Inhibition of PCAF Histone Acetyltransferase, Cytotoxicity and Cell Permeability of 2-Acylamino-1-(3- or 4-Carboxy-phenyl)benzamides
Abstract
:1. Introduction
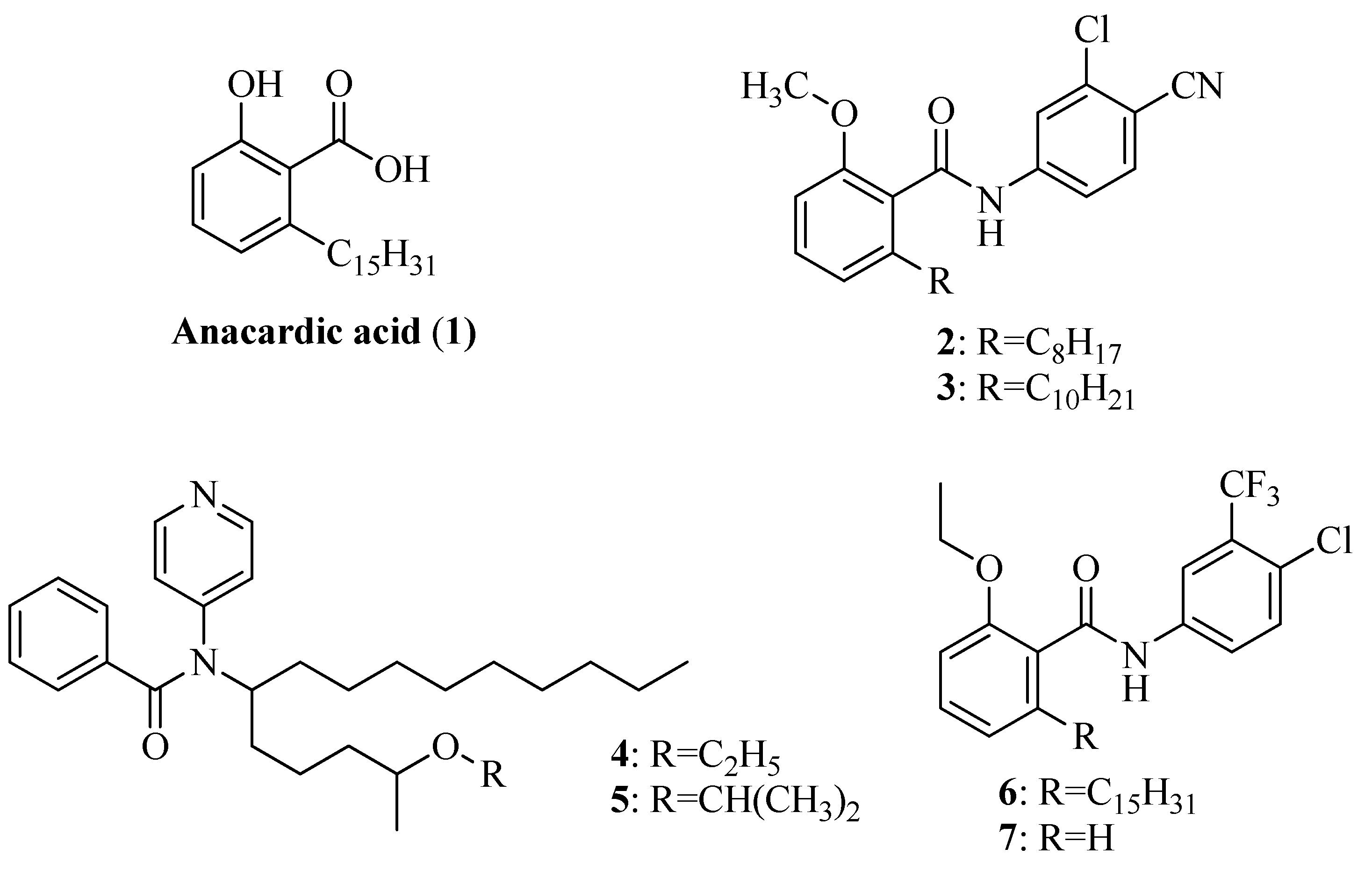
2. Results and Discussion
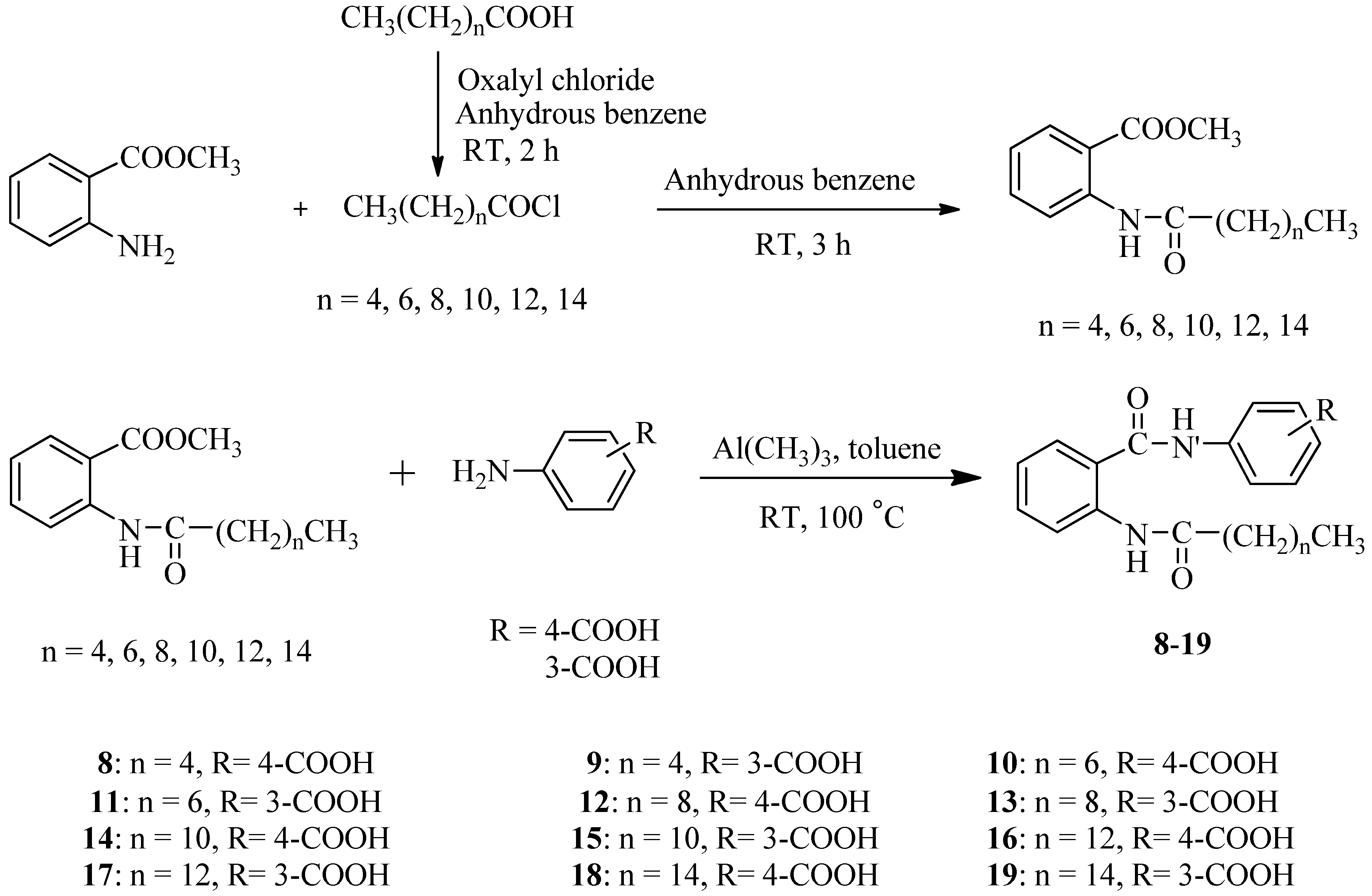
2.1. Synthesis
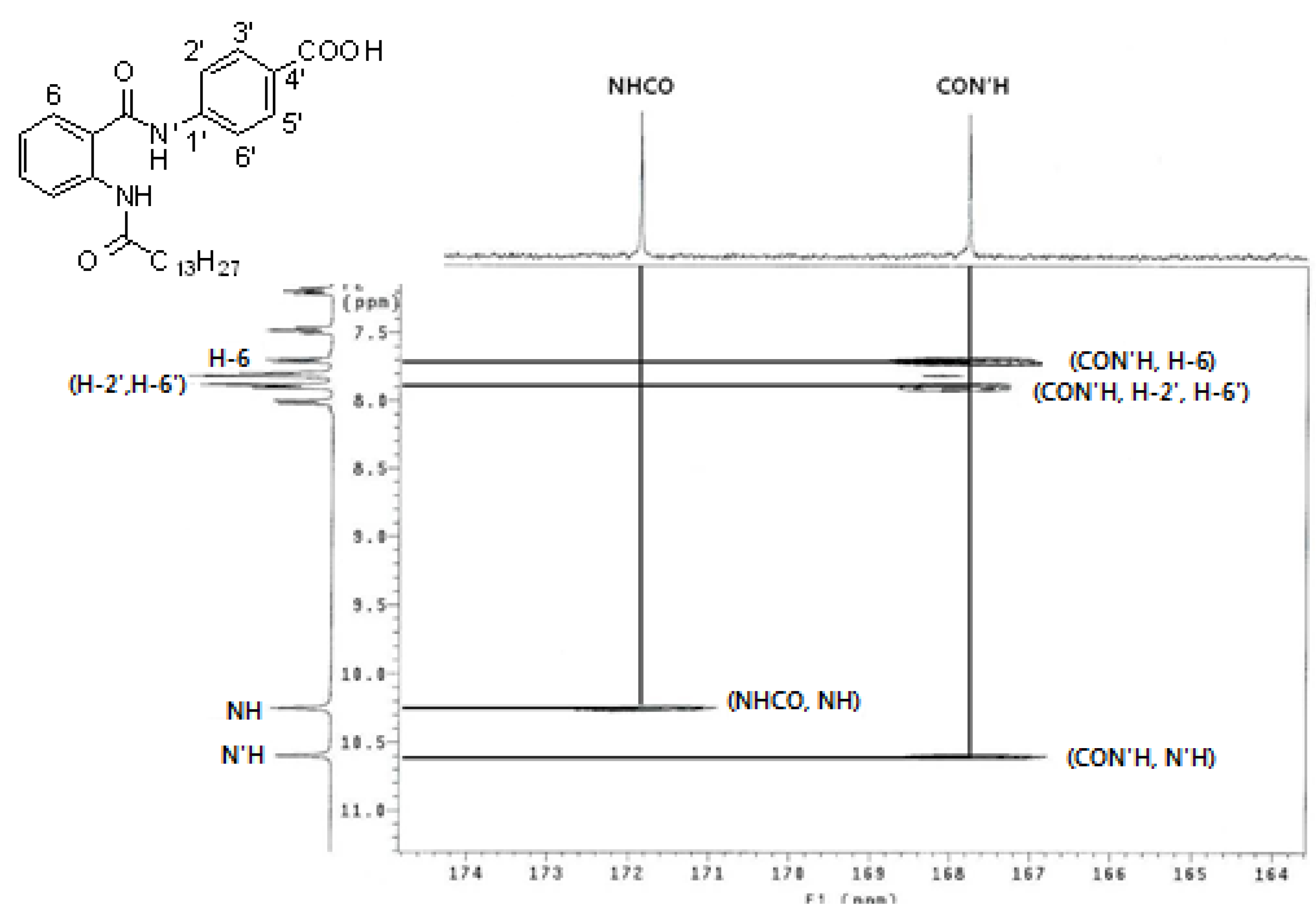
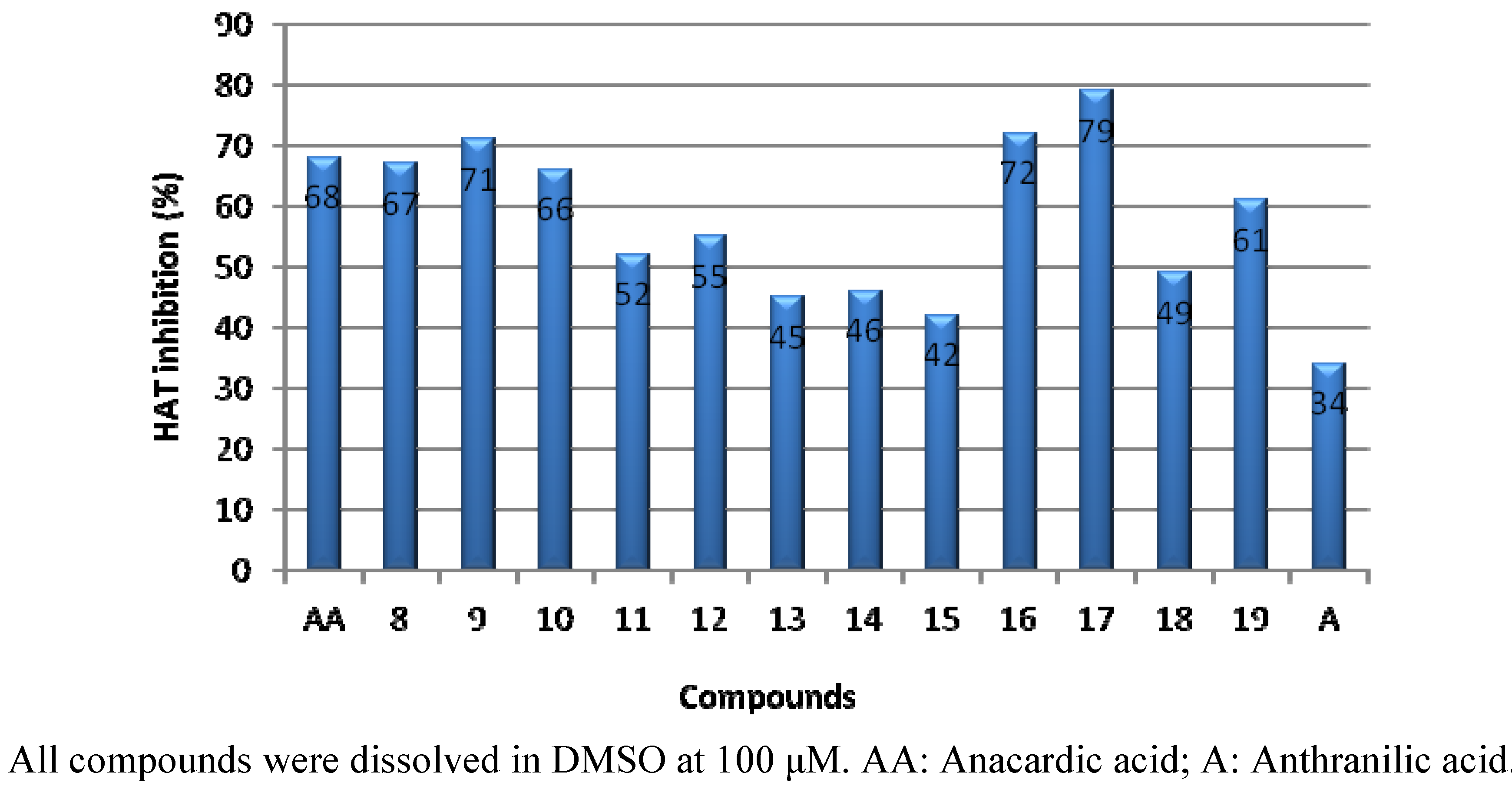
2.2. PCAF HAT Inhibition Assay
2.3. Cytotoxic Activity by SRB Assay
