Ultrasound-Promoted Greener Synthesis of Novel Trifurcate 3-Substituted-chroman-2,4-dione Derivatives and Their Drug-Likeness Evaluation
Abstract
:1. Introduction

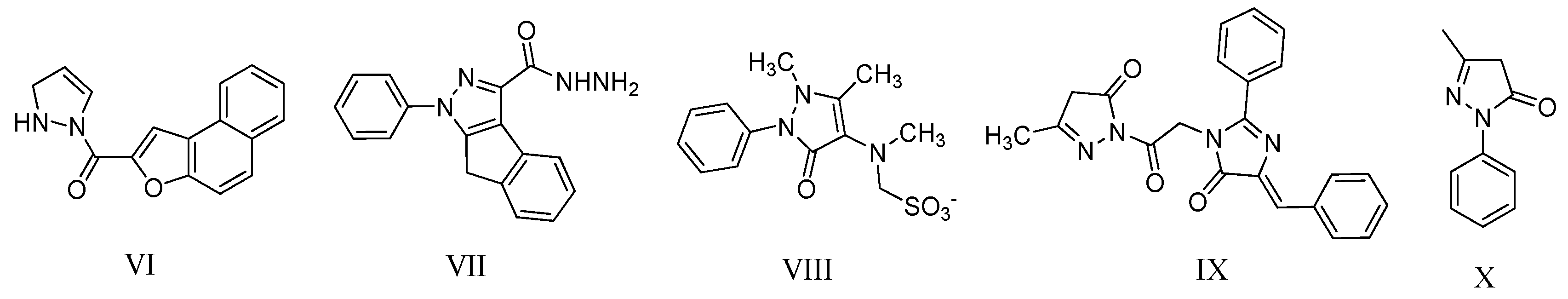
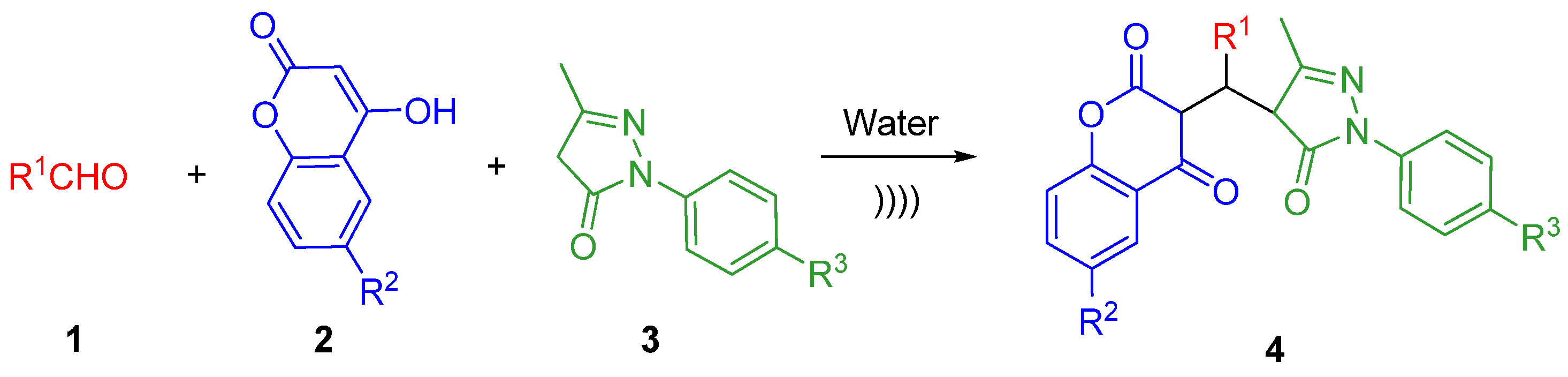
2. Results and Discussion
| Entry | Solvent | Catalyst (mol%) | Temprature (°C) | With sonication a | Without sonication b | ||
|---|---|---|---|---|---|---|---|
| Time (h) | Yield (%) c | Time (h) | Yield (%) c | ||||
| 1 | EtOH | ——— | 70 | 1 | 55 | 4 | 23 |
| 2 | Acetonitrile | ——— | 80 | 1 | 14 | 4 | <5 |
| 3 | Toluene | ——— | 80 | 0.5 | — | 2 | — |
| 4 | Water | ——— | 60 | 0.5 | 81 | 2 | 67 |
| 5 | EtOH | ZnCl2(5) | 70 | 0.5 | — | 2 | — |
| 6 | Acetonitrile | Et3N(5) | 80 | 1 | Trace | 4 | Trace |
| 7 | Water | Morpholine(5) | 60 | 1 | 36 | 6 | 15 |
| 8 | Water | ZnCl2(5) | 60 | 0.5 | — | 2 | — |
| 9 | Water | AlCl3(5) | 60 | 0.5 | — | 2 | — |
| 10 | Water | Morpholine(5) | 60 | 0.5 | 51 | 2 | 36 |
| 11 | Water | Et3N(5) | 60 | 0.5 | 35 | 2 | 17 |
| 12 | Water | L-proline(5) | 60 | 0.5 | 42 | 2 | 30 |
