Assessment of Genetic Fidelity in Rauvolfia serpentina Plantlets Grown from Synthetic (Encapsulated) Seeds Following in Vitro Storage at 4 °C
Abstract
:1. Introduction
2. Results and Discussion

| Storage duration (Weeks) | Encapsulated buds | Non-encapsulated buds |
|---|---|---|
| 0 | 91.6 ± 2.7 a | 93.0 ± 3.0 a |
| 1 | 85.0 ± 2.3 ab | 57.0 ± 2.6 b |
| 2 | 81.4 ± 2.6 b | 40.2 ± 2.3 c |
| 4 | 80.0 ± 2.3 b | 21.0 ± 1.8 d |
| 6 | 57.3 ± 2.0 c | 17.1 ± 1.6 d |
| 8 | 50.0 ± 1.8 d | 7.0 ± 1.1 e |
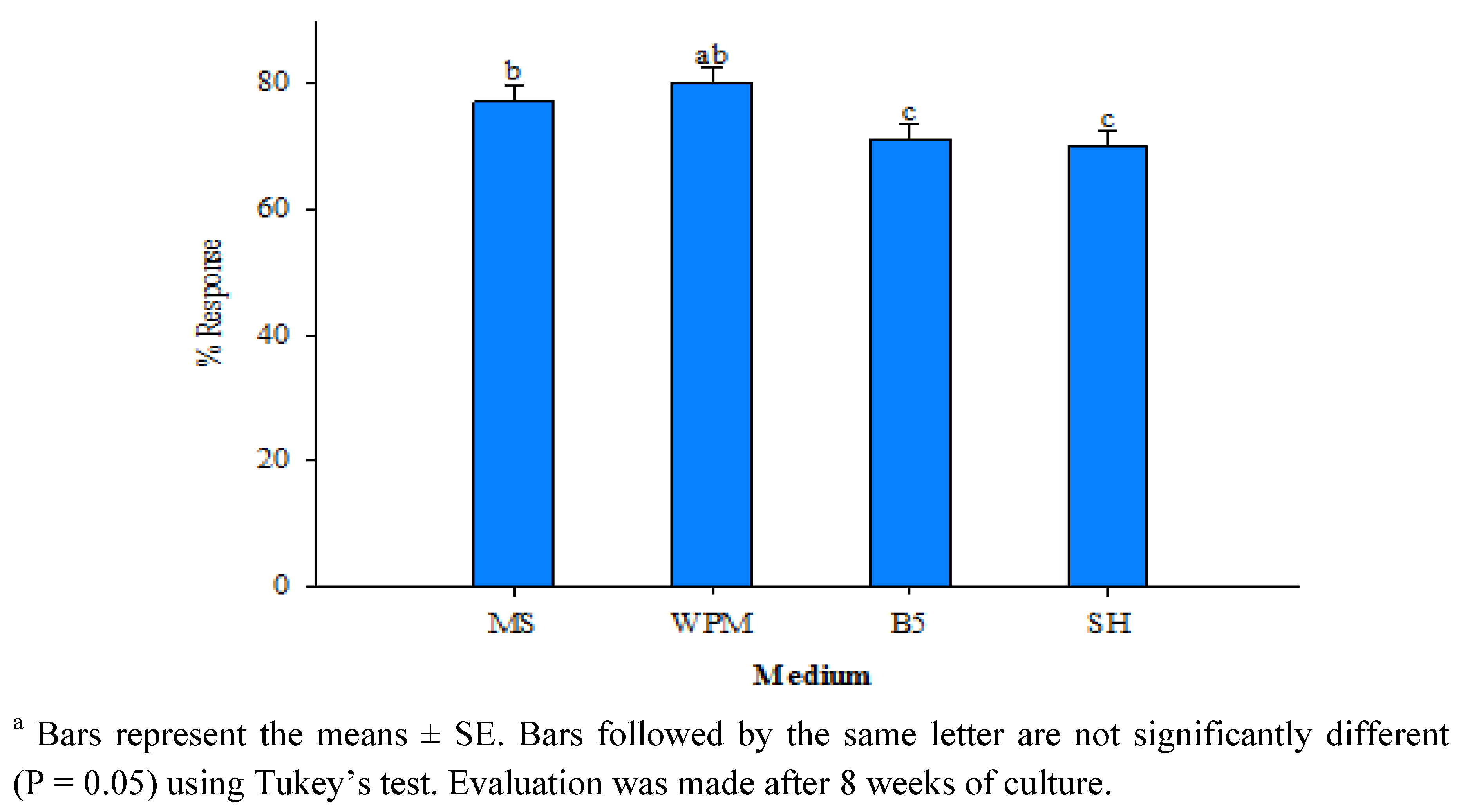
| Planting substrate | Number of plants transferred | Number of plants survived | Plant survival (%) |
|---|---|---|---|
| Garden soil | 30 | 16 | 53.3 |
| Soil-rite | 30 | 27 | 90.0 |
| Vermiculite | 30 | 25 | 76.6 |
| S. No. | Name of primers | Primer sequence (5´ – 3´) | Number of bands |
