Controlled Fabrication of Flower-like Nickel Oxide Hierarchical Structures and Their Application in Water Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Flower-Like Hierarchical Structures
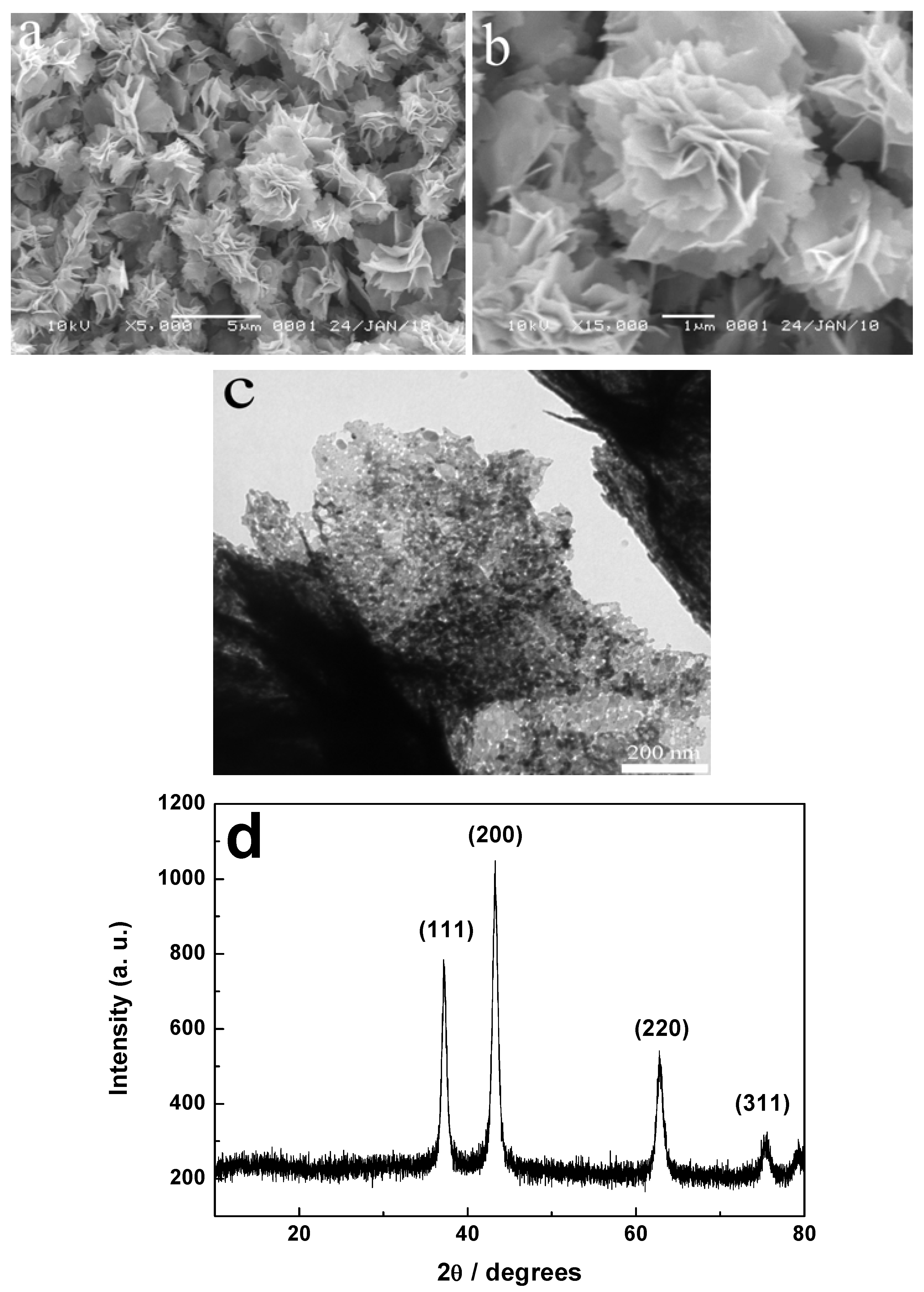
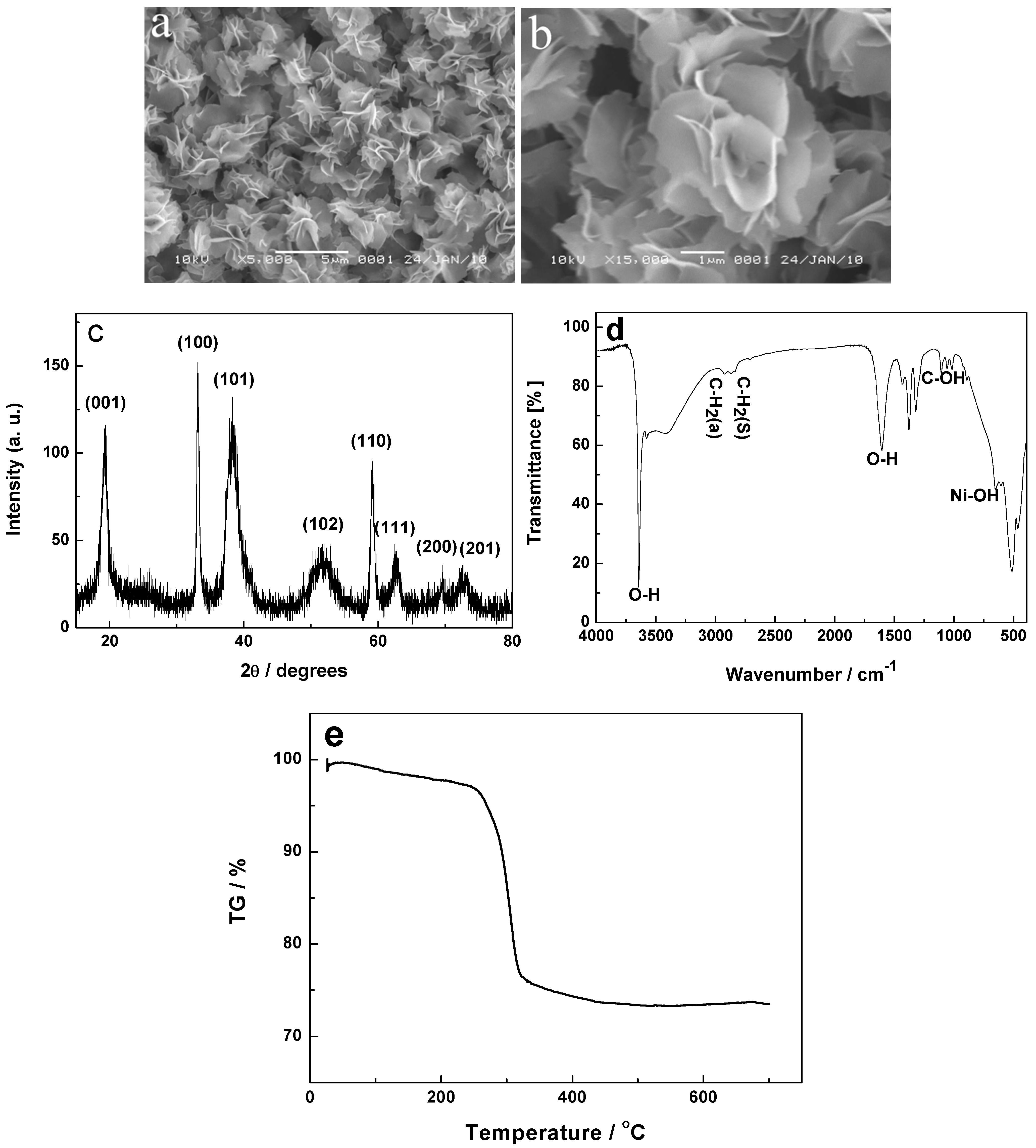
2.2. Research on the Formation Process of NiO Hierarchical Structures


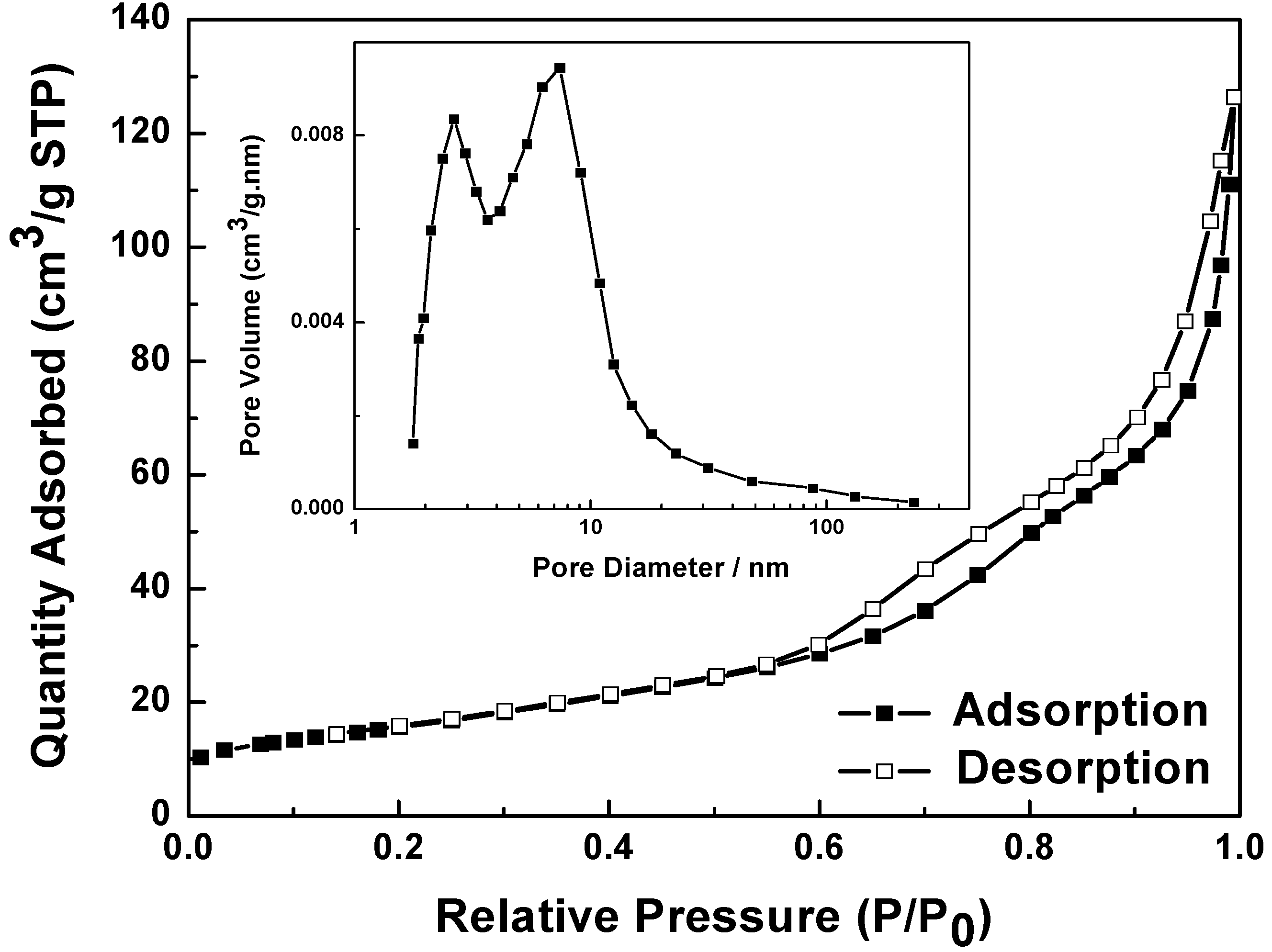
2.3. Nitrogen Physisorption Isotherm of NiO Hierarchical Structures
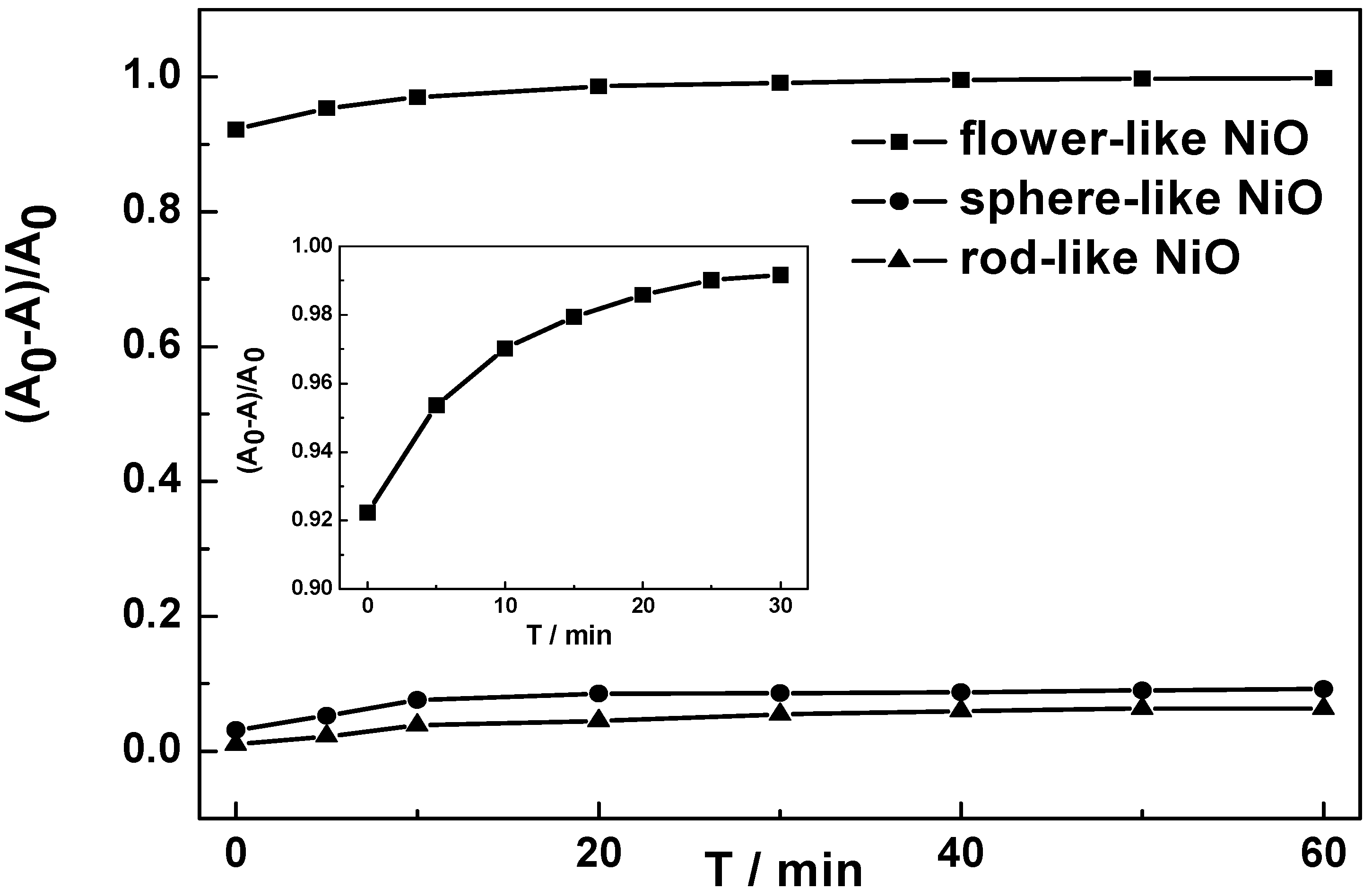
2.4. Adsorption Properties

| Pollutant | λmax/nm | NiO | Adsorption time/min | Elimination rates/% |
|---|---|---|---|---|
| Reactive Red | 519 | flower-like NiO | 10 | 92.1 |
| sphere-like NiO | 10 | 3.12 | ||
| rod-like NiO | 10 | 1.56 | ||
| Reactive Brilliant Blue | 598 | flower-like NiO | 5 | 97.6 |
| sphere-like NiO | 5 | 5.61 | ||
| rod-like NiO | 5 | 2.28 | ||
| Reactive Black | 572 | Flower-like NiO | 8 | 96.7 |
| Sphere-like NiO | 8 | 9.61 | ||
| Rod-like NiO | 8 | 5.72 |
