Secondary Metabolites from Two Species of Tolpis and Their Biological Activities
Abstract
:1. Introduction

2. Results and Discussion
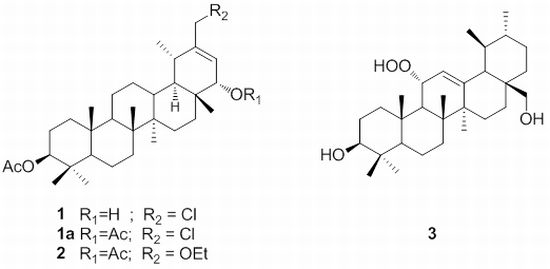
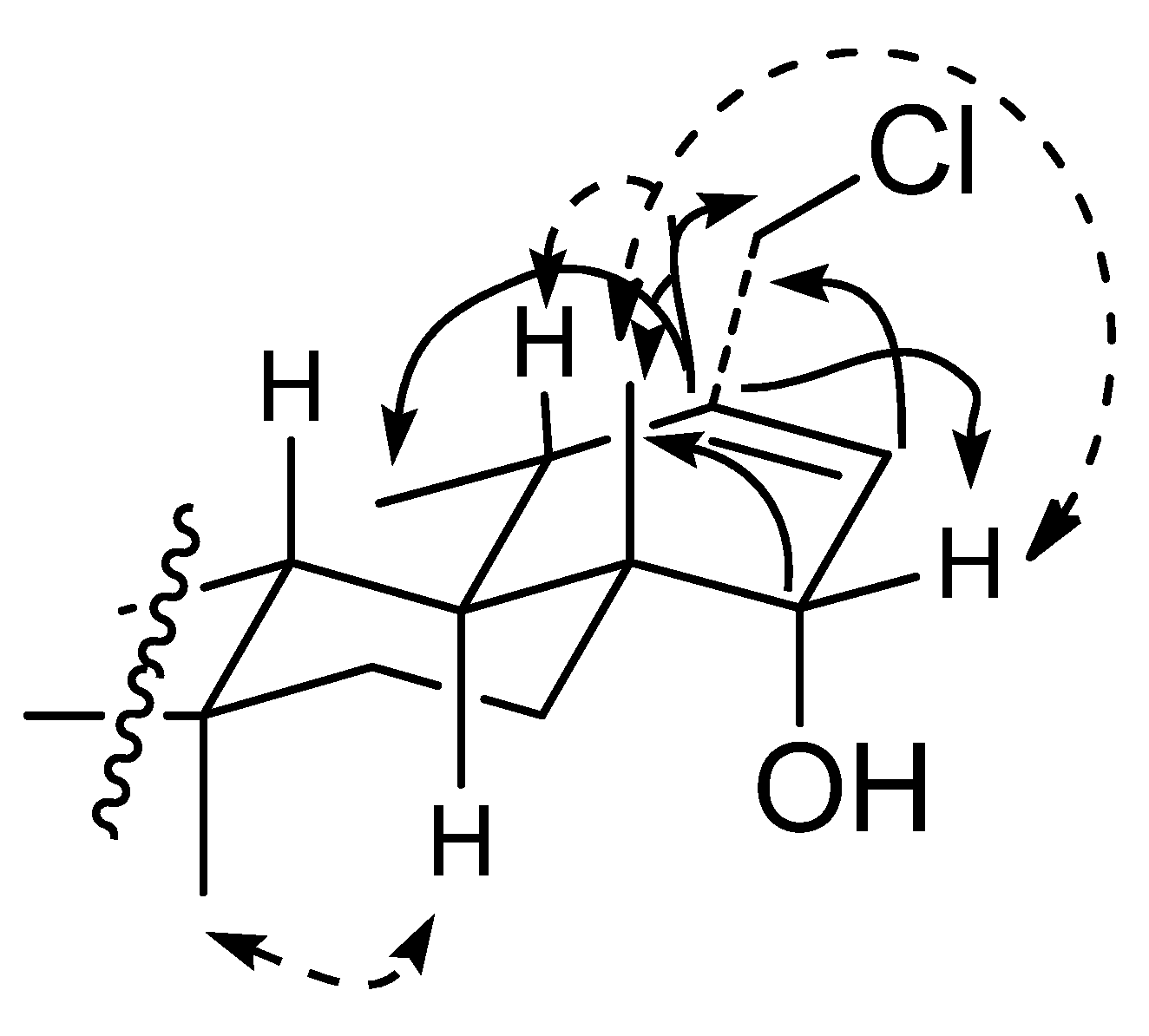
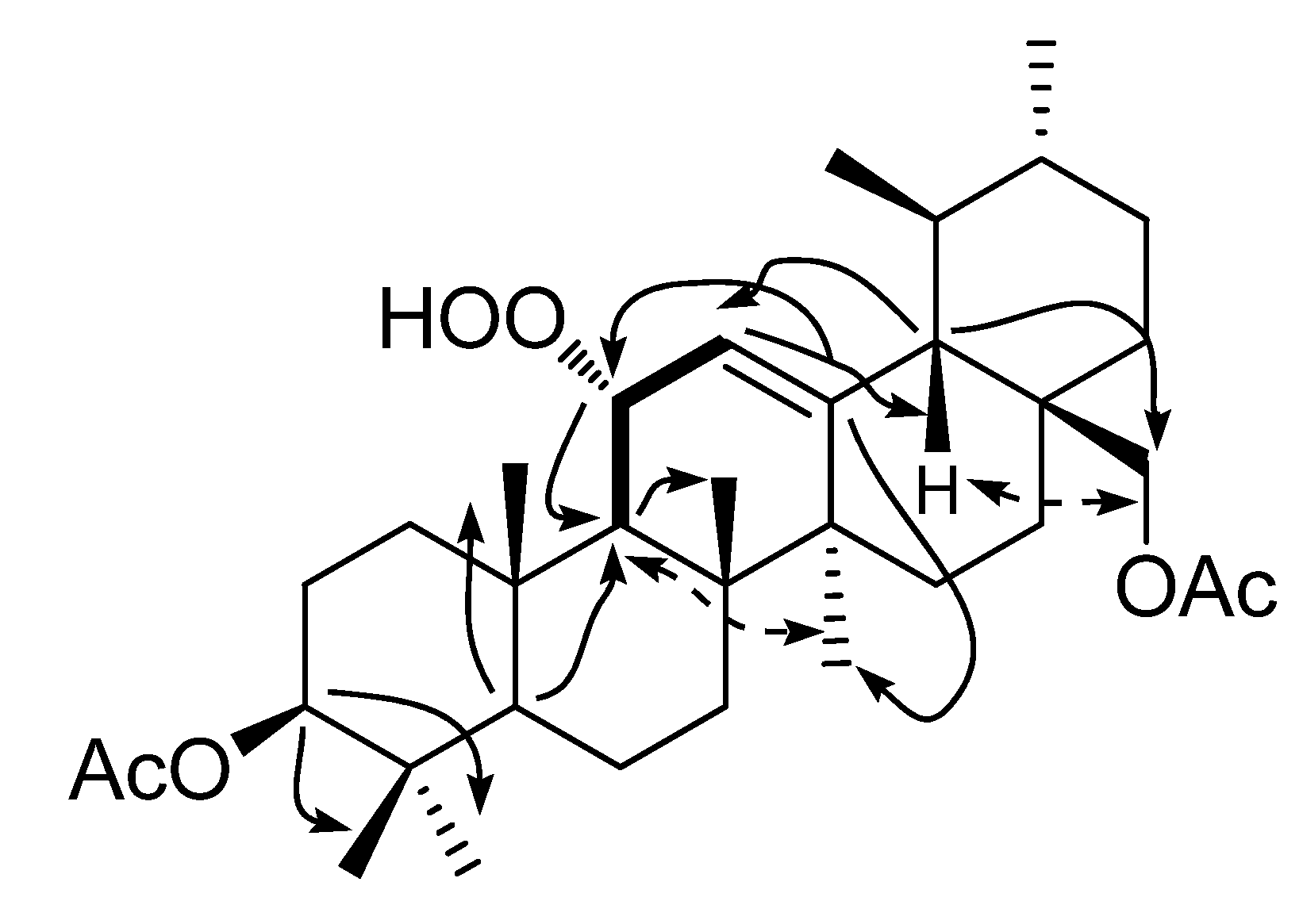
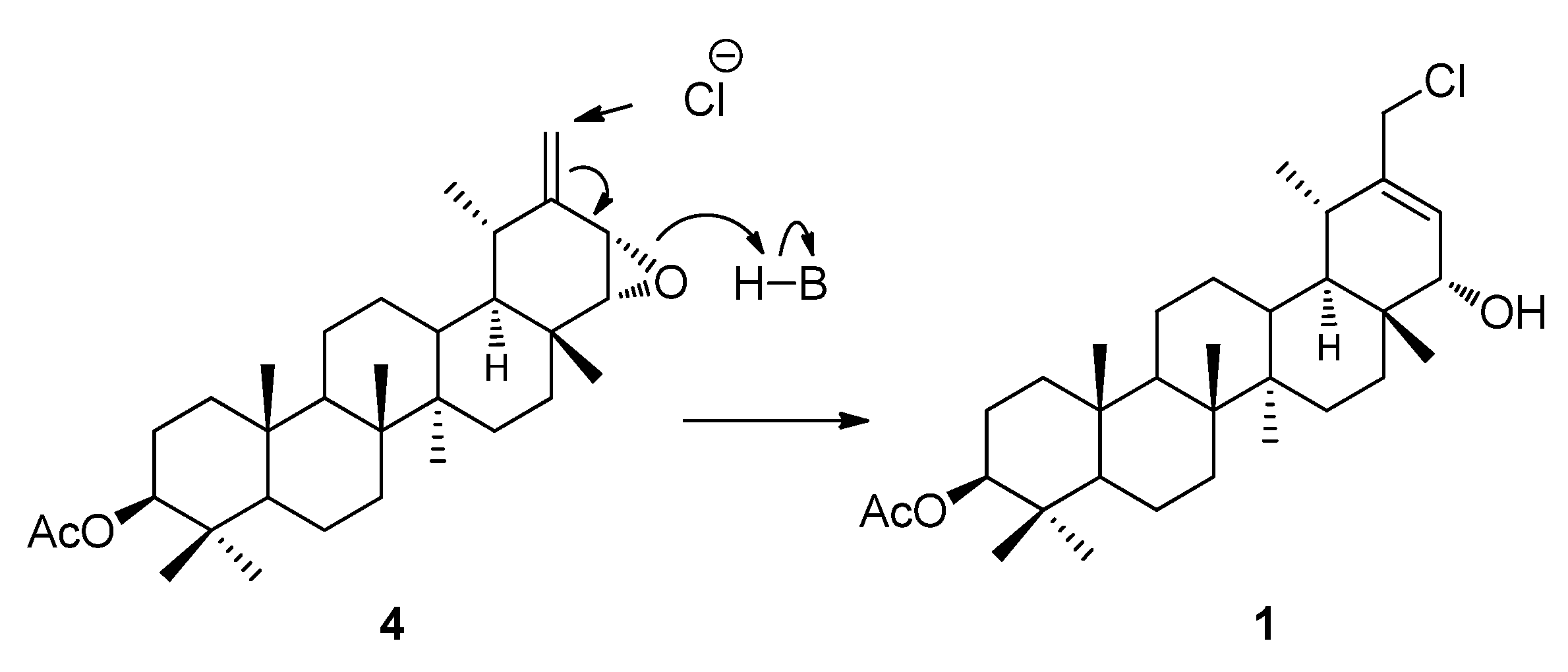
2.1. Structure Elucidation of Compounds 1–3
| 1 | 1a | 2 | ||||
|---|---|---|---|---|---|---|
| Position | δH | δC | δH | δC | δH | δC |
| 1 | 1.65 * | 38.5 | 1.65 * | 38.7 | 1.60 * | 38.5 |
| 2 | 1.50 * | 23.7 | 1.54 * | 21.7 | 1.55 * | 23.7 |
| 3 | 4.41 dd (6.1,10.8) | 81.0 | 4.43 dd (6.4,10.0) | 81.1 | 4.43 dd (5.1 10.4) | 81.0 |
| 4 | - | 38.3 | - | 38.0 | - | 37.8 |
| 5 | 0.75 * | 55.4 | 0.74 * | 55.6 | 0.75 * | 55.4 |
| 6 | 1.45 * | 18.2 | 1.44 * | 18.0 | 1.30 * | 18.2 |
| 1.35 * | 1.34 * | |||||
| 7 | 1.35 * | 34.2 | 1.34 * | 34.4 | 1.35 * | 34.2 |
| 8 | - | 41.2 | - | 41.3 | - | 41.1 |
| 9 | 1.25 * | 50.3 | 1.26 * | 50.6 | 1.25 * | 50.4 |
| 10 | - | 37.1 | - | 37.3 | - | 37.1 |
| 11 | 1.20 * | 21.6 | 1.21 * | 21.4 | 1.20 * | 21.6 |
| 12 | 1.60 * | 27.6 | 1.60 * | 27.7 | 1.58 * | 27.5 |
| 1.20 * | ||||||
| 13 | 0.95 * | 38.6 | 0.95 * | 1.66 * | 38.6 | |
| 14 | - | 42.3 | - | 42.4 | - | 42.3 |
| 15 | 1.72 * | 26.7 | 1.71 * | 29.9 | 1.50 * | 26.6 |
| 1.05ddd (2.5,4.0,13.05) | 1.02 * | |||||
| 16 | 0.95 * | 29.7 | 0.95 * | 29.9 | 1.60 * | 29.9 |
| 1.85 dt (9.0,13.0) | ||||||
| 17 | - | 37.8 | - | 37.4 | - | 37.2 |
| 18 | 1.45 * | 40.5 | 1.44 * | 41.5 | 1.45 * | 41.5 |
| 19 | 2.00 t (7.0) | 31.6 | 2.03 tbr (7.0) | 31.7 | 1.79 q (6.6) | 32.3 |
| 20 | - | 144.9 | - | 146.5 | - | 147.7 |
| 21 | 5.89 d (6.5) | 126.3 | 5.87 d (6.4) | 123.1 | 5.75 d (6.3) | 119.2 |
