Degradation Kinetics of Anthocyanins from European Cranberrybush (Viburnum opulus L.) Fruit Extracts. Effects of Temperature, pH and Storage Solvent
Abstract
:1. Introduction
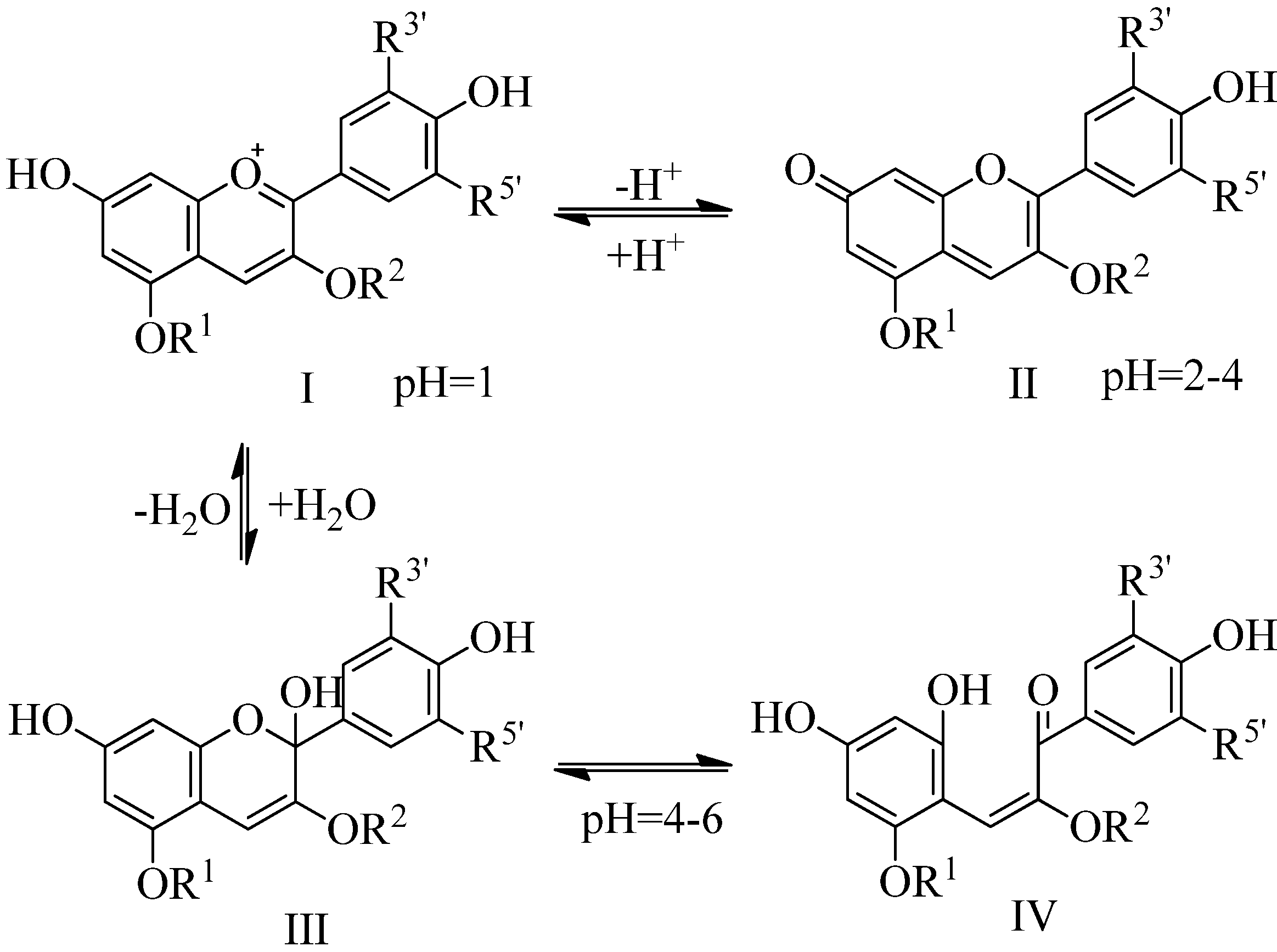
2. Results and Discussion
2.1. Total Anthocyanins Content
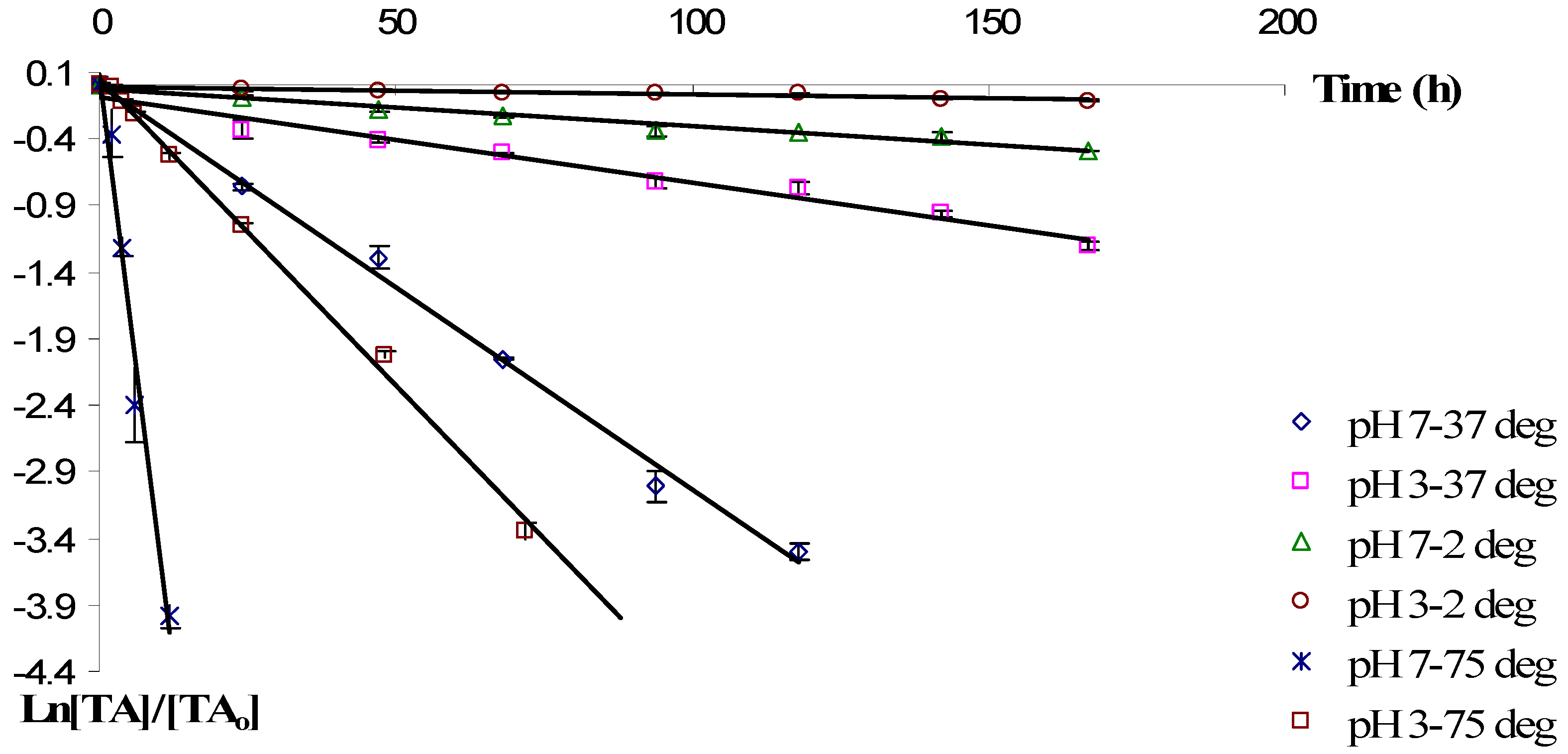
2.2. Degradation Kinetics of Anthocyanins during Storage
| pH | Temp. (°C) | Solvent | k × 103 (h−1) a | t1/2 (h) |
|---|---|---|---|---|
| 3 | 2 | Water | 0.6 (0.9503) | 1,155 |
| 3 | 37 | Water | 6.4 (0.9740) | 108.28 |
| 3 | 75 | Water | 46.1 (0.9971) | 15.03 |
| 7 | 2 | Water | 2.8 (0.9680) | 247.5 |
| 7 | 37 | Water | 30.5 (0.9944) | 22.72 |
| 7 | 75 | Water | 348.8 (0.9762) | 1.98 |
| 3 | 2 | Ethanol/Water | 1.3 (0.9575) | 533.07 |
| 3 | 37 | Ethanol/Water | 13.6 (0.9602) | 50.95 |
| 3 | 75 | Ethanol/Water | 53.9 (0.9948) | 12.86 |
| 7 | 2 | Ethanol/Water | 2.5 (0.9298) | 277.2 |
| 7 | 37 | Ethanol/Water | 38.5 (0.9867) | 18.0 |
| 7 | 75 | Ethanol/Water | 189.3 (0.9824) | 3.66 |



| pH | Solvent | Ea (kJ/mol)a | Ko (h−1) | Q10 | |
|---|---|---|---|---|---|
| 2-37 °C | 37-75 °C | ||||
| 3 | Water | 47.39 (0.9886) | 5.94 × 105 | 1.018 | 1.681 |
| 7 | 52.47 (0.9975) | 2.43 × 107 | 1.978 | 1.898 | |
| 3 | Ethanol/Water | 40.79 (0.9890) | 8.08 × 104 | 1.956 | 1.436 |
| 7 | 47.34 (0.9999) | 2.84 × 106 | 2.184 | 1.52 | |


3. Experimental
3.1. Plant Material
3.2. Chemicals and Reagents
3.3. Preparation of Ethanolic and Aqueous Anthocyanin Extracts
3.4. Determination of Anthocyanin Content

3.5. Degradation Studies
| pH | Temp. (°C) | Solvent | Total storage time (h) |
|---|---|---|---|
| 3 | 2 | Water | 167 |
| 3 | 37 | Water | 48 |
| 3 | 75 | Water | 72 |
| 7 | 2 | Water | 167 |
| 7 | 37 | Water | 14 |
| 7 | 75 | Water | 12 |
| 3 | 2 | Ethanol/Water | 167 |
| 3 | 37 | Ethanol/Water | 145 |
| 3 | 75 | Ethanol/Water | 72 |
| 7 | 2 | Ethanol/Water | 145 |
| 7 | 37 | Ethanol/Water | 97 |
| 7 | 75 | Ethanol/Water | 14 |
3.6. Data Analysis
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the extracts are available from the authors.
