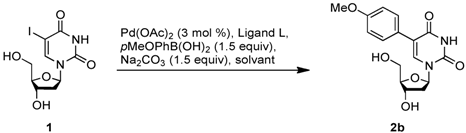
Efficient Synthesis of Unprotected C-5-Aryl/Heteroaryl-2'-deoxyuridine via a Suzuki-Miyaura Reaction in Aqueous Media
Abstract
:1. Introduction
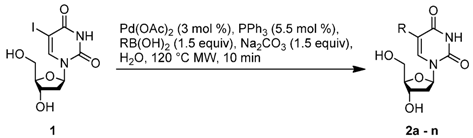
2. Results and Discussion
| Entry | Ligand (L) a | Solvent | Conditions | Yield (%) b |
|---|---|---|---|---|
| 1 | PPh3 | H2O:CH3CN 2:1 | 80 °C, 4 h | 62 |
| 2 | PPh3 | H2O (5 mL) | 80 °C, 4 h | 67 |
| 3 | PPh3c | H2O (5 mL) | 80 °C, 4 h | 69 |
| 4 | PPh3 | H2O (2.5 mL) | 80 °C, 4 h | 75 (74) d |
| 5 | TXPTS | H2O (2.5 mL) | 80 °C, 4 h | 71 |
| 6 | CataCXium F sulf | H2O (2.5 mL) | 80 °C, 4 h | traces |
| 7 | P(CH2N(C2H4OH)2)3 | H2O (2.5 mL) | 80 °C, 4 h | 70 |
| 8 | PPh3 | H2O (2.5 mL) | 120 °C, 10 min MW | 75 (66) e |
| 9 | PPh3 | H2O (2.5 mL) | 120 °C, 10 min MW | 70 f |
| Entry | RB(OH)2 | Products | Yield (%) a | |
|---|---|---|---|---|
| 1 |  | 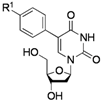 | 2a: R1 = H | 70 (62) b |
| 2b: R1 = OMe | 75 | |||
| 2c: R1 = Ac | 72 | |||
| 2d: R1 = CHO | 79 | |||
| 2e: R1 = F | 74 | |||
| 2f: R1 = NO2 | 68 | |||
| 2 |  | 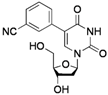 | 2g | 70 |
| 3 |  | 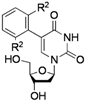 | 2h: R2 = Me | - |
| 2i: R2 = OMe | - | |||
| 4 | 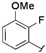 | 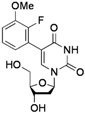 | 2j | 53 c |
| 5 | 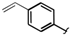 | 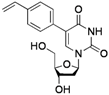 | 2k | 30 |
| 6 | 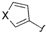 | 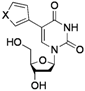 | 2l: X = O | 67 (73) d |
| 2m: X = S | 81 | |||
| 7 | 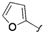 | 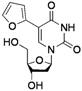 | 2n | 44c |
3. Experimental
3.1. General
3.2. General procedure
4. Conclusions
- Sample Availability: Samples of the compounds 2a–g, j, l, m are available from the authors.