| 22 | 3.35 dbr (6.6) | 73.3 | 4.51 d (6.4) | 75.3 | 4.55 d (6.3) | 75.3 |
| 23 | 0.78 s | 28.0 | 0.79 s | 28.1 | 0.81 s | 28.0 |
| 24 | 0.77 s | 16.6 | 0.78 s | 16.7 | 0.80 s | 16.5 |
| 25 | 0.82 s | 16.4 | 0.82 s | 16.7 | 0.81 s | 16.4 |
| 26 | 0.99 s | 16.1 | 0.98 s | 16.2 | 0.96 s | 16.1 |
| 27 | 0.93 s | 14.7 | 0.91 s | 14.7 | 0.92 s | 14.6 |
| 28 | 0.63 s | 17.8 | 0.70 s | 17.9 | 0.70 s | 18.2 |
| 29 | 0.99 d (6.5) | 22.2 | 0.97 d (7.6) | 22.1 | 0.96 d (6.6) | 22.6 |
| 30 | 4.15 d (11.2) | 47.6 | 4.14 d (11.2) | 47.5 | 3.96 d (12.6) | 72.3 |
| 3.89 d (11.2) | 3.89 d (11.6) | 3.72 d (12.6) | ||||
| OH | 3.14 m | |||||
| OAc | 1.97 s | 21.4 | 1.97 s | 21.3 | 1.97 s | 21.3 |
| 171.4 | 1.98 s | 21.3 | 1.98 s | 21.3 | ||
| 171.1 | 171.1 | |||||
| 171.1 | 171.1 | |||||
| OEt | 1.13 t (6.9) | 15.2 | ||||
| 3.35 m | 65.9 | |||||
| Position | δH | δC |
|---|---|---|
| 1 | 0.85 m | 39.4 |
| 2.08 dt (3.5, 7.0) | ||
| 2 | 1.58 * | 23.7 |
| 3 | 4.45 dd (3.0,9.6) | 80.6 |
| 4 | - | 38.0 |
| 5 | 0.88 m | 55.3 |
| 6 | 1.45 * | 18.1 |
| 1.31 * | ||
| 7 | 1.45 m | 33.3 |
| 1.25 m | ||
| 8 | - | 43.2 |
| 9 | 1.81 d (9.5) | 48.8 |
| 10 | - | 37.8 |
| 11 | 4.46 dd (5.0, 9.5) | 81.6 |
| 12 | 5.30 d (3.1) | 125.8 |
| 13 | - | 144.5 |
| 14 | - | 42.0 |
| 15 | 1.60 * | 26.2 |
| 0.90 m | ||
| 16 | 1.16 * | 23.3 |
| 1.92 td (3.5,9.0) | ||
| 17 | - | 36.9 |
| 18 | 1.45 m | 53.7 |
| 19 | 1.35 m | 39.0 |
| 20 | 1.28 m | 39.3 |
| 21 | 1.40 m | 30.4 |
| 22 | 1.31 m1.52 dt (3.0, 6.5) | 35.5 |
| 23 | 0.81 s | 28.2 |
| 24 | 0.81 s | 16.7 |
| 25 | 1.02 s | 16.8 |
| 26 | 1.00 s | 18.0 |
| 27 | 1.11 s | 22.2 |
| 28 | 3.56 d (11.0) | 71.0 |