2.1. Effects of the Catalysts under Ultrasound Irradiation

2.2. Effects of the Solvents under Ultrasound Irradiation
2.3. Comparison of Ultrasonic Irradiation and Conventional Method
| Entry | Frequency (kHz) | Temprature (°C) | Time (h) | Yield (%) b |
|---|---|---|---|---|
| 30 | 60 | 0.5 | 67 | |
| 2 | 40 | 60 | 0.5 | 81 |
| 3 | 50 | 60 | 0.5 | 79 |
2.4. High Efficiency and Generality of Synthesis by Ultrasound Irradiation
| Entry | R1 | R2 | R3 | Time (min) | Product | Isolated Yield (%) | Mp (°C) | logP a |
|---|---|---|---|---|---|---|---|---|
| 1 |  | H | H | 30 | 4a | 81 | 151–153 | 3.34 ± 0.04 |
| 2 |  | H | H | 30 | 4b | 89 | 168–170 | 3.60 ± 0.04 |
| 3 |  | H | H | 30 | 4c | 83 | 163–165 | 3.73 ± 0.05 |
| 4 |  | H | H | 30 | 4d | 77 | 166–168 | 3.89 ± 0.04 |
| 5 |  | H | H | 30 | 4e | 82 | 184–186 | 3.47 ± 0.04 |
| 6 |  | H | H | 30 | 4f | 71 | 177–179 | 4.17 ± 0.05 |
| 7 |  | H | H | 30 | 4g | 78 | 149–151 | 4.34 ± 0.04 |
| 8 |  | H | H | 30 | 4h | 83 | 133–135 | 3.86 ± 0.04 |
| 9 |  | H | H | 30 | 4i | 65 | 130–132 | 2.45 ± 0.06 |