2.5. Photocatalytic Activity
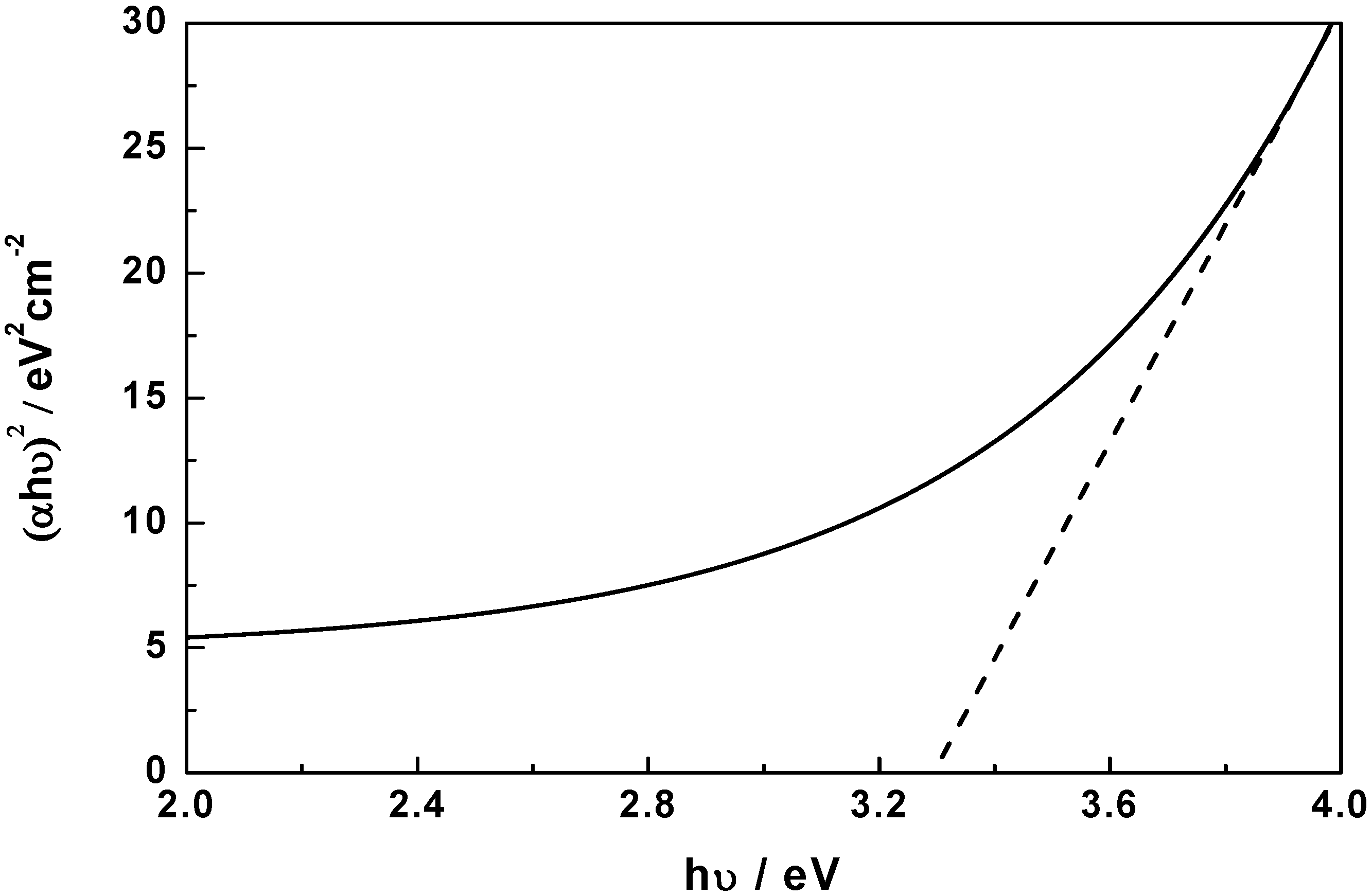
3. Experimental
3.1. Preparation of NiO Samples
3.2. Characterization
3.3. Adsorption Activity
3.4. Photocatalytic Property
4. Conclusions
Acknowledgements
References and Notes
- Rueckes, T.; Kim, K.; Joselevich, E.; Tseng, G.Y.; Cheung, C.L.; Lieber, C.M. Carbon nanotube-based nonvolatile random access memory for molecular computing. Science 2000, 289, 94–97. [Google Scholar] [CrossRef]
- Cui, Y.; Lieber, C.M. Functional nanoscale electronic devices assembled using silicon nanowire building blocks. Science 2001, 291, 851–853. [Google Scholar] [CrossRef]
- Yang, W.; Cui, C.; Sun, J.; Wang, B. Fabrication and magnetic properties of Fe3Co7 alloy nanowire arrays. J. Mater. Sci. 2010, 45, 1523–1527. [Google Scholar] [CrossRef]
- Gu, F.; Zhang, L.; Yin, X.; Tong, L. Polymer single-nanowire optical sensors. Nano Lett. 2008, 8, 2757–2761. [Google Scholar] [CrossRef]
