Evaluation of Antioxidant Activities of Aqueous Extracts and Fractionation of Different Parts of Elsholtzia ciliata
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Yield and Total Phenolics Content of Different Parts of E. ciliata Plant

| Yield | Leaf (g) | Inflorescence (g) | Stem (g) | Root (g) |
|---|---|---|---|---|
| A a | 30.64 ± 1.04 | 15.26 ± 0.34 | 10.56 ± 0.43 | 5.60 ± 0.16 |
| B b | 2.38 ± 0.09 | 2.49 ± 0.09 | 2.49 ± 0.09 | 1.41 ± 0.05 |
| C b | 2.39 ± 0.07 | 2.28 ± 0.09 | 2.29 ± 0.06 | 3.49 ± 0.09 |
| D c | 0.13 ± 0.01 | 0.09 ± 0.01 | 0.12 ± 0.01 | 0.29 ± 0.01 |
| E c | 0.54 ± 0.02 | 0.48 ± 0.01 | 0.65 ± 0.02 | 0.20 ± 0.01 |
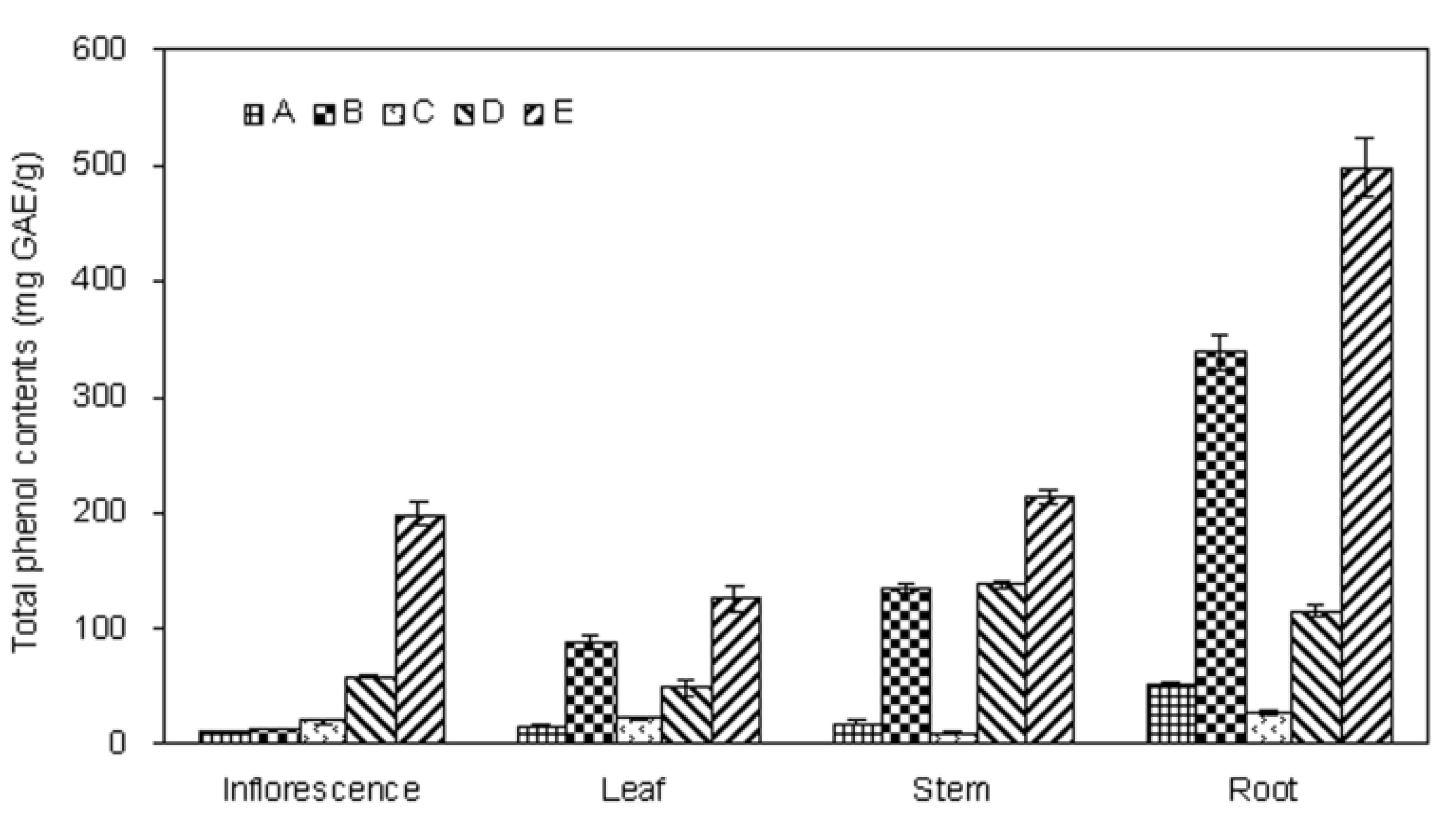
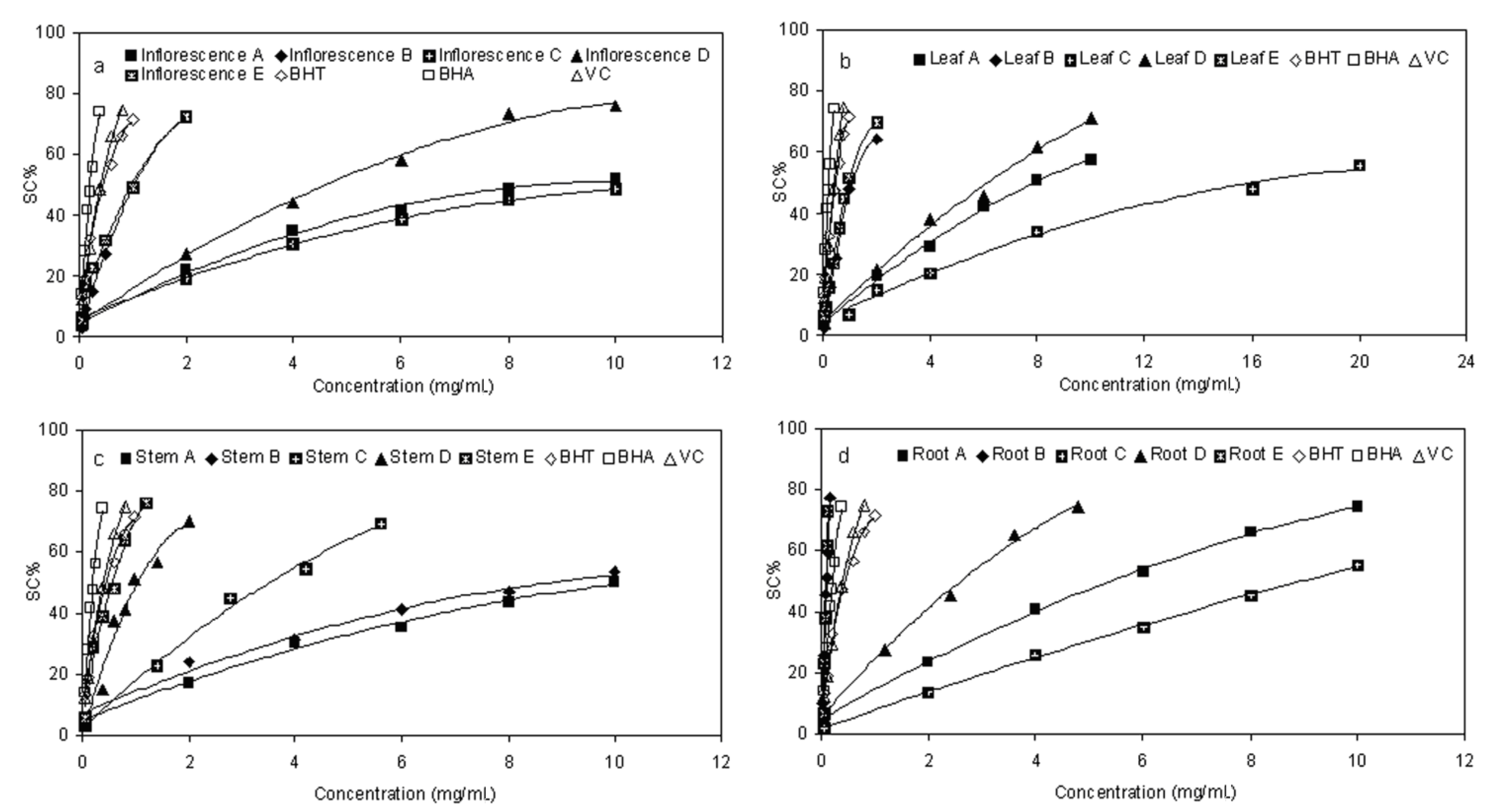
2.2. Scavenging Effect on DPPH Radical
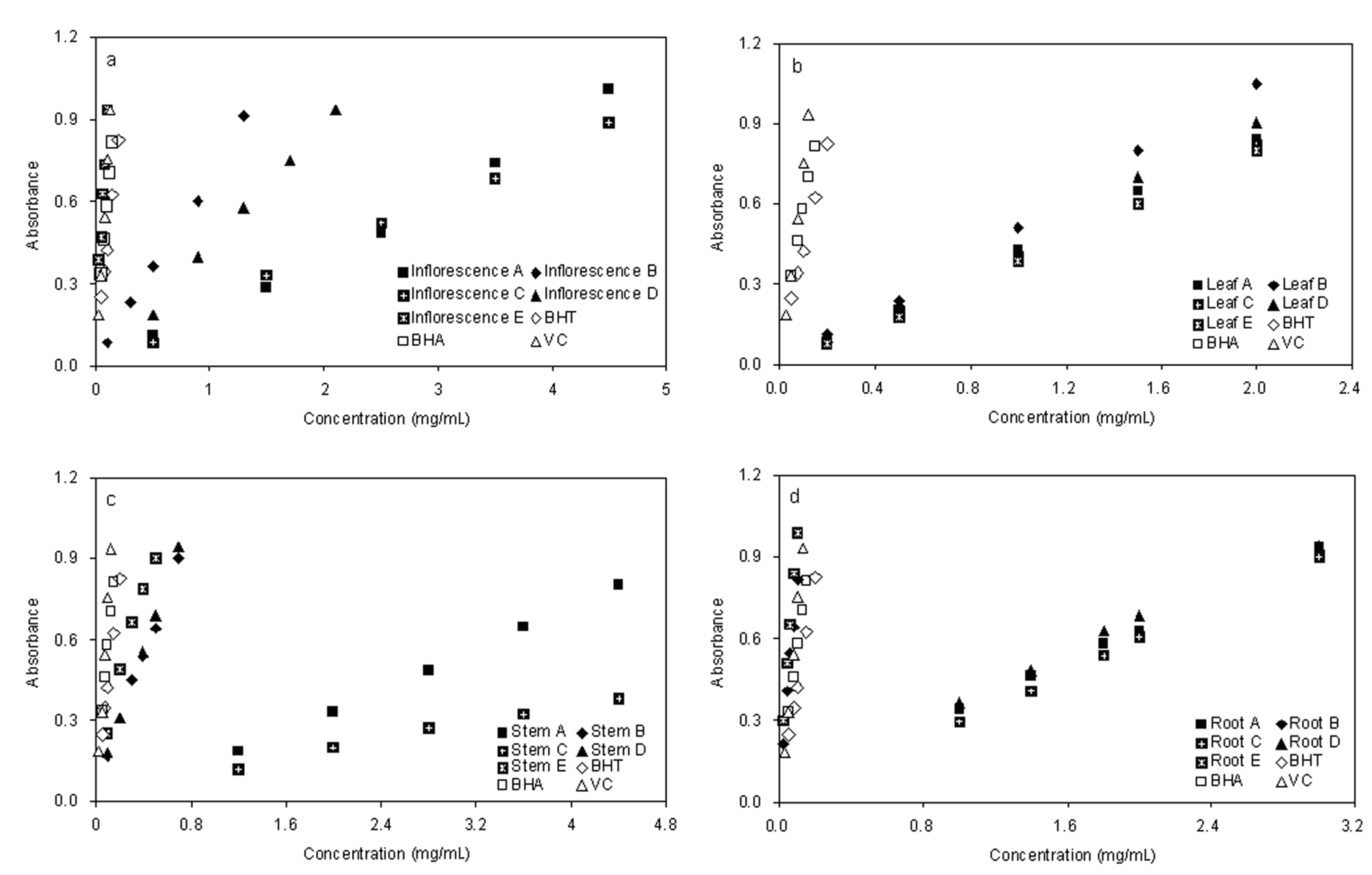
2.3. Reducing Power
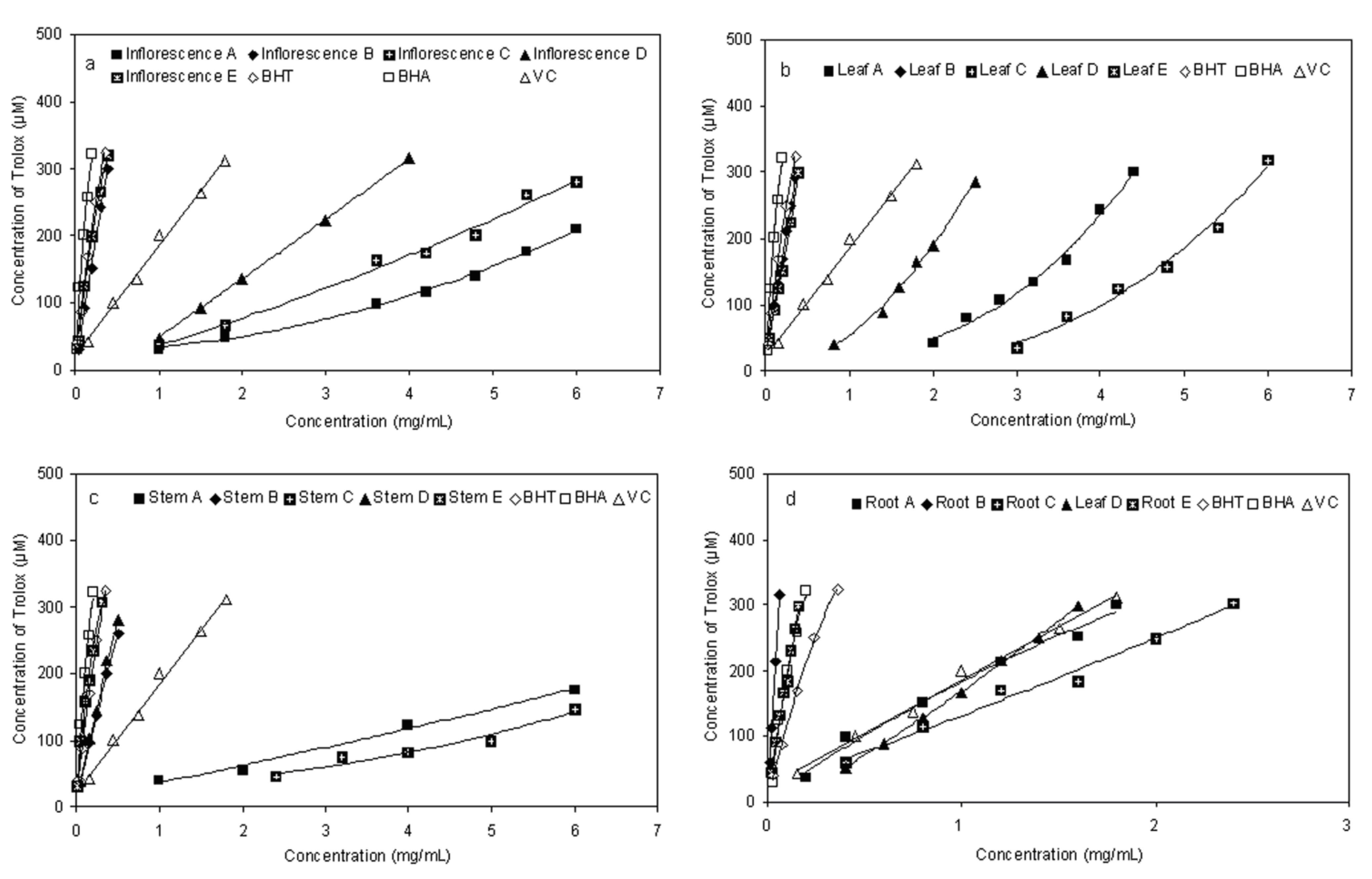
2.4. Ferric-reducing Antioxidant Power (FRAP)
3. Experimental Section
3.1. Plant Materials
3.2. Chemicals
3.3. Preparation of the Crude Extract and Fractionation
3.4. Determination of Total Phenolics
3.5. DPPH free Radical-Scavenging Assay

3.6. Measurement of Reducing Power
3.7. Total Antioxidant Capacity by FRAP Assay
3.8. Statistical Analysis
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–396. [Google Scholar]
- Bursal, E.; Gülçin, İ. Polyphenol contents and in vitro antioxidant activities of lyophilized aqueous extract of kiwifruit (Actinidia deliciosa). Food Res. Int. 2011, 44, 1482–1489. [Google Scholar]