| 10 |  | Cl | H | 30 | 4j | 79 | 195–197 | 3.90 ± 0.04 |
| 11 |  | F | H | 30 | 4k | 58 | 189–191 | 3.51 ± 0.04 |
| 12 |  | H | Me | 30 | 4l | 87 | 193–196 | 3.83 ± 0.05 |
| 13 |  | H | F | 30 | 4m | 73 | 187–189 | 3.50 ± 0.04 |
| 14 |  | H | Cl | 30 | 4n | 64 | 185–187 | 4.02 ± 0.04 |
| 15 |  | F | Me | 30 | 4o | 81 | 199–201 | 3.98 ± 0.06 |
| 16 |  | F | F | 30 | 4p | 83 | 162–164 | 4.06 ± 0.04 |
2.5. the Study of Acceleration Mechanism under Irradiation of Ultrasound
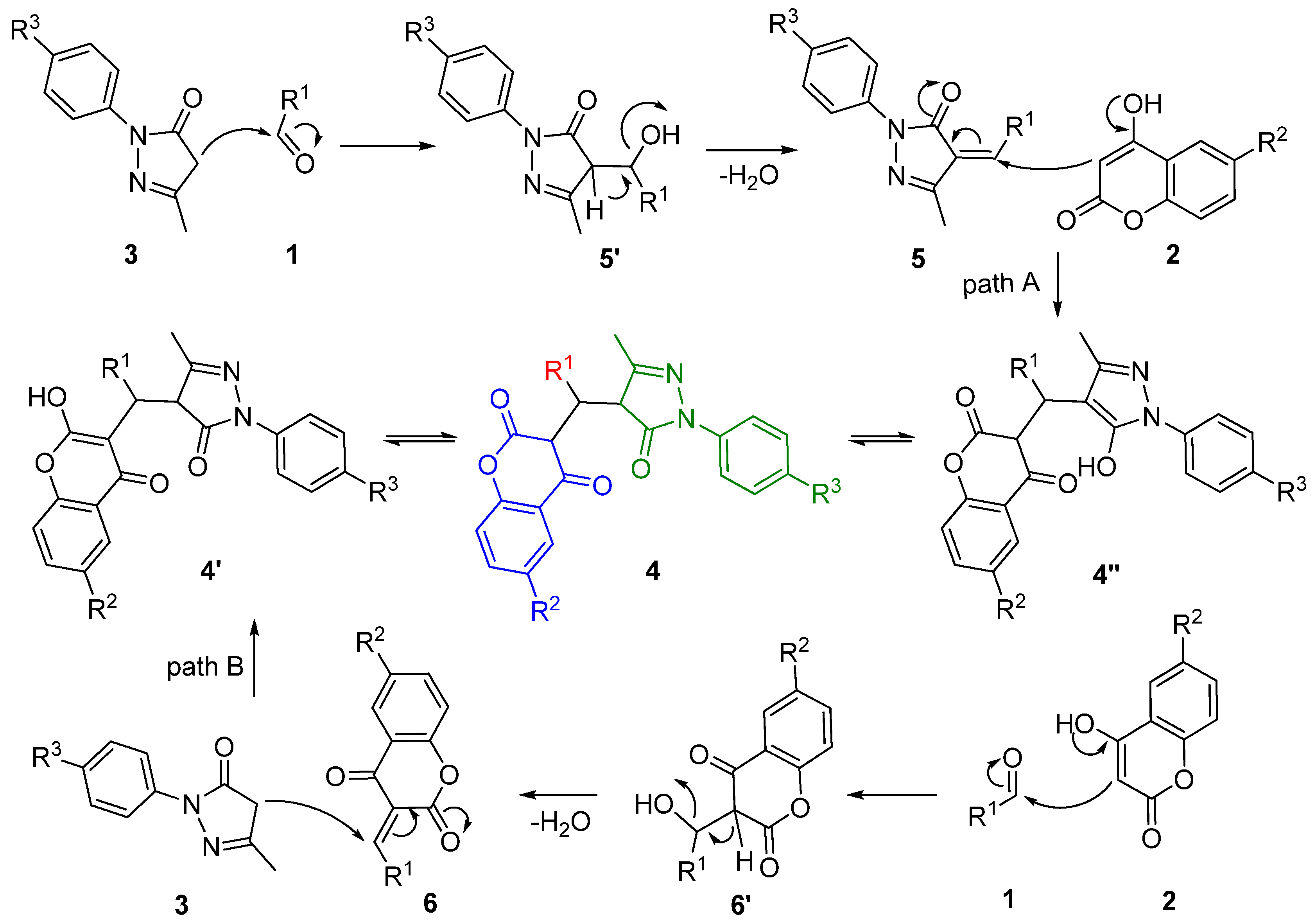
2.6. Drug-Likeness Evaluation of Obtained 3-substituted-chroman-2,4-Diones
3. Experimental
General
3.1. Typical Procedure for the Synthesis of Trifurcate 3-substituted-chroman-2,4-Dione (4a) in Water
3.2. Ultrasound-promoted Typical Procedure for Synthesis of 3-substituted-chroman-2,4-dione (4a)
3.3. Spectral Data for New Derivatives of Trifurcate 3-substituted-chroman-2,4-diones 4a–p
4. Conclusions
Supplementary Materials
Acknowledgments
- Sample Availability: Samples of the compounds 4a–p and 5a are available from the authors.