| HT-29 | HCT-116 | MDA-231 | A549 | Hep3B | HeLa | Caki | HSF | |
|---|---|---|---|---|---|---|---|---|
| 8 | >100 | >100 | >100 | >100 | >100 | NT | >100 | >100 |
| 9 | >100 | >100 | >100 | >100 | >100 | NT | >100 | >100 |
| 10 | >100 | >100 | 51.83 ± 0.68 | >100 | >100 | >100 | 69.49 ± 7.16 | >100 |
| 11 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 12 | >100 | >100 | >100 | >100 | >100 | >100 | 63.75 ± 2.39 | >100 |
| 13 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 14 | >100 | 53.30 ± 2.05 | >100 | >100 | >100 | >100 | >100 | >100 |
| 15 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| 16 | 54.79 ± 1.05 | 43.90 ± 2.39 | 79.11 ± 3.27 | 46.18 ±0.83 | 73.30 ± 9.61 | 56.53 ± 0.87 | 63.08 ± 0.21 | >100 |
| 17 | 54.90 ± 3.49 | 29.17 ± 3.86 | 62.43 ± 0.58 | 32.09 ±1.36 | 57.52 ± 0.26 | 54.97 ± 3.14 | 55.88 ± 5.09 | >100 |
| 18 | 35.49 ± 2.45 | 27.56 ± 0.28 | >100 | 30.69 ± 2.41 | 39.10 ± 6.52 | 34.41 ± 2.19 | 50.29 ± 1.49 | >100 |
| 19 | 44.76 ± 2.98 | 40.27 ± 4.30 | >100 | 40.52 ± 3.59 | 43.82 ± 5.91 | 36.92 ± 3.76 | 82.62 ± 5.89 | >100 |
| A | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| AA | 60.42 ± 6.36 | 96.31 ± 4.33 | 44.32 ± 4.20 | 70.76 ± 0.41 | >100 | NT | >100 | >100 |
2.4. Caco-2 Cell Permeabilty

| Initial Concentration (μM) | Measured Papp (×10−6 cm/s) | |
|---|---|---|
| 16 | 50 | 68.21 ± 3.56 |
| 17 | 50 | 71.48 ± 4.67 |
3. Experimental
3.1. General
3.2. General Synthetic Methods
3.3. PCAF HAT Inhibition Assay

3.4. SRB Assay
3.4.1. Cells
3.4.2. Cell Inoculation
3.4.3. Fixation
3.4.4. SRB Assay Protocol
3.5. Caco-2 Cell Permeability Test
3.5.1. Caco-2 Cell Culture
3.5.2. Caco-2 Cell Permeability
4. Conclusions
- Sample Availability: Samples of 8–19 are available from the authors.