- Cornwell, D.G.; Jones, K.H.; Jiang, Z.; Lantry, L.E.; Southwell-Keely, P.; Kohar, I.; Thornton, D.E. Cytotoxicity of tocopherols and their quinones in drug-sensitive and multidrug-resistant leukemia cells. Lipids 1998, 33, 295–301. [Google Scholar] [CrossRef]
- Namiki, M. Antioxidants / antimutagens in food. Crit. Rev. Food Sci. 1990, 29, 273–300. [Google Scholar] [CrossRef]
- Namiki, M.; Osawa, T. Antioxidants / antimutagens in foods. Basic Life Sci. 1986, 39, 131–142. [Google Scholar]
- Stich, H.F. The beneficial and hazardous effects of simple phenolic compounds. Mutat. Res-Gen. Tox. 1991, 259, 307–324. [Google Scholar]
- Kouri, G.; Tsimogiannis, D.; Bardouki, H.; Oreopoulou, V. Extraction and analysis of antioxidant components from Origanum dictamnus. Innov. Food Sci. Emerg. Tech. 2007, 8, 155–162. [Google Scholar] [CrossRef]
- Oke, F.; Aslim, B.; Ozturk, S.; Altundag, S. Essential oil composition, antimicrobial and antioxidant activities of Satureja cuneifolia Ten. Food Chem. 2009, 112, 874–879. [Google Scholar] [CrossRef]
- Politeo, O.; Jukic, M.; Milos, M. Chemical composition and antioxidant capacity of free volatile aglycones from basil (Ocimum basilicum L.) compared with its essential oil. Food Chem. 2007, 101, 379–385. [Google Scholar]