- Ding, Y.; Wan, Y.; Min, Y.L.; Zhang, W.; Yu, S.H. General synthesis and phase control of metal molybdate hydrates MMoO4·nH2O (M=Co, Ni, Mn, n=0, 3/4, 1) nano/microcrystals by a hydrothermal approach: Magnetic, photocatalytic, and electrochemical properties. Inorg. Chem. 2008, 47, 7813–7823. [Google Scholar] [CrossRef]
- Zhu, J.; Gui, Z.; Ding, Y.; Wang, Z.; Hu, Y.; Zou, M. A facile route to oriented nickel hydroxide nanocolumns and porous nickel oxide. J. Phys. Chem. C 2007, 111, 5622–5627. [Google Scholar]
- Bita, I.; Yang, J.K.W.; Jung, Y.S.; Ross, C.A.; Thomas, E.L.; Berggren, K.K. Graphoepitaxy of self-assembled block copolymers on two-dimensional periodic patterned templates. Science 2008, 321, 939–943. [Google Scholar]
- Cheng, J.Y.; Mayes, A.M.; Ross, C.A. Nanostructure engineering by templated self-assembly of block copolymers. Nat. Mater. 2004, 3, 823–828. [Google Scholar] [CrossRef]
- Moh, K.; Werner, U.; Koch, M.; Veith, M. Silver nanoparticles with controlled dispersity and their assembly into superstructures. Adv. Eng. Mater. 2010, 12, 368–373. [Google Scholar] [CrossRef]
