Synthesis and Preliminary Antimicrobial Activities of New Arylideneamino-1,3,4-thiadiazole-(thio/dithio)-acetamido Cephalosporanic Acids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
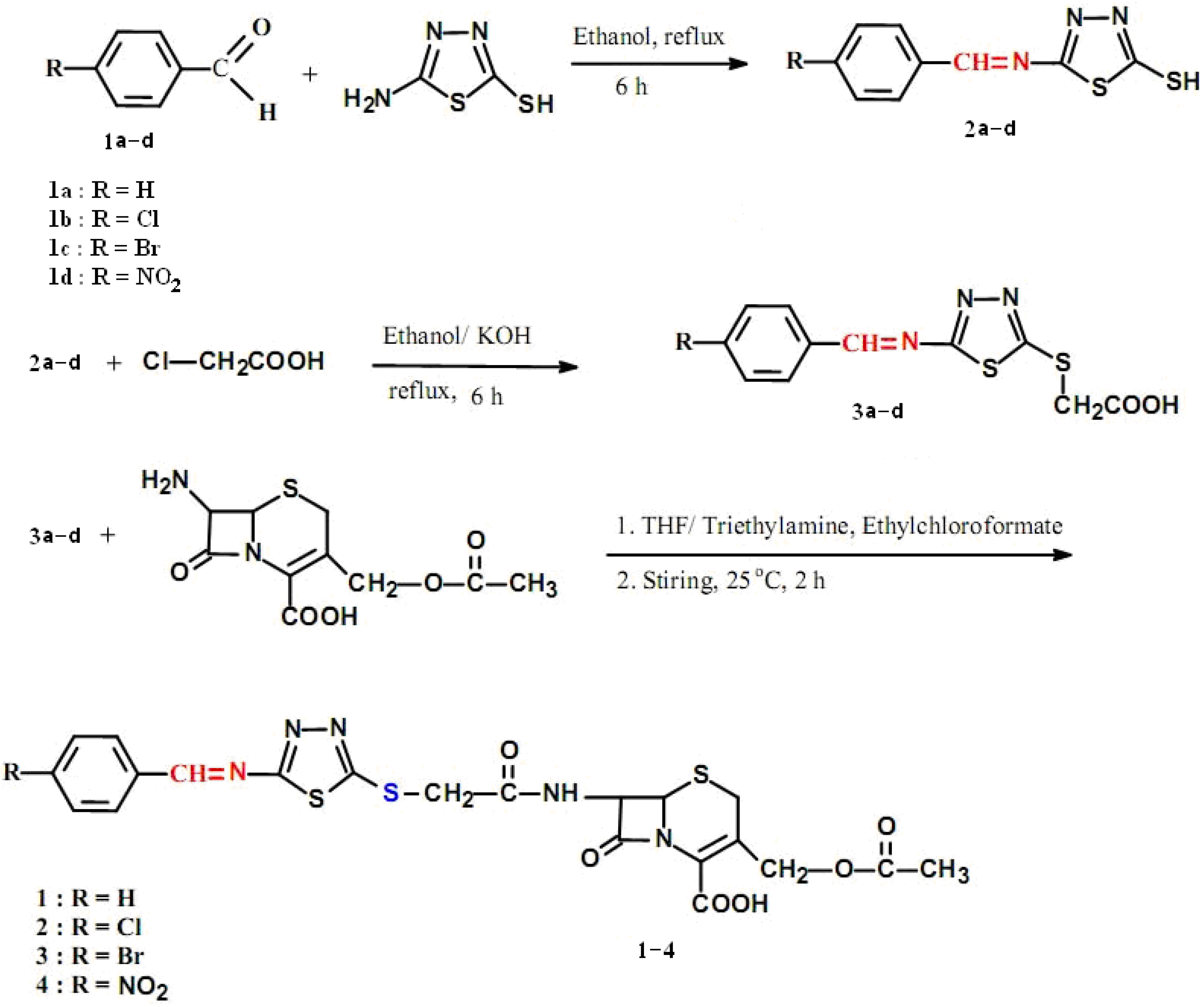
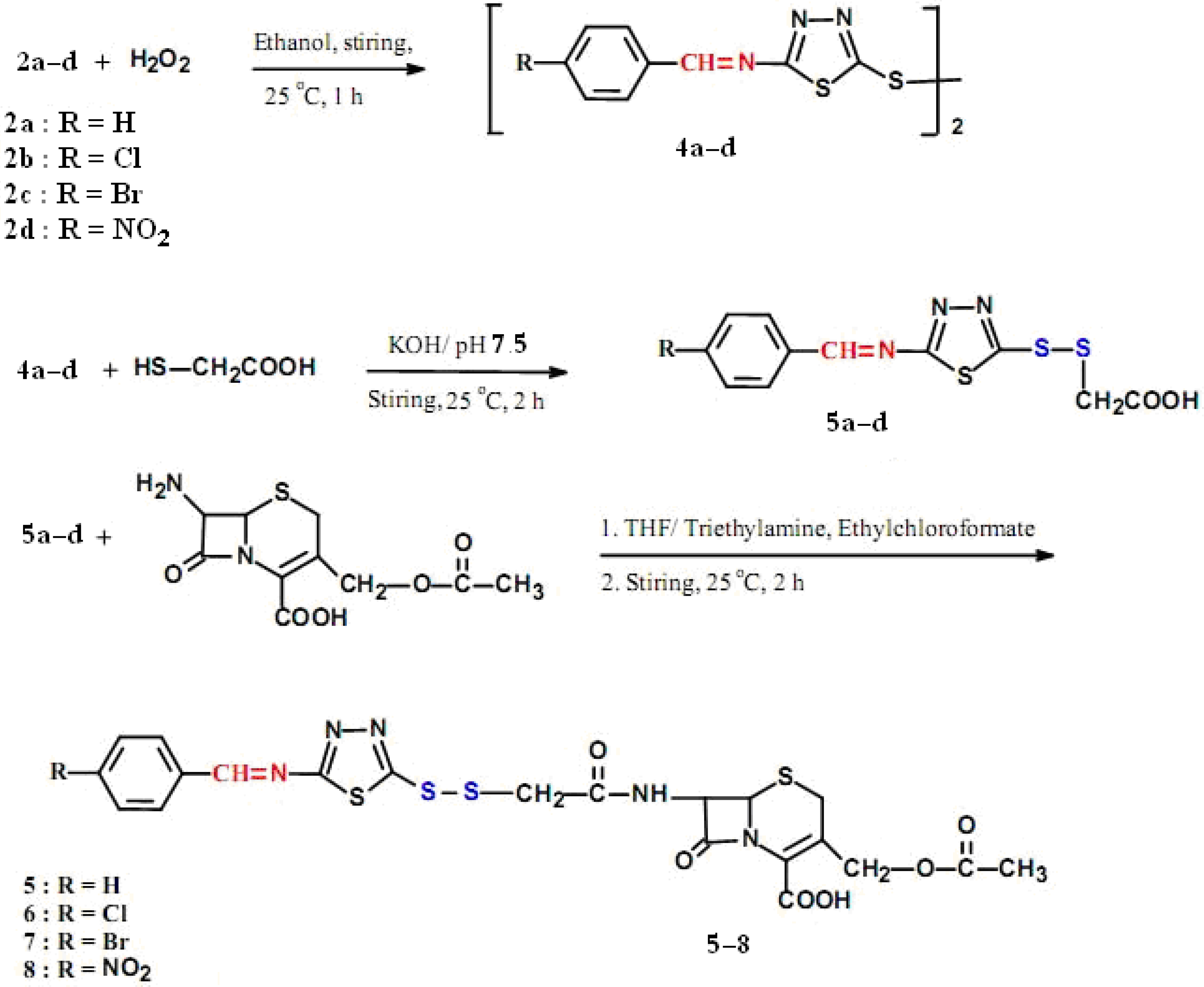
2.2. Antimicrobial Activities
| Compound | Antimicrobial Activity (zone of inhibition mm) | |||
|---|---|---|---|---|
| M. letus ATCC 9341 | S. aureus ATCC 25923 | E. coli ATCC 29522 | P. aeruginosa ATCC27853 | |
| 1 | 18 | 17 | 16 | - |
| 2 | 19 | 17 | 18 | 13 |
| 3 | 18 | 16 | 16 | 15 |
| 4 | 17 | 20 | 18 | 17 |
| 5 | 21 | 22 | 20 | - |
| 6 | 25 | 21 | 19 | 16 |
| 7 | 25 | 24 | 21 | 16 |
| 8 | 25 | 23 | 24 | 17 |
| Cephalexin | 26 | 22 | 20 | - |
3. Experimental
3.1. General
3.2. Chemical Methods
3.2.1. General procedure for the preparation of 5-(arylideneamino)-1,3,4-thiadiazole-2-thiols 2a–d
3.2.2. General procedure for the preparation of 2-(5-(arylideneamino)-1,3,4-thiadiazol-2-ylthio)acetic acids 3a–d