|---|---|---|---|
| 1 | OPA-01 | CAGGCCCTTC | 12 |
| 2 | OPA-02 | TGCCGAGCTG | 12 |
| 3 | OPA-03 | AGTCAGCCAC | 7 |
| 4 | OPA-04 | AATCGGGCTG | 14 |
| 5 | OPA-05 | AGGGGTCTTG | 8 |
| 6 | OPA-06 | GGTCCCTGAC | 1 |
| 7 | OPA-07 | GAAACGGGTG | 7 |
| 8 | OPA-08 | GTGACGTAGG | 5 |
| 9 | OPA-09 | GGGTAACGCC | 10 |
| 10 | OPA-10 | GTGATCGCAG | 13 |
| 11 | OPA-11 | CAATCGCCGT | 6 |
| 12 | OPA-12 | TCGGCGATAG | 7 |
| 13 | OPA-13 | CAGCACCCAC | 15 |
| 14 | OPA-14 | TCTGTGCTGG | 12 |
| 15 | OPA-15 | TTCCGAACCC | 5 |
| 16 | OPA-16 | AGCCAGCGAA | 0 |
| 17 | OPA-17 | GACCGCTTGT | 3 |
| 18 | OPA-18 | AGGTGACCGT | 11 |
| 19 | OPA-19 | CAAACGTCGG | 4 |
| 20 | OPA-20 | GTTGCGATCC | 8 |
| S. No. | Name of primers | Primer sequence (5′ – 3′) | Annealing temperature °C | Number of bands |
|---|---|---|---|---|
