klockow, A. Detection of carbohydrates in capillary electrophoresis. J. Chromatogr. A 1996, 720, 353–376. [Google Scholar] [CrossRef]
- Ijiri, S.; Todoroki, K.; Yoshida, H.; Yoshitake, T.; Nohta, H.; Yamaguchi, M. Sensitive determination of rhodamine 110-labeled monosaccharides in glycoprotein by capillary electrophoresis with laser-induced fluorescence detection. J. Chromatogr. A 2010, 1217, 3161–3166. [Google Scholar] [CrossRef]
- Harvey, D.J. Derivatization of carbohydrates for analysis by chromatography; electrophoresis and mass spectrometry. J. Chromatogr. B 2011, 879, 1196–1225. [Google Scholar] [CrossRef]
- Taga, A.; Suzuki, S.; Honda, S. Capillary electrophoretic analysis of carbohydrates derivatized by in-capillary condensation with 1-phenyl-3-methyl-5-pyrazolone. J. Chromatogr. A 2001, 911, 259–267. [Google Scholar] [CrossRef]
- Evangelista, R.A.; Guttman, A.; Chen, F.A. Acid-catalyzed reductive amination of aldoses with 8-aminopyrene-1,3,6-trisulfonate. Electrophoresis 1996, 17, 347–351. [Google Scholar] [CrossRef]
- Lin, C.; Hung, W.T.; Kuo, C.Y.; Liao, K.S.; Liu, Y.C.; Yang, W.B. I2-catalyzed oxidative condensation of aldoses with diamines: Synthesis of aldo-naphthimidazoles for carbohydrate analysis. Molecules 2010, 15, 1340–1353. [Google Scholar] [CrossRef]
- Lin, C.; Kuo, C.Y.; Liao, K.S.; Yang, W.B. Monosaccharide-NAIM derivatives for D-, L-configuration analysis. Molecules 2011, 16, 652–664. [Google Scholar] [CrossRef]
- Lin, C.; Hung, W.T.; Chen, C.H.; Fang, J.M.; Yang, W.B. A new naphthimidazole derivative for saccharide labeling with enhanced sensitivity in mass spectrometry detection. Rapid Commun. Mass Spectrom. 2010, 24, 85–94. [Google Scholar]
- Chang, Y.L.; Liao, K.S.; Chen, Y.C.; Hung, W.T.; Yu, H.M.; Yang, W.B.; Fang, J.M.; Chen, C.H.; Lee, Y.C. Tagging saccharides for signal enhancement in mass spectrometric analysis. J. Mass. Spectrom. 2011, 46, 247–255. [Google Scholar] [CrossRef]
- Lin, C.; Lai, P.T.; Liao, S.K.S.; Hung, W.T.; Yang, W.B.; Fang, J.M. Using molecular iodine in direct oxidative condensation of aldoses with diamines: An improved synthesis of aldo-benzimidazoles and aldo-naphthimidazoles for carbohydrate analysis. J. Org. Chem. 2008, 73, 3848–3853. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Xiao, Y.; Ng, C.H.; Oh, T.S.; Tan, T.T.Y.; Ng, S.C. Preparation of cyclodextrin chiral stationary phases by organic soluble catalytic ‘click’ chemistry. Nat. Protoc. 2011, 6, 935–942. [Google Scholar] [CrossRef]
- Lin, B.; Shi, Z.G.; Zhang, H.J.; Ng, S.C.; Feng, Y.Q. Perphenylcarbamoylated β-cyclodextrin bonded-silica particles as chiral stationary phase for enantioseparation by pressure-assisted capillary electrochromatography. Electrophoresis 2006, 27, 3057–3065. [Google Scholar] [CrossRef]
- Scriba, G.K.E. Selected fundamental aspects of chiral electromigration techniques and their application to pharmaceutical and biomedical analysis. J. Pharm. Biomed. Anal. 2002, 27, 373–399. [Google Scholar] [CrossRef]
- Cheng, K.C.; Huang, H.C.; Chen, J.H.; Hsu, J.W.; Cheng, H.C.; Ou, C.H.; Yang, W.B.; Wong, C.H.; Juan, H.F. Ganoderma lucidum polysaccharides in human monocytic leukemia cells: From gene expression to network construction. BMC Genomics 2007, 8, 411. [Google Scholar] [CrossRef]
- Hua, K.F.; Hsu, H.Y.; Chao, L.K.; Chen, S.T.; Yang, W.B.; Hsu, J.; Wong, C.H. Ganoderma lucidum polysaccharides enhance CD14 endocytosis of LPS and promote TLR4 signal transduction of cytokine expression. J. Cell. Physiol. 2007, 212, 537–550. [Google Scholar] [CrossRef]
- Lin, K.I.; Kao, Y.Y.; Kuo, H.K.; Yang, W.B.; Chou, A.; Lin, H.H.; Yu, A.L.; Wong, C.H. Reishi polysaccharides induce immunoglobulin production through the TLR4/TLR2-mediated induction of transcription factor Blimp-1. J. Biol. Chem. 2006, 281, 24111–24123. [Google Scholar]
- Whiton, R.S.; Lau, P.; Morgan, S.L.; Gilbart, J.; Fox, A. Modifications in the alditol acetate method for analysis of muramic acid and other neutral and amino sugars by capillary gas chromatography–mass spectrometry with selected ion monitoring. J. Chromatogr. A 1985, 347, 109–120. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Khoo, K.H.; Chen, S.T.; Lin, C.C.; Wong, C.H.; Lin, C.H. Studies on the immuno-modulating and antitumor activities of Ganoderma lucidum (Reishi) polysaccharides: Functional and proteomic analyses of a fucose-containing glycoprotein fraction responsible for the activities. Bioorg. Med. Chem. 2002, 10, 1057–1062. [Google Scholar] [CrossRef]