- Wang, S.; Ballington, J. Free radical scavenging capacity and antioxidant enzyme activity in deerberry (Vaccinium stamineum L.). LWT-Food Sci. Tech. 2007, 40, 1352–1361. [Google Scholar]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT-Food Sci. Tech. 2008, 41, 1060–1066. [Google Scholar]
- Jia, Z.; Tao, F.; Guo, L.; Tao, G.; Ding, X. Antioxidant properties of extracts from juemingzi (Cassia tora L.) evaluated in vitro. LWT-Food Sci. Tech. 2007, 40, 1072–1077. [Google Scholar]
- Melkani, A.; Dev, V.; Beauchamp, P.; Negi, A.; Mehta, S.; Melkani, K. Constituents of the essential oil of a new chemotype of Benth. Biochem. Syst. Ecol. 2005, 33, 419–425. [Google Scholar] [CrossRef]
- Peng, H.Y.; Yang, X.E. Volatile constituents in the flowers of Elsholtzia argyi and their variation: A possible utilization of plant resources after phytoremediation. J. Zhejiang Univ. Sci. B 2005, 6, 91–95. [Google Scholar]
- She, G.; Guo, Z.; Lv, H.; She, D. New flavonoid glycosides from Elsholtzia rugulosa Hemsl. Molecules 2009, 14, 4190–4196. [Google Scholar] [CrossRef]
- Liu, A.L.; Liu, B.; Qin, H.L.; Lee, S.; Wang, Y.T.; Du, G.H. Anti-influenza virus activities of flavonoids from the medicinal plant Elsholtzia rugulosa. Planta Med. 2008, 74, 847–851. [Google Scholar] [CrossRef]
- Zheng, S.; Lü, R.; Shen, X. Studies on the flavonoids of Elsholtzia ciliata Hyland. Chem. J. Chin. Univ. 1989, 10, 866–868. [Google Scholar]
- Kim, H.H.; Yoo, J.S.; Lee, H.S.; Kwon, T.K.; Shin, T.Y.; Kim, S.H. Elsholtzia ciliata inhibits mast cell-mediated allergic inflammation: Role of calcium, p38 mitogen-activated protein kinase and nuclear factor-κB. Exp. Biol. Med. 2011, 236, 1070–1077. [Google Scholar] [CrossRef]