| 1 | ISSR-01 | ACA CAC ACA CAC ACA CT | 45.7 | 8 |
| 2 | ISSR-02 | ACA CAC ACA CAC ACA CG | 49.0 | 12 |
| 3 | ISSR-03 | AGA GAG AGA GAG AGA GYT | 49.0 | 12 |
| 4 | ISSR-04 | GAG AGA GAG AGA GAG AYC | 49.0 | 13 |
| 5 | ISSR-05 | ACA CAC ACA CAC ACA CYT | 49.0 | 15 |
| 6 | ISSR-06 | DBD ACA CAC ACA CAC AC | 45.7 | 17 |
| 7 | ISSR-07 | HVH TGT GTG TGT GTG TG | 45.7 | 13 |
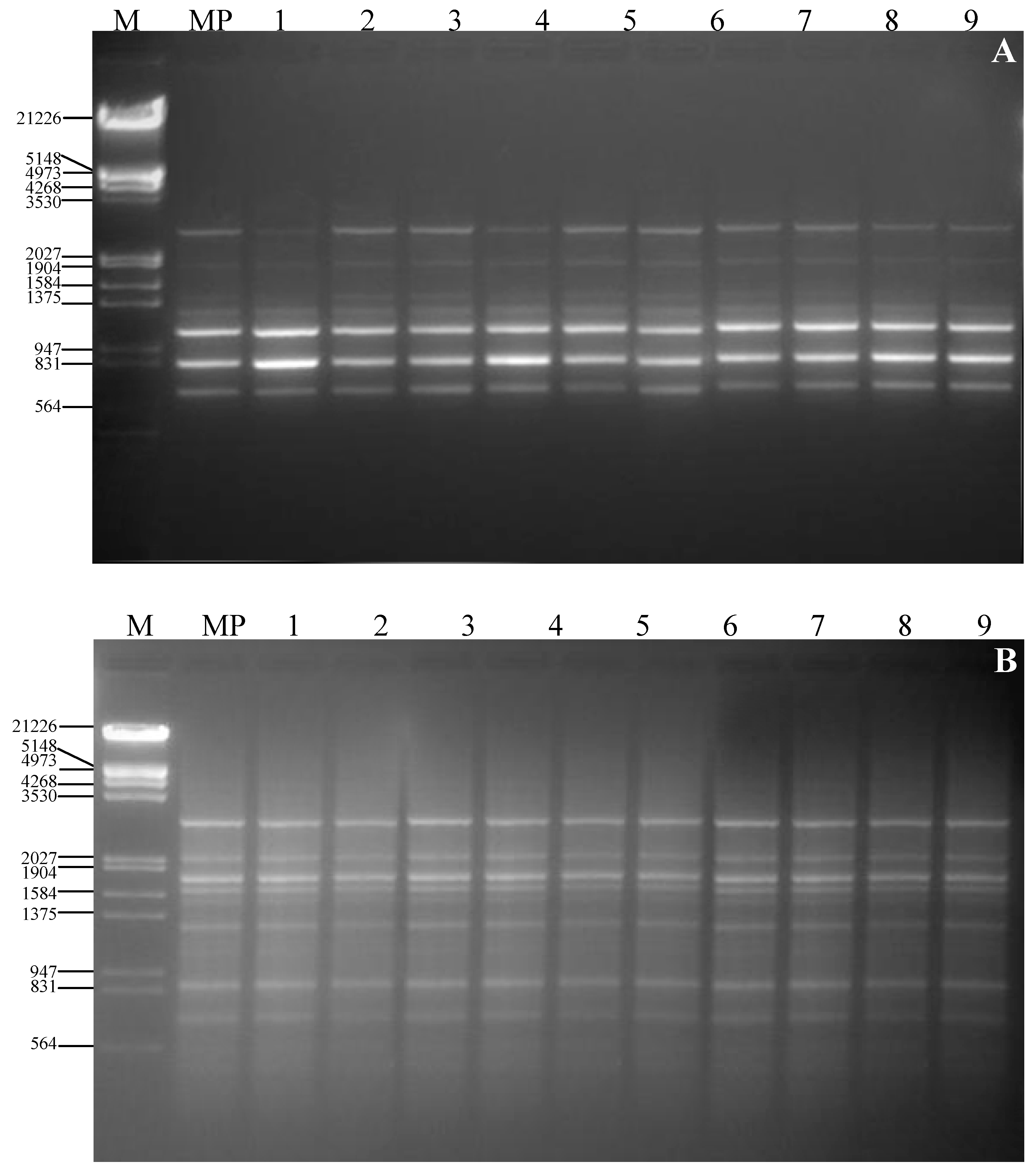
3. Experimental
3.1. Plant Material and Explant Source
3.2. Encapsulation and Low Temperature Storage
Plant regeneration and culture condition
3.3. Acclimatization
3.4. Genomic DNA Extraction and PCR Amplification
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
References and Notes
- Anonymous, The wealth of India: A Dictionary of Indian Raw Materials and Industrial Products; CSIR: New Delhi, India, 2003.