References
- Jordheim, M.; Giske, N.H.; Andersen, Ø.M. Anthocyanins in caprifoliaceae. Biochem. Syst. Ecol. 2007, 35, 153–159. [Google Scholar] [CrossRef]
- Dennehy, C. The Use of Herbs and Dietary Supplements in Gynecology: An Evidence-Based Review. J. Midwifery Womens Health 2006, 51, 402–409. [Google Scholar] [CrossRef]
- Altun, M.L.; Citoğlu, G.S.; Yilmaz, B.S.; Özbek, H. Antinociceptive and anti-inflammatory activities of Viburnum opulus. Pharm. Biol. 2009, 47, 653–658. [Google Scholar] [CrossRef]
- Velioğlu, Y.S.; Ekici, L.; Poyrazoglu, E.S. Phenolic composition of European cranberrybush (Viburnum opulus L.) berries and astringency removal of its commercial juice. Int. J. Food Sci. Technol. 2006, 41, 1011–1015. [Google Scholar] [CrossRef]
- Sagdic, O.; Aksoy, A.; Ozkan, G. Evaluation of the antibacterial and antioxidant potentials of cranberry (gilaburu, Viburnum opulus L.) fruit extract. Acta Aliment. Hung. 2006, 35, 487–492. [Google Scholar] [CrossRef]
- Moldovan, B.; Ghic, O.; David, L.; Chişbora, C. The Influence of Storage on the Total Phenols Content and Antioxidant Activity of the Cranberrybush (Viburnum opulus L.) Fruits Extract. Rev. Chim. Bucharest 2012, 63, 463–464. [Google Scholar]
- Rop, O.; Reznicek, V.; Valsikova, M.; Jurikova, T.; Mlcek, J.; Kramarova, D. Antioxidant properties of european cranberrybush fruit (Viburnum opulus var. edule). Molecules 2010, 15, 4467–4477. [Google Scholar]
- Yoshimoto, M.; Okuno, S.; Yamaguchi, M.; Yamakawa, O. Antimutagenicity of Deacylated Anthocyanins in Purple-fleshed Sweetpotato. Biosci. Biotechnol. Biochem. 2001, 65, 1652–1655. [Google Scholar] [CrossRef]
- Smith, M.A.L.; Marley, K.A.; Seigler, D.; Singletary, K.W.; Meline, B. Bioactive Properties of Wild Blueberry Fruits. J. Food Sci. 2000, 65, 352–356. [Google Scholar] [CrossRef]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary cyanidin 3-O-β-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar]