| 3.93 d (11.0) | ||
| 29 | 0.86 d (6.4) | 17.4 |
| 30 | 0.88 d (7.3) | 21.3 |
| OAc | 21.0 | |
| 1.97 s | 21.3 | |
| 1.97 s | 171.0 | |
| 171.3 |
2.2. Antioxidant Activities
| Assays | T. proustii | T. lagopoda |
|---|---|---|
| RSA a | 59.6 ± 0.4 | 41.4 ± 0.1 |
| FRAP b | 18.1 ± 0.4 | 4.1 ± 0.2 |
| FRAP c | 93 ± 2 | 41 ± 1 |
| Assays | Aesculetin | α-Tocopherol | BHA |
|---|---|---|---|
| 0.1 mg mL−1 | 0.1 mg mL−1 | 0.1 mg mL−1 | |
| DPPH a | 100 ± 0 | 17.7 ± 0.1 | 21.9 ± 0.6 |
| FRAP b | 9.4 ± 0.7 | 0.97 ± 0.03 | 3.13 ± 0.05 |
2.3. Cytotoxic Activity
| Compound | IC50 (μM) | |
|---|---|---|
| K562 | K562/ADR | |
| Ursolic acid | 40.6 ± 3.6 | 49.2 ± 3.1 |
| Ursolic acid methyl ester | 59.3 ± 15.5 | 64.0 ± 14.5 |
| Acetyl ursolic acid | 99.5 ± 20.5 | >100 |
| Acetyl ursolic acid methyl ester | >100 | >100 |
| Aesculetin | 63.2 ± 3.2 | 77.0 ± 5.1 |
| Aesculetin acetyl | 68.6 ± 17.1 | 70.3 ± 19.2 |
| Aesculetin diacetyl | 62.3 ± 6.6 | 59.5 ± 4.5 |
| 11-Oxo-β-amyrin | >100 | >100 |
| 22-Oxo-20-taraxasten-3β-ol | 30.0 ± 10.0 | 43.0 ± 7.0 |
| 1,2-Diacetoxyphytene | >100 | >100 |
3. Experimental
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Antioxidant Experiments
3.4.1. Chemicals
3.4.2. Preparation of Extracts for Antioxidant Assays
3.4.3. Free Radical Scavenging Activity on DPPH
3.4.4. Ferric Reducing Antioxidant Power Assay (FRAP)
3.5. Cytotoxic Experiments
3.5.1. Cell Culture
3.5.2. Assay for Growth Inhibition and Cell Viability
4. Conclusions
Acknowledgments
References
- Kunkel, G.; Beltrán, E.; Bañares, A.J.; Gil, M.C.; González, M.N.; González, J.M.; Haroun, R.J.; Hernández, C.; Losada, A.; Marrero, A.; Rodríguez, J.L.; Rodrigo, J. Flora del Archipiélago Canario (in Spanish). In Tratado florístico, primera parte; Editora Regional Canaria: Las Palmas de Gran Canaria, Spain, 1992. [Google Scholar]