- Jain, S.P.; Singh, J.; Singh, S.C. Rare and endangered medicinal and aromatic plants of Madhya Pradesh. J. Econ. Taxon. Bot. 2003, 27, 925–932. [Google Scholar]
- Standardi, A.; Piccioni, E. Recent perspective on synthetic seed technology using nonembryogenic in vitro-derived explants. Int. J. Plant Sci. 1998, 159, 968–978. [Google Scholar]
- Ara, H.; Jaiswal, U.; Jaiswal, V.S. Synthetic seed: prospects and limitations. Curr. Sci. 2003, 78, 1438–1444. [Google Scholar]
- Mandal, A.B.; Thomas, V.A.; Elanchezhian, R. RAPD pattern of Costus speciosus Koen ex. Retz., an important medicinal plant from the Andaman and Nicobar Islands. Curr. Sci. 2003, 93, 369–373. [Google Scholar]
- Anand, Y.; Bansal, Y.K. Synthetic seeds: A novel approach of in vitro plantlet formation in Vasaka (Adhatoda vasica. Nees.). Plant Biotech. 2002, 19, 159–162. [Google Scholar] [CrossRef]
- Singh, A.K.; Sharma, M.; Varshney, R.; Agarwal, S.S.; Bansal, K.C. Plant regeneration from alginate to encapsulated shoot tips of Phyllanthus amarus Schum and Thonn, a medicinally important plant species. In Vitro Cell Dev. Biol. Plant 2006, 42, 109–113. [Google Scholar]
- Narula, A.; Kumar, S.; Srivastava, P.S. Genetic fidelity of in vitro regenerants, encapsulation of shoot tips and high diosgenin content in Dioscorea bulbifera L., a potential alternative source of diosgenin. Biotechnol. Lett. 2007, 29, 623–629. [Google Scholar] [CrossRef]
- Faisal, M.; Anis, M. Regeneration of plants from alginate-encapsulated shoots of Tylophora indica (Burm. f.) Merrill, an endangered medicinal plant. J. Hort. Sci. Biotech. 2007, 82, 351–354. [Google Scholar]
- Ray, A.; Bhattacharyaa, S. Storage and plant regeneration from encapsulated shoot tips of Rauvolfia serpentina-an effective way of conservation and mass propagation. S. Afr. J. Bot. 2008, 74, 776–779. [Google Scholar] [CrossRef]
- Lata, H.; Chandra, S.; Khan, I.A.; Elsohly, M.A. Propagation through alginate encapsulation of axillary buds of Cannabis sativa L.-an important medicinal plant. Physiol. Mol. Biol. Plants 2009, 15, 79–86. [Google Scholar] [CrossRef]
- Gangopadhyay, G.; Bandyopadhyay, T.; Poddar, R.; Gangopadhyaym, S.B.; Mukherjee, K.K. Encapsulation of pineapple microshoots in alginate beads for temporary storage. Curr. Sci. 2005, 88, 972–977. [Google Scholar]
- Srivastava, V.; Khan, S.A.; Banerjee, S. An evaluation of genetic fidelity of encapsulated microshoots of the medicinal plant: Cineraria maritima following six months of storage. Plant Cell Tiss. Org. Cult. 2009. [Google Scholar] [CrossRef]
- Mishra, J.; Singh, M.; Palni, L.M.S.; Nandi, S.K. Assessment of genetic fidelity of encapsulated microshoots of Picrorhiza kurrooa. Plant Cell Tiss. Org. Cult. 2011, 104, 181–186. [Google Scholar] [CrossRef]
- Salvi, N.D.; George, L.; Eapen, S. Plant regeneration from leaf base callus of turmeric and random amplified polymorphic DNA analysis of regenerated plants. Plant Cell Tiss. Organ. Cult. 2001, 66, 113–119. [Google Scholar] [CrossRef]
- Lakshmanan, V.; Venkataramareddy, S.R.; Neelwarne, B. Molecular analysis of genetic stability in long-term micropropagated shoots of banana using RAPD and ISSR markers. Electron. J. Biotech. 2007, 10, 1–8. [Google Scholar]
- Joshi, P.; Dhawan, V. Assessment of genetic fidelity of micropropagated Swertia chirayita plantlets by ISSR marker assay. Biol. Plant 2007, 51, 22–26. [Google Scholar] [CrossRef]