3.2.3. General procedure for the preparation of 3-(acetoxymethyl)-7-(2-(5-(arylideneamino)-1,3,4-thiadiazol-2-ylthio)acetamido)-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acids 1–4
3.2.4. General procedure for the preparation of 5,5'-disulfanyl-bis-(N-arylideneamino-1,3,4-thiadiazoles) 4a–d
3.2.5. General procedure for the preparation of (E)-2-(2-(5-(arylideneamino)-1,3,4-thiadiazol-2-yl)disulfanyl) acetic acids 5a–d
3.2.6. General procedure for the preparation of 3-(acetoxymethyl)-7-(2-((5-(arylideneamino)-1,3,4-thiadiazol-2-yl)disulfanyl)acetamido)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acids 5–8
3.3. Preliminary Antimicrobial Assay
| Compound | M. letus ATCC 9341 | S. aureus ATCC 25923 | E. coli ATCC 29522 | P. aeruginosa ATCC27853 |
|---|---|---|---|---|
| 1 | 34 | 32 | 50 | Not Tested |
| 2 | 33 | 35 | 38 | 88 |
| 3 | 30 | 34 | 38 | 90 |
| 4 | 28 | 30 | 36 | 92 |
| 5 | 25 | 30 | 38 | Not Tested |
| 6 | 26 | 28 | 28 | 82 |
| 7 | 16 | 17 | 25 | 82 |
| 8 | 13 | 23 | 24 | 80 |
| Cephalexin | 15 | 25 | 30 | Not Tested |