- Renaud, S.; Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. The Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Heredia, F.J.; Francia-Aricha, E.M.; Rivas-Gonzalo, J.C.; Vicario, I.M.; Santos-Buelga, C. Chromatic characterization of anthocyanins from red grapes—I. pH effect. Food Chem. 1998, 63, 491–498. [Google Scholar] [CrossRef]
- Cemeroğlu, B.; Velioğlu, S.; Işik, S. Degradation kinetics of anthocyanins in sour cherry juice and concentrate. J. Food Sci. 1994, 59, 1216–1218. [Google Scholar] [CrossRef]
- Cevallos-Casalas, B.A.; Cisneros-Zevallos, L. Stability of anthocyanin-based aqueous extracts of Andean purple corn and red-fleshed sweet potato compared to synthetic and natural colorants. Food Chem. 2004, 86, 69–77. [Google Scholar] [CrossRef]
- Dyrby, M.; Westergaard, N.; Stapelfeldt, H. Light and heat sensitivity of red cabbage extract in soft drink model systems. Food Chem. 2001, 72, 431–437. [Google Scholar] [CrossRef]
- Özkan, M.; Yemenicioğlu, A.; Asefi, N.; Cemeroğlu, B. Degradation Kinetics of Anthocyanins from Sour Cherry, Pomegranate, and Strawberry Juices by Hydrogen Peroxid. J. Food Sci. 2002, 67, 525–529. [Google Scholar] [CrossRef]
- Hernández-Herrero, J.A.; Frutos, M.J. Degradation kinetics of pigment, colour and stability of the antioxidant capacity in juice model systems from six anthocyanin sources. Int. J. Food Sci. Technol. 2011, 46, 2550–2557. [Google Scholar] [CrossRef]
- Deineka, V.I.; Sorokopudov, V.N.; Deineka, L.A.; Shaposhnik, E.I.; Kol’tsov, S.V. Anthocyans from Fruit of Some Plants of the Caprifoliaceae Family. Chem. Nat. Compd. 2005, 41, 162–164. [Google Scholar] [CrossRef]
- Kraemer-Schafhalter, A.; Fuchs, H.; Strigl, A.; Silhar, S.; Kovac, M.; Pfannhauser, W. Process consideration for anthocyanin extraction from black chokeberry (Aronia melanocarpa ELL). In Proceedings of the Second International Symposium on Natural Colorants; S.I.C. Publishing Col.: Hamden, CT, USA, 1996; pp. 153–160. [Google Scholar]
- Mazza, G.; Miniati, E. Introduction. In Anthocyanins in Fruits, Vegetables and Grains; Mazza, G., Miniati, E., Eds.; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Timberlake, C.F.; Henry, B.S. Anthocyanins as natural food colorants. Prog. Clin. Biol. Res. 1988, 280, 107–121. [Google Scholar]