- Bapat, V.A.; Rao, P.S. Plantlet regeneration fromencapsulated and non-encapsulated desiccated somaticembryos of forest tree, sandalwood (Santalum album L). J. Plant Biochem. Biotech. 1992, 1, 109–113. [Google Scholar]
- Antonietta, G.M.; Manuel, P.; Alvaro, S. Effect of encapsulation on Citrus reticulata ‘Blanco’ somatic embryo conversion. Plant Cell Tiss. Org. Cult. 1999, 55, 235–237. [Google Scholar]
- Danso, K.E.; Ford-Lloyd, B.V. Encapsulation of nodal cuttings and shoot tips for storage and exchange of cassava germplasm. Plant Cell Rep. 2003, 21, 718–725. [Google Scholar]
- Faisal, M.; Ahmad, N.; Anis, M. In vitro plant regeneration from alginate-encapsulated microcuttings of Rauvofia tetraphylla L. American-Eurasina J. Agric. Environ. Sci. 2006, 1, 1–6. [Google Scholar]
- Singh, S.K.; Rai, M.K.; Asthana, P.; Sahoo, L. Alginate-encapsulation of nodal segments for propagation, short-term conservation and germplasm exchange and distribution of Eclipta alba (L.). Acta Physiol. Plant 2010, 32, 607–610. [Google Scholar] [CrossRef]
- Martins, M.; Sarmento, D.; Oliveira, M.M. Genetic stability of micropropagated almond plantlets, as assessed by RAPD and ISSR markers. Plant Cell Rep. 2004, 23, 492–496. [Google Scholar] [CrossRef]
- Devarumath, R.M.; Nandy, S.; Rani, V.; Marimuthu, S.; Muraleedharan, N. RAPD, ISSR and RFLP fingerprints as useful markers to evaluate genetic integrity of micropropagated plants of three diploid and triploid elite tea clones representing Camellia sinensis (China type) and C. assamica ssp. assamica (Assam- India type). Plant Cell Rep. 2002, 21, 166–173. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of Mountain laurel, Kalmia latifolia, by use of shoot tip culture. Int. Plant Prop. Soc. Proc. 1981, 30, 421–427. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrients requirement of Suspension cultures of Soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Schenk, R.U.; Hildebrandt, A. Medium and techniques for induction and growth of monocotyledonous and dicotyle- donous plant cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Weising, K.; Nybom, H.; Wolff, K.; Meyer, W. DNA Fingerprinting in Plants and Fungi; CRC: Boca Raton, FL, USA, 1995. [Google Scholar]
- Williams, K.; Kubelik, A.R.; Rafalski, J.A.; Tingey, S.V. DNA polymorphisms amplified by arbitary primers are useful asgenetic markers. Nucleic Acid Res. 1990, 8, 1631–1635. [Google Scholar]
- Sample Availability: Not Available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Faisal, M.; Alatar, A.A.; Ahmad, N.; Anis, M.; Hegazy, A.K. Assessment of Genetic Fidelity in Rauvolfia serpentina Plantlets Grown from Synthetic (Encapsulated) Seeds Following in Vitro Storage at 4 °C. Molecules 2012, 17, 5050-5061. https://doi.org/10.3390/molecules17055050
Faisal M, Alatar AA, Ahmad N, Anis M, Hegazy AK. Assessment of Genetic Fidelity in Rauvolfia serpentina Plantlets Grown from Synthetic (Encapsulated) Seeds Following in Vitro Storage at 4 °C. Molecules. 2012; 17(5):5050-5061. https://doi.org/10.3390/molecules17055050
Chicago/Turabian StyleFaisal, Mohammad, Abdulrahman A. Alatar, Naseem Ahmad, Mohammad Anis, and Ahmad K. Hegazy. 2012. "Assessment of Genetic Fidelity in Rauvolfia serpentina Plantlets Grown from Synthetic (Encapsulated) Seeds Following in Vitro Storage at 4 °C" Molecules 17, no. 5: 5050-5061. https://doi.org/10.3390/molecules17055050