References
- Biel, M.; Wascholowski, V.; Giannis, A. Epigenetics-an epicenter of gene regulation: Histones and histone-modifying enzymes. Angew. Chem. Int. Ed. Engl. 2005, 44, 3186–3216. [Google Scholar] [CrossRef]
- Feinberg, A.P.; Vogelstein, B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature 1983, 301, 89–92. [Google Scholar] [CrossRef]
- Nakao, M. Epigenetics: Interaction of DNA methylation and chromatin. Gene 2001, 278, 25–31. [Google Scholar] [CrossRef]
- Hake, S.B.; Xiao, A.; Allis, C.D. Linking the epigenetic “language” of covalent histone modification to cancer. Br. J. Cancer 2004, 90, 761–769. [Google Scholar] [CrossRef]
- Inche, A.G.; La thangue, N.B. Chromatin control and cancer-drug discovery: Realizing the promise. Drug Discov. Today 2006, 11, 97–109. [Google Scholar] [CrossRef]
- Davis, C.D.; Ross, S.A. Dietary components impact histone modifications and cancer risk. Nutr. Rev. 2007, 65, 88–94. [Google Scholar] [CrossRef]
- Ghizzoni, M.; Boltjes, A.; de Graaf, C.; Haisma, H.J.; Dekker, F.J. Improved inhibition of the histone acetyl transferase PCAF by an anacardic acid derivative. Bioorg. Med. Chem. 2010, 18, 5826–5834. [Google Scholar]
- Carrozza, M.J.; Utley, R.T.; Workman, J.L.; Cote, J. The diverse functions of histone acetyl transferase complexes. Trends Genet. 2003, 19, 321–329. [Google Scholar] [CrossRef]
- Sterner, D.V.; Berger, S.L. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 2000, 64, 435–459. [Google Scholar] [CrossRef]
- Dekker, F.J.; Haisma, H.J. Histone acetyl transferases as emerging drug targets. Drug Discov. Today 2009, 14, 942–948. [Google Scholar] [CrossRef]
- Lau, O.D.; Kundu, T.K.; Soccio, R.E. HATs off: Selective synthetic inhibitors of the histone acetyltransferase p300 and PCAF. Mol. Cell 2000, 5, 589–595. [Google Scholar] [CrossRef]
- Balasubramanyam, K.; Altaf, M.; Varier, R.A.V.; Swaminathan, V.; Ravindran, A.; Sadhale, P.P.; Kundu, T.K. Polyisoprenylated benzophenone, Garcinol, A natural histone acetyltransferase inhibitor, Represses chromatin transcription and alters global gene expression. J. Biol. Chem. 2004, 279, 33716–33726. [Google Scholar]
- Balasubramanyam, K.; Varier, R.A.; Altaf, M.V.; Swaminathan, V.; Siddappa, N.B.; Ranga, U.; Kundu, T.K. Curcumin, A novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, Represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J. Biol. Chem. 2004, 279, 51163–51171. [Google Scholar]
- Stimson, L.; Rowlands, M.G.; Newbatt, Y.M.; Smith, N.F.; Raynaud, F.I.; Rogers, P.; Bavetsias, V.; Gorsuch, S.; Jarman, M.; Bannister, A.; et al. Isothiazolones as inhibitors of PCAF and p300 histone acetyltransferase activity. Mol. Cancer Ther. 2005, 4, 1521–1532. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Santhosh, M.S.; Kemparaju, K.; Girish, K.S. Emerging roles of anacardic acid and its derivatives: A pharmacological overview. Basic Clin. Pharmacol. Toxcol. 2012, 110, 122–132. [Google Scholar] [CrossRef]
- Eliseeva, E.; Valkov, V.; Jung, M.; Jung, M.O. Characterization of novel inhibitors of histone acetyltransferases. Mol. Cancer Ther. 2007, 6, 2391–2398. [Google Scholar]
- Souto, J.A.; Conte, M.; Alvarez, R.; Nebbioso, A.; Carafa, V.; Altucci, L.; Lera, A.R. Synthesis of benzamides related to anacardic acid and their histone acetyltransferase (HAT) inhibitory activities. ChemMedChem 2008, 3, 1435–1442. [Google Scholar]
- Chandregowda, V.; Kush, A.; Reddy, G.C. Synthesis of benzamide derivatives of anacardic acid and their cytotoxic activity. Eur. J. Med. Chem. 2009, 44, 2711–2719. [Google Scholar] [CrossRef]