- Soundararajan, S.; Badawi, M.; Kohlrust, C.M.; Hageman, J.H. Boronic acids for affinity chromatography: Spectral methods for determinations of ionization and diol-binding constants. Anal. Biochem. 1989, 178, 125–134. [Google Scholar]
- Kodama, S.; Aizawa, S.; Taga, A.; Yamashita, T.; Yamamoto, A. Chiral resolution of monosaccharides as 1-phenyl-3-methyl-5-pyrazolone derivatives by ligand-exchange CE using borate anion as a central ion of the chiral selector. Electrophoresis 2006, 27, 4730–4734. [Google Scholar] [CrossRef]
- De Jesus, L.I.; Albuquerque, N.C.P.; Borges, K.B.; Simões, R.A.; Calixto, L.A.; Furtado, N.A.J.C.; De Gaitani, C.M.; Pupo, M.T.; De Oliveira, A.R.M. Enantioselective fungal biotransformation of risperidone in liquid culture medium by capillary electrophoresis and hollow fiber liquid-phase microextraction. Electrophoresis 2011, 32, 2765–2775. [Google Scholar]
- Hsieh, S.Y.; Chien, C.; Liao, K.S.; Liao, S.F.; Hung, W.T.; Yang, W.B.; Lin, C.C.; Cheng, T.J.; Chang, C.C.; Fang, J.M.; et al. Structure and bioactivity of the polysaccharides in medicinal plant Dendrobium huoshanense. Bioorg. Med. Chem. 2008, 16, 6054–6068. [Google Scholar]
- Gübitz, G.; Schmid, M.G. Chiral separation by capillary electromigration techniques. J. Chromatogr. A 2008, 1204, 140–156. [Google Scholar] [CrossRef]
- Koval, D.; Severa, L.; Adriaenssens, L.; Lávra, J.; Teplý, F.; Kašička, V. Chiral analysis of helquats by capillary electrophoresis: Resolution of helical N-heteroaromatic dications using randomly sulfated cyclodextrins. Electrophoresis 2011, 32, 2683–2692. [Google Scholar] [CrossRef]
- Chuang, Y.J.; Swanson, R.; Raja, S.M.; Olson, S.T. Heparin enhances the specificity of antithrombin for thrombin and factor Xa independent of the reactive center loop sequence. J. Biol. Chem. 2001, 276, 14961–14971. [Google Scholar]
- Malavaki, C.L.; Theocharis, A.D.; Lamari, F.N.; Kanakis, I.; Tsegenidis, T.; Tzanakakis, G.N.; Karamanos, N.K. Heparan sulfate: Biological significance, tools for biochemical analysis and structural characterization. Biomed. Chromatogr. 2011, 25, 11–20. [Google Scholar] [CrossRef]
- Jones, C.J.; Beni, S.; Limtiaco, J.F.K.; Langeslay, D.J.; Larive, C.K. Heparin characterization: Challenges and solutions. Ann. Rev. Anal. Chem. 2011, 4, 439–465. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Vanky, P.; Tzanakakis, G.N.; Hjerpe, A. High performance capillary electrophoresis method to characterize heparin and heparan sulfate disaccharides. Electrophoresis 1996, 17, 391–395. [Google Scholar] [CrossRef]
- Korir, A.K.; Larive, C.K. Advances in the separation, sensitive detection, and characterization of heparin and heparin sulfate. Anal. Bioanal. Chem. 2009, 393, 155–169. [Google Scholar] [CrossRef]
- Sudor, J.; Novotny, M.V. End-label free-solution electrophoresis of the low molecular weight heparins. Anal. Chem. 1997, 69, 3199–3204. [Google Scholar] [CrossRef]
- Bendrazzoli, C.; Liverani, L.; Spelta, F.; Prandi, M.; Fiori, J.; Gotti, R. Determination of dermatan sulfate and chondroitin sulfate as related substances in heparin by capillary electrophoresis. J. Pharm. Biomed. Anal. 2010, 53, 1193–1200. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kuo, C.-Y.; Wang, S.-H.; Lin, C.; Liao, S.K.-S.; Hung, W.-T.; Fang, J.-M.; Yang, W.-B. Application of 2,3-Naphthalenediamine in Labeling Natural Carbohydrates for Capillary Electrophoresis. Molecules 2012, 17, 7387-7400. https://doi.org/10.3390/molecules17067387
Kuo C-Y, Wang S-H, Lin C, Liao SK-S, Hung W-T, Fang J-M, Yang W-B. Application of 2,3-Naphthalenediamine in Labeling Natural Carbohydrates for Capillary Electrophoresis. Molecules. 2012; 17(6):7387-7400. https://doi.org/10.3390/molecules17067387
Chicago/Turabian StyleKuo, Chien-Yuan, Shwu-Huey Wang, Chunchi Lin, Sylvain Kuo-Shiang Liao, Wei-Ting Hung, Jim-Min Fang, and Wen-Bin Yang. 2012. "Application of 2,3-Naphthalenediamine in Labeling Natural Carbohydrates for Capillary Electrophoresis" Molecules 17, no. 6: 7387-7400. https://doi.org/10.3390/molecules17067387