References
- Amann, N.; Pandurski, E.; Fiebig, T.; Wagenknecht, H.-A. Electron Injection into DNA: Synthesis and Spectroscopic Properties of Pyrenyl-Modified Oligonucleotides. Chem. Eur. J. 2002, 8, 4877–4883. [Google Scholar] [CrossRef]
- Amann, N.; Pandurski, E.; Fiebig, T.; Wagenknecht, H.-A. A model nucleoside for electron injection into DNA: 5-Pyrenyl-2-deoxyribose. Angew. Chem. Int. Ed. Engl. 2002, 41, 2978–2980. [Google Scholar] [CrossRef]
- Okamoto, A.; Inasaki, T.; Saito, I. Synthesis and ESR studies of nitronyl nitroxide-tethered oligodeoxynucleotides. Tetrahedron Lett. 2005, 46, 791–795. [Google Scholar]
- Wagner, C.; Wagenknecht, H.-A. Reductive Electron Transfer in Phenothiazine-Modified DNA Is Dependent on the Base Sequence. Chem. Eur. J. 2005, 11, 1871–1876. [Google Scholar] [CrossRef]
- Roy, V.; Zerrouki, R.; Krausz, P. New dinucleoside analogues via cross-coupling metathesis. Nucleos. Nucleot. Nucl. Acid. 2005, 24, 289–301. [Google Scholar]
- Okamoto, A.; Tainaka, K.; Unzai, T.; Saito, I. Synthesis and fluorescence properties of dimethylaminonaphthalene-deoxyuridine conjugates as micropolarity-sensitive probes. Tetrahedron 2007, 63, 3465–3470. [Google Scholar] [CrossRef]
- Capek, P.; Cahová, H.; Pohl, R.; Hocek, M.; Gloeckner, C.; Marx, A. An Efficient Method for the Construction of Functionalized DNA Bearing Amino Acid Groups through Cross-Coupling Reactions of Nucleoside Triphosphates Followed by Primer Extension or PCR. Chem. Eur. J. 2007, 13, 6196–6203. [Google Scholar]
- Ehrenschwender, T.; Wagenknecht, H.-A. Synthesis and Spectroscopic Characterization of BODIPY-Modified Uridines as Potential Fluorescent Probes for Nucleic Acids. Synthesis 2008, 2008, 3657–3662. [Google Scholar] [CrossRef]
- Wanninger-Weiß, C.; Wagenknecht, H.-A. Synthesis of 5-(2-pyrenyl)-2'-deoxyuridine as a DNA Modification for Electron-Transfer Studies: The Critical Role of the Position of the Chromophore Attachment. Eur. J. Org. Chem. 2008, 2008, 64–71. [Google Scholar] [CrossRef]
- Colombeau, L.; Teste, K.; Hadj-Bouazza, A.; Chaleix, V.; Zerrouki, R.; Kreamer, M.; Sainte Catherine, O. Synthesis and biological activity of chloroethyl pyrimidine nucleosides. Nucleos. Nucleot. Nucl. Acid. 2008, 27, 110–120. [Google Scholar] [CrossRef]
- Jacobsen, M.F.; Ferapontova, E.E.; Gothelf, K.V. Synthesis and electrochemical studies of an anthraquinone-conjugated nucleoside and derived oligonucleotides. Org. Biomol. Chem. 2009, 7, 905–908. [Google Scholar] [CrossRef]
- Kalachova, L.; Pohl, R.; Hocék, M. Synthesis of 2'-Deoxyuridineand 2'-Deoxycytidine Nucleosides Bearing Bipyridine and Terpyridine Ligands at Position 5. Synthesis 2009, 2009, 105–112. [Google Scholar] [CrossRef]
- Raindlová, V.; Pohl, R.; Šanda, M.; Hocek, M. Direct Polymerase Synthesis of Reactive Aldehyde-Functionalized DNA and Its Conjugation and Staining with Hydrazines. Angew. Chem. Int. Ed. Engl. 2010, 49, 1064–1066. [Google Scholar]
- Macíčková-Cahová, H.; Pohl, R.; Horáková, P.; Havran, L.; Špaček, J.; Fojta, M.; Hocek, M. Alkylsulfanylphenyl Derivatives of Cytosine and 7-Deazaadenine Nucleosides, Nucleotides and Nucleoside Triphosphates: Synthesis, Polymerase Incorporation to DNA and Electrochemical Study. Chem. Eur. J. 2011, 17, 5833–5841. [Google Scholar]