References
- Blaskovicova, M.; Gaplovsky, A.; Blasko, J. Synthesis and Photochemistry of 1-Iodocyclohexene: Influence of Ultrasound on Ionic vs. Radical Behaviour. Molecules 2007, 12, 188–193. [Google Scholar] [CrossRef]
- Doan, N.; Le, T.; Nguyen, H.; Hansen, P.; Duus, F. Ultrasound Assisted Synthesis of 5,9-Dimethylpentadecane and 5,9-Dimethylhexadecane-The Sex Pheromones of Leucoptera coffeella. Molecules 2007, 12, 2080–2088. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, C.-J.; Li, Y.-P. Ultrasound Promoted Synthesis of Bis (substituted pyrazol-4-ylcarbonyl)-Substituted Thioureas. Molecules 2009, 14, 1423–1428. [Google Scholar] [CrossRef]
- Liu, C.-J.; Wang, J.-D. Ultrasound-Assisted Synthesis of Novel 4-(2-Phenyl-1,2,3-Triazol-4-yl)-3,4-Dihydropyrimidin-2(1H)-(Thio)ones Catalyzed by Sm(ClO4)3. Molecules 2010, 15, 2087–2095. [Google Scholar] [CrossRef]
- Wang, J.; Bai, X.; Xu, C.; Wang, Y.; Lin, W.; Zou, Y.; Shi, D. Ultrasound-Promoted One-Pot, Three-Component Synthesis of Spiro[indoline-3,1'-pyrazolo[1,2-b]phthalazine] Derivatives. Molecules 2012, 17, 8674–8686. [Google Scholar] [CrossRef]
- Gao, D.-M.; Ma, W.-L.; Li, T.-R.; Huang, L.-Z.; Du, Z.-T. An Improved Synthesis of 1,2-Diarylethanols under Conventional Heating and Ultrasound Irradiation. Molecules 2012, 17, 10708–10715. [Google Scholar] [CrossRef]
- Gomha, S.M.; Khalil, K.D. A Convenient Ultrasound-Promoted Synthesis of Some New Thiazole Derivatives Bearing a Coumarin Nucleus and Their Cytotoxic Activity. Molecules 2012, 17, 9335–9347. [Google Scholar] [CrossRef]
- Aggeler, P.M.; O'Reilly, R.A.; Leong, L.; Kowitz, P.E. Potentiation of anticoagulant effect of warfarin by phenylbutazone. N. Engl. J. Med. 1967, 276, 496–501. [Google Scholar] [CrossRef]
- O'Reilly, R.A.; Aggeler, P.M. Studies on coumarin anticoagulant drugs: Initiation of warfarin therapy without a loading dose. Circulation 1968, 38, 169–177. [Google Scholar] [CrossRef]
- Huang, L.; Yuan, X.; Yu, D.; Lee, K.; Ho Chen, C. Mechanism of action and resistant profile of anti-HIV-1 coumarin derivatives. Virology 2005, 332, 623–628. [Google Scholar] [CrossRef]
- Spino, C.; Dodier, M.; Sotheeswaran, S. Anti-HIV coumarins from Calophyllum seed oil. Bioorg. Med. Chem. Lett. 1998, 8, 3475–3478. [Google Scholar] [CrossRef]
- Baba, M.; Jin, Y.; Mizuno, A.; Suzuki, H.; Okada, Y.; Takasuka, N.; Tokuda, H.; Nishino, H.; Okuyama, T. Studies on Cancer Chemoprevention by Traditional Folk Medicines XXIV.-Inhibitory Effect of a Coumarin Derivative, 7-Isopentenyloxycoumarin, against Tumor-Promotion. Biol. Pharm. Bull. 2002, 25, 244–246. [Google Scholar] [CrossRef]
- Murakami, A.; Kuki, W.; Takahashi, Y.; Yonei, H.; Nakamura, Y.; Ohto, Y.; Ohigashi, H.; Koshimizu, K. Auraptene, A Citrus Coumarin, Inhibits 12-o-Tetradecanoylphorbol-13-acetate-induced Tumor Promotion in ICR Mouse Skin, Possibly through Suppression of Superoxide Generation in Leukocytes. Cancer Sci. 1997, 88, 443–452. [Google Scholar] [CrossRef]
- Kelly, R.A.; Gelfand, J.A.; Pincus, S.H. Cutaneous necrosis caused by systemicallyadministered heparin. JAMA 1981, 246, 1582–1583. [Google Scholar]
- Stanchev, S.; Momekov, G.; Jensen, F.; Manolov, I. Synthesis, computational study and cytotoxic activity of new 4-hydroxycoumarin derivatives. Eur. J. Med. Chem. 2008, 43, 694–706. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Shaikh, A.U.; Rauf, A.; Supuran, C.T. Antibacterial, antifungal and cytotoxic properties of novel N-substituted sulfonamides from 4-hydroxycoumarin. J. Enzym. Inhib. Med. Chem. 2006, 21, 741–748. [Google Scholar] [CrossRef]