- Kirca, A.; Özkan, M.; Cemeroğlu, B. Effects of temperature, solid content and pH on the stability of black carrot anthocyanins. Food Chem. 2007, 101, 212–218. [Google Scholar] [CrossRef]
- Wang, W.-D.; Xu, S.-Y. Degradation kinetics of anthocyanins in blackberry juice and concentrate. J. Food Eng. 2007, 82, 271–275. [Google Scholar] [CrossRef]
- Moldovan, B.; David, L.; Donca, R.; Chişbora, C. Degradation kinetics of anthocyanins from crude ethanolic extract from sour cherries. Stud. U. Babeş-Bol. Che. 2011, 56, 243–248. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. UNIT F1.2 Characterization and measurement of anthocyanins by UV-Visible spectroscopy. Current Protocols in Food Analytical Chemistry 2001. [Google Scholar] [CrossRef]
- Kirca, A.; Cemeroğlu, B. Thermal degradation of blood orange anthocyanins. Food Chem. 2003, 81, 583–587. [Google Scholar] [CrossRef]
- Reyes, L.F., Cisneros-Zevallos. Degradation kinetics and colour of anthocyanins in aqueous extracts of purple- and red-flesh potatoes (Solanum tuberosum L.). Food Chem. 2007, 100, 885–894. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Moldovan, B.; David, L.; Chişbora, C.; Cimpoiu, C. Degradation Kinetics of Anthocyanins from European Cranberrybush (Viburnum opulus L.) Fruit Extracts. Effects of Temperature, pH and Storage Solvent. Molecules 2012, 17, 11655-11666. https://doi.org/10.3390/molecules171011655
Moldovan B, David L, Chişbora C, Cimpoiu C. Degradation Kinetics of Anthocyanins from European Cranberrybush (Viburnum opulus L.) Fruit Extracts. Effects of Temperature, pH and Storage Solvent. Molecules. 2012; 17(10):11655-11666. https://doi.org/10.3390/molecules171011655
Chicago/Turabian StyleMoldovan, Bianca, Luminiţa David, Cristian Chişbora, and Claudia Cimpoiu. 2012. "Degradation Kinetics of Anthocyanins from European Cranberrybush (Viburnum opulus L.) Fruit Extracts. Effects of Temperature, pH and Storage Solvent" Molecules 17, no. 10: 11655-11666. https://doi.org/10.3390/molecules171011655