- Segal, M.; Fischer, B. Analogues of uracil nucleosides with intrinsic fluorescence (NIF-analogues): Synthesis and photophysical properties. Org. Biomol. Chem. 2012, 10, 1571–1580. [Google Scholar] [CrossRef]
- Riedl, J.; Pohl, R.; Ruíšek, L.; Hocek, M. Synthesis and Photophysical Properties of Biaryl-Substituted Nucleos(t)ides. Polymerase Synthesis of DNA Probes Bearing Solvatochromic and pH-Sensitive Dual Fluorescent and 19F-NMR Labels. J. Org. Chem. 2012, 77, 1026–1044. [Google Scholar] [CrossRef]
- Peyrat, S.; Xie, J. Synthesis of Thymidine Dimers from 5'-O-Aminothymidine. Synthesis 2012, 2012, 1718–1724. [Google Scholar]
- De Clercq, E. Antiviral and Antitumor Activities of 5-Substituted 2'-Deoxyuridines. Meth. Find. Exp. Clin. Pharmacol. 1980, 2, 253–267. [Google Scholar]
- De Clercq, E. Biochemical aspects of the selective antiherpes activity of Nucleoside Analogues. Biochem. Pharmacol. 1984, 33, 2159–2169. [Google Scholar] [CrossRef]
- De Winter, H.; Herdewijn, P. Understanding the Binding of 5-Substituted 2'-Deoxyuridine Substrates to Thymidine Kinase of Herpes Simplex Virus Type-1. J.Med. Chem. 1996, 39, 4727–4737. [Google Scholar] [CrossRef]
- Wigerinck, P.; Snoeck, R.; Claes, P.; de Clercq, E.; Herdewijn, P. Synthesis and Antiviral Activity of 5-Heteroaryl-Substituted 2'-Deoxyuridines. J. Med. Chem. 1991, 34, 1767–1772. [Google Scholar] [CrossRef]
- Wigerinck, P.; Pannecouque, C.; Snoeck, R.; Claes, P.; de Clercq, E.; Herdewijn, P. 5-(5-Bromothien-2-yl)-2'-deoxyuridine and 5-(5-chlorothien-2-yl)-2'-deoxyuridine are equipotent to (E)-5-(2-bromovinyl)-2'-deoxyuridine in the inhibition of herpes simplex virus type I replication. J. Med. Chem. 1991, 34, 2383–2389. [Google Scholar] [CrossRef]
- Herdewijn, P. 5-Substituted-2'-deoxyuridines as anti-HSV agents: Synthesis and structure activity relationship. Antivir. Chem. Chemother. 1994, 5, 131–146. [Google Scholar]
- Kögler, M.; Vanderhoydonck, B.; de Jonghe, S.; Rozenski, J.; Van Belle, K.; Herman, J.; Louat, T.; Parchina, A.; Sibley, C.; Lescrinier, E.; Herdewijn, P. Synthesis and Evaluation of 5-Substituted 2'-deoxyuridine Monophosphate Analogues As Inhibitors of Flavin-Dependent Thymidylate Synthase in Mycobacterium tuberculosis. J. Med. Chem. 2011, 54, 4847–4862. [Google Scholar] [CrossRef]
- De Clercq, E.; Descamp, J.; DeSomer, P.; Barr, P.J.; Jones, A.S.; Walker, R.T. (E)-5-(2-bromovinyl)-2'-Deoxyuridine, A potent ans Selective Antiherpes Agent. Proc. Natl. Acad. Sci. USA 1979, 76, 2947–2951. [Google Scholar] [CrossRef]
- De Clercq, E.; Descamps, J.; Verhelst, G.; Walker, R.T.; Jones, A.S.; Torrence, P.F.; Shugar, D. Comparative Efficacy of Antiherpes Drugs Against Different Strains of Herpes Simplex Virus. J. Infect. Dis. 1980, 141, 563–574. [Google Scholar] [CrossRef]
- Kundu, N.G.; Dasgupta, S.K. Synthesis of 5-(acylethynyl)uracils and their corresponding 2'-Deoxyribonucleosides through Palladium-Catalyzed Reactions. J. Chem. Soc. Perkin Trans. I 1993, 21, 2657–2663. [Google Scholar]
- Lakshman, M.K. Palladium-catalyzed C-N and C-C cross-coupling as versatile, new avenues for modification of purine 2'-deoxynucleosides. J. Organomet. Chem. 2002, 653, 234–251. [Google Scholar] [CrossRef]