- Hayase, F.; Kato, H. Antioxidative components of sweet potatoes. J. Nutr. Sci. Vitaminol. 1984, 30, 37–46. [Google Scholar] [CrossRef]
- Kedage, V.V.; Tilak, J.C.; Dixit, G.B.; Devasagayam, T.P.; Mhatre, M. A study of antioxidant properties of some varieties of grapes (Vitis vinifera L.). Crit. Rev. Food Sci. Nutr. 2007, 47, 175–185. [Google Scholar]
- Sarikurkcu, C.; Tepe, B.; Daferera, D.; Polissiou, M.; Harmandar, M. Studies on the antioxidant activity of the essential oil and methanol extract of Marrubium globosum subsp. globosum (lamiaceae) by three different chemical assays. Bioresource Technol. 2008, 99, 4239–4246. [Google Scholar]
- Gülçin, İ. Antioxidant activity of eugenol: A structure and activity relationship study. J. Med. Food 2011, 14, 975–985. [Google Scholar]
- Gülçin, İ.; Topal, F.; Çakmakçı, R.; Bilsel, M.; Gören, A.C.; Erdogan, U. Pomological features, nutritional quality, polyphenol content analysis and antioxidant properties of domesticated and three wild ecotype forms of raspberries (Rubus idaeus L.). J. Food Sci. 2011, 76, C585–C593. [Google Scholar]
- Liu, J.; Wang, C.; Wang, Z.; Zhang, C.; Lu, S.; Liu, J. The antioxidant and free-radical scavenging activities of extract and fractions from corn silk (Zea mays L.) and related flavone glycosides. Food Chem. 2011, 126, 261–269. [Google Scholar]