- Archibald, J.K.; Crawford, D.J.; Santos-Guerra, A.; Mort, M.E. The utility of automated analysis of inter-simple sequence repeat (ISSR) loci for resolving relationships in the Canary Islands species of Tolpis (Asteraceae). Am. J. Bot. 2006, 93, 1154–1162. [Google Scholar] [CrossRef]
- Crawford, D.J.; Archibald, J.K.; Santos-Guerra, A.; Mort, M.E. Allozyme diversity within and divergente among species of Tolpis (Asteraceae Lactuceae) in the Canary Islands: Systematic, Evolutionary and Biogeographical implications. Am. J. Bot. 2006, 93, 656–664. [Google Scholar] [CrossRef]
- Triana, J.; López, M.; Pérez, F.J.; González-Platas, J.; Estévez, F.; León, F.; Hernández, J.C.; Brouard, I.; Bermejo, J. Chemical constituenst of Tolpis species. Fitoterapia 2009, 80, 437–441. [Google Scholar] [CrossRef]
- Southon, S. Increased fruit and vegetable consumption within the EU: Potential health benefits. Food Res. Int. 2000, 33, 211–217. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Chiang, Y.M.; Kuo, Y.H. Taraxastane type triterpenes from the aerial roots of Ficus microcarpa. J. Nat. Prod. 2000, 63, 898–901. [Google Scholar] [CrossRef]
- Menichini, F.; Di-Benedetto, R.; Delle-Monache, F. A triterpene epoxide and a guainolide from Ptilostemmon gnaphaloides. Phytochemistry 1996, 41, 1377–1379. [Google Scholar]
- Kuo, Y.H.; Chaiang, Y.M. Five new taraxastane-type triterpenes from the aerial roots of Ficus microcarpa. Chem. Pharm. Bull. 1999, 47, 498–500. [Google Scholar] [CrossRef]