- Agrofoglio, L.A.; Gillaizeau, I.; Saito, Y. Palladium-assisted routes to nucleosides. Chem. Rev. 2003, 103, 1875–1916. [Google Scholar] [CrossRef]
- Lakshman, M.K. Synthesis of biologically important nucleoside analogs by palladium-catalyzed C-N bond formation. Curr. Org. Synth. 2005, 2, 83–112. [Google Scholar] [CrossRef]
- Thoresen, L.H.; Jiao, G.-S.; Haaland, W.C.; Metzker, M.L.; Burgess, K. Rigid, Conjugated, Fluoresceinated Thymidine Triphosphates: Syntheses and Polymerase Mediated Incorporation into DNA Analogues. Chem. Eur. J. 2003, 9, 4603–4610. [Google Scholar] [CrossRef]
- Ogino, M.; Yoshimura, Y.; Nakazawa, A.; Saito, I.; Fujimoto, K. Template-directed DNA photoligation via a 5-cyanovinyldeoxyuridine. Org. Lett. 2005, 7, 2853–2856. [Google Scholar]
- Takeda, S.; Tsukiji, S.; Nagamune, T. A cysteine-appended deoxyuridine for the postsynthetic DNA modification using native chemical ligation. Tetrahedron Lett. 2005, 46, 2235–2238. [Google Scholar] [CrossRef]
- Ding, H.; Greenberg, M.M. Hole Migration is the Major Pathway Involved in Alkali-Labile Lesion Formation in DNA by the Direct Effect of Ionizing Radiation. J. Am. Chem. Soc. 2007, 129, 772–773. [Google Scholar] [CrossRef]
- Ikonen, S.; Macíčková-Cahová, H.; Pohl, R.; Šanda, M.; Hocek, M. Synthesis of nucleoside and nucleotide conjugates of bile acids, and polymerase construction of bile acid-functionalized DNA. Org. Biomol. Chem. 2010, 8, 1194–1201. [Google Scholar]
- Cho, J.H.; Prickett, C.D.; Shaughnessy, K.H. Efficient Sonogashira Coupling of Unprotected halonucleosides in Aqueous Solvents Using Water-Soluble Palladium Catalysts. Eur. J. Org. Chem. 2010, 2010, 3678–3683. [Google Scholar] [CrossRef]
- Kielkowski, P.; Pohl, R.; Hocek, M. Synthesis of Acetylene Linked Double-Nucleobase Nucleos(t)ide Building Blocks and Polymerase Construction of DNA Containing Cytosines in the Major Groove. J. Org. Chem. 2011, 76, 3457–3462. [Google Scholar] [CrossRef]
- Cho, J.H.; Shaughnessy, K.H. Aqueous-Phase Heck Coupling of 5-Iodouridine and Alkenes under Phosphine-Free Conditions. Synlett 2011, 2011, 2963–2960. [Google Scholar] [CrossRef]
- Casalnuovo, A.L.; Calabrese, J.C. Palladium-catalyzed alkylations in aqueous media. J. Am. Chem. Soc. 1990, 112, 4324–4326. [Google Scholar] [CrossRef]
- Western, E.C.; Daft, J.R.; Johnson, E.M.; Gannett, P.M.; Shaughnessy, K.H. Efficient One-Step Suzuki Arylation of Unprotected Halonucleosides using Water-Soluble Palladium Catalysts. J. Org. Chem. 2003, 68, 6767–6774. [Google Scholar] [CrossRef]
- Daku, K.M.L.; Newton, R.F.; Pearce, S.P.; Vile, J.; Williams, J.M.J. Suzuki cross-coupling reactions using reverse-phase glass beads in aqueous media. Tetrahedron Lett. 2003, 44, 5095–5098. [Google Scholar]
- Pesnot, T.; Wagner, G.K. Novel derivatives of UDP-glucose: Concise synthesis and fluorescent properties. Org. Biomol. Chem. 2008, 6, 2884–2891. [Google Scholar] [CrossRef]
- Sartori, G.; Enderlin, G.; Hervé, G.; Len, C. Highly Effective Synthesis of C-5-Substituted 2'-Deoxyuridine Using Suzuki-Miyaura Cross-Coupling in Water. Synthesis 2012, 2012, 767–772. [Google Scholar]