- Gülçin, I.; Bursal, E.; Sehitoğlu, M.H.; Bilsel, M.; Gören, A.C. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem. Toxicol. 2010, 48, 2227–2238. [Google Scholar] [CrossRef]
- Aaby, K.; Wrolstad, R.E.; Ekeberg, D.; Skrede, G. olyphenol composition and antioxidant activity in strawberry purees; impact of achene level and storage. J. Agric. Food Chem. 2007, 55, 5156–5166. [Google Scholar] [CrossRef]
- Asadi, S.; Khodagholi, F.; Esmaeili, M.A.; Tusi, S.K.; Ansari, N.; Shaerzadeh, F.; Sonboli, A.; Ghahremanzamaneh, M. Chemical composition analysis, antioxidant, antiglycating activities and neuroprotective effects of S. choloroleuca, S. mirzayanii and S. santolinifolia from Iran. Am. J. Chin. Med. 2011, 39, 615–638. [Google Scholar]
- Bakasso, S.; Lamien-Meda, A.; Lamien, C.E.; Kiendrebeogo, M.; Millogo, J.; Ouedraogo, A.G.; Nacoulma, O.G. Polyphenol contents and antioxidant activities of five Indigofera species (Fabaceae) from Burkina Faso. Pakistan J. Biol. Sci. 2008, 11, 1429–1435. [Google Scholar] [CrossRef]
- Su, L.; Yin, J.J.; Charles, D.; Zhou, K.; Moore, J.; Yu, L. Total phenolic contents, chelating capacities, and radical-scavenging properties of black peppercorn, nutmeg, rosehip, cinnamon and oregano leaf. Food Chem. 2007, 100, 990–997. [Google Scholar] [CrossRef]