- Gribble, G.W. Naturally Occurring Organohalogen Compounds—A Comprehensive Update, Progress in the Chemistry Of Organic Natural Products; Springer-Verlag: Wien, Austria, 2010; p. 577. [Google Scholar]
- Monte, F.J.Q.; Kintzinger, J.P.; Braz-Filho, R. Total assignment of 1H and 13C spectra of the chlorinated triterpenoid (Methyl 2α,3β,24-Tri-O-acetylolean-12α-chloro-28,13β-olide) by NMR spectroscopy. Magn. Reson. Chem. 1998, 36, 381–384. [Google Scholar]
- Luo, X.D.; Wu, S.H.; Ma, Y.B.; Wu, D.G. Dammarane triterpenoids from Amoora yunnanensis. Heterocycles 2000, 53, 2795–2802. [Google Scholar] [CrossRef]
- Xu, Y.X.; Xiang, Z.B.; Jin, Y.S.; Shen, Y.; Chen, H.S. Two new triterpenoids from the roots of Actinidia chinensis. Fitoterapia 2010, 81, 920–924. [Google Scholar] [CrossRef]
- Chen, S.N.; Lankin, D.C.; Nikolic, D.; Fabricant, D.S.; Lu, Z.Z.; Ramirez, B.; van Breemen, R.B.; Fong, H.H.S.; Farnsworth, N.R.; Pauli, G.F. Chlorination diversifies Cimicifuga racemosa triterpene glycoside. J. Nat. Prod. 2007, 70, 1016–1023. [Google Scholar] [CrossRef]
- Thuong, P.T.; Hung, T.M.; Ngoc, T.M.; Ha, D.T.; Min, B.S.; Kwack, S.J.; Kang, T.S.; Choi, J.S.; Bae, K. Antioxidant activities of coumarins from Korean medicinal plants and their structure-activity relationships. Phytother. Res. 2010, 24, 101–106. [Google Scholar] [CrossRef]
- Van-Loo, P.; De-Bruyn, A.; Budesinsky, M. Reinvestigation of the structural assignment of signals in the proton and carbon NMR spectra of the flavone apigenin. Magn. Reson. Chem. 1986, 24, 879–882. [Google Scholar]
- Brown, G.D.; Liang, G.Y.; Sy, L.K. Terpenoids from the seeds of Artemisia annua. Phytochemistry 2003, 64, 303–323. [Google Scholar]