- El Kazzouli, S.; Berteina-Raboin, S.; Agrofoglio, L.A. Supported synthesis and functionnalization of 2"-deoxyuridine by suzuki-miyaura cross-coupling. Nucleos. Nucleot. Nucl. Acid. 2007, 26, 1395–1398. [Google Scholar] [CrossRef]
- Fresneau, N.; Hiebel, M.-A.; Agrofoglio, L.A.; Berteina-Raboin, S. One-pot Sonogashira-cyclization protocol to obtain substituted furopyrimidine nucleosides in aqueous conditions. Tetrahedron Lett. 2012, 53, 1760–1763. [Google Scholar] [CrossRef]
- Huang, R.; Shaughnessy, K.H. Water-Soluble Palladacycles as Precursors to Highly Recyclable Catalysts for the Suzuki Coupling of Aryl Bromides in Aqueous Solvents. Organometallics 2006, 25, 4105–4112. [Google Scholar] [CrossRef]
- Moore, L.R.; Western, E.C.; Craciun, R.; Spruell, J.M.; Dixon, D.A.; O’Halloran, K.P.; Shaughnessy, K.H. Sterically Demanding, Sulfonated, Triarylphosphines: Application to Palladium-Catalyzed Cross-Coupling, Steric and Electronic Properties, and Coordination Chemistry. Organometallics 2008, 27, 576–593. [Google Scholar] [CrossRef]
- Fleckenstein, C.A.; Plenio, H. Aqueous cross-coupling: Highly efficient Suzuki-Miyaura coupling of N-heteroaryl halides and N-heteroarylboronic acids. Green Chem. 2007, 9, 1287–1291. [Google Scholar] [CrossRef]
- Krauter, J.G.E.; Beller, M. An Easy and Practical Synthetic Route to Electron Rich Water Soluble Ligands: α-Aminomethylation of Trishydroxymethylphosphine. Tetrahedron 2000, 56, 771–774. [Google Scholar] [CrossRef]
- Shaughnessy, K.H. Hydrophilic Ligands and Their Application in Aqueous-Phase Metal-Catalyzed Reactions. Chem. Rev. 2009, 109, 643–710. [Google Scholar] [CrossRef]
- Zayas, H.A.; Bowyer, M.C.; Gordon, C.P.; Holdsworth, C.I.; Mc Cluskey, A. Synthesis of biaryl-styrene monomers by microwave-assisted Suzuki coupling. Tetrahedron Lett. 2009, 50, 5894–5895. [Google Scholar]
- Chang, G.; Mertes, M.P. Linear free energy relationships studies of 5-substituted 2,4-dioxopyrimidine nucleosides. J. Org. Chem. 1987, 52, 3625–3631. [Google Scholar] [CrossRef]
- Wigerinck, P.; Kerremans, L.; Cleas, P.; Snoeck, R.; Maudgal, P.; de Clercq, E.; Herdewijn, P. Synthesis and antiviral activity of 5-thien-2-yl-2'-deoxyuridine analogs. J. Med. Chem. 1993, 36, 538–543. [Google Scholar] [CrossRef]
- Aucagne, V.; Berteina-Raboin, S.; Guenot, P.; Agrofoglio, L.A. Palladium-catalyzed parallel solid-phase synthesis of nucleosides. J. Comb. Chem. 2004, 6, 717–723. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fresneau, N.; Hiebel, M.-A.; Agrofoglio, L.A.; Berteina-Raboin, S. Efficient Synthesis of Unprotected C-5-Aryl/Heteroaryl-2'-deoxyuridine via a Suzuki-Miyaura Reaction in Aqueous Media. Molecules 2012, 17, 14409-14417. https://doi.org/10.3390/molecules171214409
Fresneau N, Hiebel M-A, Agrofoglio LA, Berteina-Raboin S. Efficient Synthesis of Unprotected C-5-Aryl/Heteroaryl-2'-deoxyuridine via a Suzuki-Miyaura Reaction in Aqueous Media. Molecules. 2012; 17(12):14409-14417. https://doi.org/10.3390/molecules171214409
Chicago/Turabian StyleFresneau, Nathalie, Marie-Aude Hiebel, Luigi A. Agrofoglio, and Sabine Berteina-Raboin. 2012. "Efficient Synthesis of Unprotected C-5-Aryl/Heteroaryl-2'-deoxyuridine via a Suzuki-Miyaura Reaction in Aqueous Media" Molecules 17, no. 12: 14409-14417. https://doi.org/10.3390/molecules171214409