- Mantelingu, K.; Kishore, A.H.; Balasubramanyam, K.; Kumar, G.V.; Altaf, M.; Swamy, S.N.; Selvi, R.; Das, C.; Narayana, C.; Rangappa, K.S.; et al. Activation of p300 histone acetyltransferase by small molecules altering enzyme structure: Probed by surface-enhanced Raman spectroscopy. J. Phys. Chem. B 2007, 111, 4527–4534. [Google Scholar]
- Sbardella, G.; Castellano, S.; Vicidomini, C.; Rotili, D.; Nebbioso, A.; Miceli, M.; Aitucci, L.; Mai, A. Identification of long chain alkylidenemalonates as novel small molecule modulators of histone acetyl transferases. Bioorg. Med. Chem. Lett. 2008, 18, 2788–2792. [Google Scholar]
- Green, I.R.; Tocoli, F.E.; Lee, S.H.; Nihei, K.; Kubo, L. Molecular design of anti-MRSA agents based on the anacardic acid scaffold. Bioorg. Med. Chem. 2007, 15, 6236. [Google Scholar] [CrossRef]
- Hird, N.W.; Milner, P.H. Synthetic and β-lactamase inhibition of anacardic acids and their analogues. Bioorg. Med. Chem. Lett. 1994, 4, 1423–1426. [Google Scholar]
- Yamagiwa, Y.; Ohashi, K.; Sakamota, Y.; Hirakawa, S.; Kamikawa, T.; Kubo, I. Syntheses of anacardic acids and ginkgoic acid. Tetrahedron 1987, 43, 3387–3394. [Google Scholar] [CrossRef]
- Levin, J.I.; Turos, E.; Weinreb, S.M. An alternative procedure for the aluminium-medated conversion of esters to amides. Syn. Commun. 1982, 12, 989–993. [Google Scholar] [CrossRef]
- Gustafsson, T.; Ponten, F.; Seeberger, P.H. Trimethylaluminum mediated amide bond formationin a continuous flow microreactor as key to the synthesis of rimonabant and efaproxiral. Chem. Commun. 2008, 9, 1100–1102. [Google Scholar]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinalbarrier: Influence of cell and culture-related factors on Caco-2 cellfunctional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- Azenha, M.A.; Evangelista, R.; Martel, F.; Vasconcelos, M.T. Estimation of the human intestinal permeability of butyltin speciesusing the Caco-2 cell line model. Food Chem. Toxicol. 2004, 42, 1431–1442. [Google Scholar] [CrossRef]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 1996, 22, 67–84. [Google Scholar] [CrossRef]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef]
- Dekker, F.J.; Ghizzoni, M.; van der Meer, N.; Wisastra, R.; Haisma, H.J. Inhibition of the PCAF histone acetyltransferase and cell proliferation by isothiazolones. Bioorg. Med. Chem. 2009, 17, 460–466. [Google Scholar]
- Trievel, R.C.; Li, F.Y.; Marmorstein, R. Application of a fluorescent histone acetyltransferase assay to probe the substrate specificity of human p300/CBP-associated factor. Anal. Biochem. 2000, 287, 319–328. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bodesh, H.; Kenny, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Park, W.J.; Ma, E. Inhibition of PCAF Histone Acetyltransferase, Cytotoxicity and Cell Permeability of 2-Acylamino-1-(3- or 4-Carboxy-phenyl)benzamides. Molecules 2012, 17, 13116-13131. https://doi.org/10.3390/molecules171113116
Park WJ, Ma E. Inhibition of PCAF Histone Acetyltransferase, Cytotoxicity and Cell Permeability of 2-Acylamino-1-(3- or 4-Carboxy-phenyl)benzamides. Molecules. 2012; 17(11):13116-13131. https://doi.org/10.3390/molecules171113116
Chicago/Turabian StylePark, Woong Jae, and Eunsook Ma. 2012. "Inhibition of PCAF Histone Acetyltransferase, Cytotoxicity and Cell Permeability of 2-Acylamino-1-(3- or 4-Carboxy-phenyl)benzamides" Molecules 17, no. 11: 13116-13131. https://doi.org/10.3390/molecules171113116
APA StylePark, W. J., & Ma, E. (2012). Inhibition of PCAF Histone Acetyltransferase, Cytotoxicity and Cell Permeability of 2-Acylamino-1-(3- or 4-Carboxy-phenyl)benzamides. Molecules, 17(11), 13116-13131. https://doi.org/10.3390/molecules171113116