- Thaisrivongs, S.; Watenpaugh, K.D.; Howe, W.J.; Tomich, P.K.; Dolak, L.A.; Chong, K.T.; Tomich, C.S.C.; Tomasselli, A.G.; Turner, S.R. Structure-based design of novel HIV protease inhibitors: carboxamide-containing 4-hydroxycoumarins and 4-hydroxy-2-pyrones as potent nonpeptidic inhibitors. J. Med. Chem. 1995, 38, 3624–3637. [Google Scholar] [CrossRef]
- Kostova, I. Synthetic and natural coumarins as cytotoxic agents. Curr. Med. Chem. 2005, 5, 29–46. [Google Scholar] [CrossRef]
- Dias, L.R.S.; Salvador, R.R.S. Pyrazole Carbohydrazide Derivatives of Pharmaceutical Interest. Pharmaceuticals 2012, 5, 317–324. [Google Scholar] [CrossRef]
- Wang, X.H.; Wang, X.K.; Liang, Y.J.; Shi, Z.; Zhang, J.Y.; Chen, L.M.; Fu, L.W. A cellbased screen for anticancer activity of 13 pyrazolone derivatives. Chin. J. Cancer 2010, 29, 980–987. [Google Scholar] [CrossRef]
- Chandrasekharan, N.; Dai, H.; Roos, K.; Evanson, N.K.; Tomsik, J.; Elton, T.S.; Simmons, D.L. COX-3, A cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: Cloning, Structure, And expression. Proc. Nat. Acad. Sci. USA 2002, 99, 13926–13931. [Google Scholar]
- Laporte, J.; Carne, X.; Vidal, X.; Moreno, V.; Juan, J. Upper gastrointestinal bleeding in relation to previous use of analgesics and non-steroidal anti-inflammatory drugs. Lancet 1991, 337, 85–89. [Google Scholar]
- Shichinohe, H.; Kuroda, S.; Yasuda, H.; Ishikawa, T.; Iwai, M.; Horiuchi, M.; Iwasaki, Y. Neuroprotective effects of the free radical scavenger Edaravone (MCI-186) in mice permanent focal brain ischemia. Brain Res. 2004, 1029, 200–206. [Google Scholar] [CrossRef]
- Toyoda, K.; Fujii, K.; Kamouchi, M.; Nakane, H.; Arihiro, S.; Okada, Y.; Ibayashi, S.; Iida, M. Free radical scavenger, edaravone, in stroke with internal carotid artery occlusion. J. Neurol. Sci. 2004, 221, 11–17. [Google Scholar] [CrossRef]
- Kokura, S.; Yoshida, N.; Sakamoto, N.; Ishikawa, T.; Takagi, T.; Higashihara, H.; Nakabe, N.; Handa, O.; Naito, Y.; Yoshikawa, T. The radical scavenger edaravone enhances the anti-tumor effects of CPT-11 in murine colon cancer by increasing apoptosis via inhibition of NF-κB. Cancer Lett. 2005, 229, 223–233. [Google Scholar] [CrossRef]
- Sueishi, K.; Mishima, K.; Makino, K.; Itoh, Y.; Tsuruya, K.; Hirakata, H.; Oishi, R. Protection by a radical scavenger edaravone against cisplatin-induced nephrotoxicity in rats. Eur. J. Pharmacol. 2002, 451, 203–208. [Google Scholar] [CrossRef]
- Iguchi, T.; Nishikawa, M.; Chang, B.J.; Muroya, O.; sato, E.F.; Nakatani, T.; Inoue, M. Edaravone inhibits acute renal injury and cyst formation in cisplatin-treated rat kidney. Free Radic. Res. 2004, 38, 333–341. [Google Scholar] [CrossRef]
- Hopkins, A.L.; Groom, C.R. The druggable genome. Nat. Rev. Drug Discov. 2002, 1, 727–730. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Method. 2000, 44, 235–249. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liang, C.; Jiang, H.; Zhou, Z.; Lei, D.; Xue, Y.; Yao, Q. Ultrasound-Promoted Greener Synthesis of Novel Trifurcate 3-Substituted-chroman-2,4-dione Derivatives and Their Drug-Likeness Evaluation. Molecules 2012, 17, 14146-14158. https://doi.org/10.3390/molecules171214146
Liang C, Jiang H, Zhou Z, Lei D, Xue Y, Yao Q. Ultrasound-Promoted Greener Synthesis of Novel Trifurcate 3-Substituted-chroman-2,4-dione Derivatives and Their Drug-Likeness Evaluation. Molecules. 2012; 17(12):14146-14158. https://doi.org/10.3390/molecules171214146
Chicago/Turabian StyleLiang, Chengyuan, Hailong Jiang, Zhiguang Zhou, Dong Lei, Yu Xue, and Qizheng Yao. 2012. "Ultrasound-Promoted Greener Synthesis of Novel Trifurcate 3-Substituted-chroman-2,4-dione Derivatives and Their Drug-Likeness Evaluation" Molecules 17, no. 12: 14146-14158. https://doi.org/10.3390/molecules171214146