- Kojima, H.; Sato, N.; Hatano, A.; Ogura, H. Constituents of the labiatae plants. Part 5. Sterol glucosides from Prunella vulgaris. Phytochemistry 1990, 29, 2351–2355. [Google Scholar]
- González, A.G.; Bermejo, J.; Rodríguez, E.M.; Hernández, C.E. Chemical constituents of the lichen Ramalina hierrensis. Planta Med. 1992, 58, 214–218. [Google Scholar] [CrossRef]
- Seebacher, W.; Simic, N.; Weis, R.; Saf, R.; Kunert, O. Complete assignments of 1H and 13C-NMR resonances of oleanolic acid, 18α-oleanolic acid, ursolic acid and their 11-oxo derivatives. Magn. Reson. Chem. 2003, 41, 636–638. [Google Scholar]
- Burns, D.; Reynolds, W.F.; Buchanan, G.; Reese, P.B.; Enriquez, R.G. Assignment of 1H and 13C spectra and investigation of hindered side-chain rotation in lupeol derivatives. Magn. Reson. Chem. 2000, 38, 488–493. [Google Scholar]
- Petrovic, S.D.; Gorunovic, M.S.; Wray, V.; Merfort, I. A taraxasterol derivative and phenolic compounds from Hieracium gymnocephalum. Phytochemistry 1999, 50, 293–296. [Google Scholar]
- Kapoor, V.K.; Chawla, A.S.; Gupta, Y.C.; Passannanti, S.; Paternostro, M.P. Constituents of Buddleia species leaves. Fitoterapia 1981, 52, 235–237. [Google Scholar]
- ksuz, S.; Ulubelen, A.; Barla, A.; Kohlbau, H.J.; Voelter, W. Triterpenoids and a Diterpene from Euphorbia iberica. Planta Med. 1999, 65, 475–477. [Google Scholar] [CrossRef]
- Chiang, Y.M.; Chang, J.Y.; Kuo, C.C.; Chang, C.Y.; Kuo, Y.H. Cytotoxic triterpenes from the aerial roots of Ficus microcarpa. Phytochemistry 2005, 66, 495–501. [Google Scholar]