- Yang, L.; Huang, J.M.; Zu, Y.G.; Ma, C.H.; Wang, H.; Sun, X.W.; Sun, Z. Preparation and radical scavenging activities of polymeric procyanidins nanoparticles by a supercritical antisolvent (SAS) process. Food Chem. 2011, 128, 1152–1159. [Google Scholar] [CrossRef]
- Li, H.; Wong, C.; Cheng, K.; Chen, F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT-Food Sci. Tech. 2008, 41, 385–390. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, X.; Jia, J.; Yang, L.; Yang, F.; Ge, H.; Zhao, C.; Zhang, L.; Zu, Y. Evaluation of Antioxidant Activities of Aqueous Extracts and Fractionation of Different Parts of Elsholtzia ciliata. Molecules 2012, 17, 5430-5441. https://doi.org/10.3390/molecules17055430
Liu X, Jia J, Yang L, Yang F, Ge H, Zhao C, Zhang L, Zu Y. Evaluation of Antioxidant Activities of Aqueous Extracts and Fractionation of Different Parts of Elsholtzia ciliata. Molecules. 2012; 17(5):5430-5441. https://doi.org/10.3390/molecules17055430
Chicago/Turabian StyleLiu, Xiangping, Jia Jia, Lei Yang, Fengjian Yang, Hongshuang Ge, Chunjian Zhao, Lin Zhang, and Yuangang Zu. 2012. "Evaluation of Antioxidant Activities of Aqueous Extracts and Fractionation of Different Parts of Elsholtzia ciliata" Molecules 17, no. 5: 5430-5441. https://doi.org/10.3390/molecules17055430
APA StyleLiu, X., Jia, J., Yang, L., Yang, F., Ge, H., Zhao, C., Zhang, L., & Zu, Y. (2012). Evaluation of Antioxidant Activities of Aqueous Extracts and Fractionation of Different Parts of Elsholtzia ciliata. Molecules, 17(5), 5430-5441. https://doi.org/10.3390/molecules17055430