- Zhang, X.; Takeuchi, M. Controlled fabrication of fullerene C60 into microspheres of nanoplates through porphyrin-polymer-assisted self-assembly. Angew. Chem. Int. Ed. Engl. 2009, 121, 9826–9831. [Google Scholar] [CrossRef]
- Zhao, B.; Ke, X.K.; Bao, J.H.; Wang, C.L.; Dong, L.; Chen, Y.W.; Chen, H.L. Synthesis of flower-like NiO and effects of morphology on its catalytic properties. J. Phys. Chem. C 2009, 113, 14440–14447. [Google Scholar]
- Park, J.; Kang, E.; Son, S.U.; Park, H.M.; Lee, M.K.; Kim, J.; Kim, K.W.; Noh, H.J.; Park, J.H.; Bae, C.J.; Park, J.G.; Hyeon, T. Monodisperse nanoparticles of Ni and NiO: Synthesis, characterization, self-assembled superlattices, and catalytic applications in the suzuki coupling reaction. Adv. Mater. Weinheim 2005, 17, 429–434. [Google Scholar] [CrossRef]
- Qiao, H.; Wu, N.; Huang, F.; Cai, Y.; Wei, Q. Solvothermal synthesis of NiO/C hybird microspheres as Li-intercalation electrode material. Mater. Lett. 2010, 64, 1022–1024. [Google Scholar] [CrossRef]
- Mattei, G.; Mazzoldi, P.; Post, M.L.; Buso, D.; Guglielmi, M.; Martucci, A. Cookio-like Au/NiO nanoparticles with optical gas-sensing properties. Adv. Mater. Weinheim 2007, 19, 561–564. [Google Scholar] [CrossRef]
- Fantini, M.C.A.; Ferreira, F.F.; Gorenstein, A. Theoretical and experimental results on Au-NiO and Au-CoO electrochromic composite films. Solid State Ionics 2002, 152–153, 867–872. [Google Scholar]
- García-Cerda, L.A.; Romo-Mendoza, L.E.; Quevedo-López, M.A. Synthesis and characterization of NiO nanoparticles and their PMMA nanocomposites obtained by in situ bulk polymerization. J. Mater. Sci. 2009, 44, 4553–4556. [Google Scholar] [CrossRef]
- Xu, C.; Xu, G.; Wang, G. Preparation and characterization of NiO nanorods by thermal decomposition of NiC2O4 precursor. J. Mater. Sci. 2003, 38, 779–782. [Google Scholar] [CrossRef]