- Boiteau, P.; Pasich, B.; Ratsimamanga, A.R. Les triterpenoides en physiologie vegetale et animale (in French); Gautier-Villars: Paris, France, 1964; p. 1370. [Google Scholar]
- Pettit, G.R.; Numata, A.; Gragg, G.; Herald, D.L.; Takada, T.; Iwamoto, C.; Riesen, R.; Schmidt, J.M.; Doubek, D.L.; Goswami, A. Isolation and structures of Schleicherastatins 1-7 and Schleicheols 1 and 2 from the teak forest medicinal tree Schleichera oleosa. J. Nat. Prod. 2000, 63, 72–78. [Google Scholar] [CrossRef]
- Dekebo, A.; Dagne, E.; Gautun, O.R.; Aasen, A.J. Triterpenes from the resin of Boswellia Neglecta. Bull. Chem. Soc. Ethiop. 2002, 16, 87–90. [Google Scholar]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. LWT Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar]
- Sample Availability: Samples of the compounds 1a, 2 and 3 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Triana, J.; López, M.; Pérez, F.J.; Rico, M.; López, A.; Estévez, F.; Marrero, M.T.; Brouard, I.; León, F. Secondary Metabolites from Two Species of Tolpis and Their Biological Activities. Molecules 2012, 17, 12895-12909. https://doi.org/10.3390/molecules171112895
Triana J, López M, Pérez FJ, Rico M, López A, Estévez F, Marrero MT, Brouard I, León F. Secondary Metabolites from Two Species of Tolpis and Their Biological Activities. Molecules. 2012; 17(11):12895-12909. https://doi.org/10.3390/molecules171112895
Chicago/Turabian StyleTriana, Jorge, Mariana López, Francisco Javier Pérez, Milagros Rico, Aroa López, Francisco Estévez, María Teresa Marrero, Ignacio Brouard, and Francisco León. 2012. "Secondary Metabolites from Two Species of Tolpis and Their Biological Activities" Molecules 17, no. 11: 12895-12909. https://doi.org/10.3390/molecules171112895