- Yang, Q.; Sha, J.; Ma, X.; Yang, D. Synthesis of NiO nanowires by a sol-gel process. Mater. Lett. 2005, 59, 1967–1970. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, K.; Chen, L.; Yang, H.; Li, Z.; Suchopar, A.; Richards, R. Preparation and surface activity of single-crystalline NiO (111) nanosheets with hexagonal holes: A semiconductor nanospanner. Adv. Mater. Weinheim 2008, 20, 267–271. [Google Scholar] [CrossRef]
- Kuang, D.B.; Lei, B.X.; Pan, Y.P.; Yu, X.Y.; Su, C.Y. Fabricaton of novel hierarchical β-Ni(OH)2 and NiO microspheres via an easy hydrothermal process. J. Phys. Chem. C 2009, 113, 5508–5513. [Google Scholar]
- Huang, D.; Luo, G.; Yang, L.; Wang, Y. Synthesis of mesoporous TiO2 materials with high specific area using inorganic acids as catalysts. China Particuology 2005, 3, 176–180. [Google Scholar] [CrossRef]
- Yong, X.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar]
- Sample Availability: Samples of the flower-like, sphere-like and rod-like NiO are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tao, F.; Shen, Y.; Wang, L. Controlled Fabrication of Flower-like Nickel Oxide Hierarchical Structures and Their Application in Water Treatment. Molecules 2012, 17, 703-715. https://doi.org/10.3390/molecules17010703
Tao F, Shen Y, Wang L. Controlled Fabrication of Flower-like Nickel Oxide Hierarchical Structures and Their Application in Water Treatment. Molecules. 2012; 17(1):703-715. https://doi.org/10.3390/molecules17010703
Chicago/Turabian StyleTao, Feifei, Yongmiao Shen, and Linxia Wang. 2012. "Controlled Fabrication of Flower-like Nickel Oxide Hierarchical Structures and Their Application in Water Treatment" Molecules 17, no. 1: 703-715. https://doi.org/10.3390/molecules17010703
APA StyleTao, F., Shen, Y., & Wang, L. (2012). Controlled Fabrication of Flower-like Nickel Oxide Hierarchical Structures and Their Application in Water Treatment. Molecules, 17(1), 703-715. https://doi.org/10.3390/molecules17010703