4. Conclusions
Acknowledgements
References and Notes
- Yong, Z.K.; Jong, C.L.; Sam, S.K.; Young, C.M.; Chang, S.L. Synthesis and antimicrobial activity of novel 1-(3-pyrimidine thio-substituted) cephalosporins. Kor. J. Med. Chem. 1996, 6, 15–21. [Google Scholar]
- Eun, S.J.; Gee, H.J.; Ghlsoo, N.; Jae, C.L.; Sung, H.K.; Heanju, S.; Dae, Y.C.; Joong, H.K. New 3-substitued cephalosporin’s(1,3-thiazole, 1,2,4-triazole-2-yl and 3-triazole)-thiomethyl derivatives. Kor. J. Med. Chem. 1995, 5, 60–63. [Google Scholar]
- Jung, F.; Boucherot, D.; Delvare, C.; Olivier, A.; Davies, G.M.; Betts, M.J.; Brown, C.; Stevenson, R.; Joseph, M.; Kingston, J.F. Synthesis and SAR of new cephalosporin’s with aminoimidazoles at C7. J. Antibiot. 1993, 46, 992–1012. [Google Scholar] [CrossRef]
- Hara, R.; Sakamoto, K.; Hisamichi, H.; Nagano, N. SAR of cephalosporin’s having a (dimethyl-isoxazolidino) vinyl moiety at their C3-position. J. Antibiot. 1996, 49, 1162–1171. [Google Scholar] [CrossRef]
- Ashour, F.A.; Habib, N.S.; El-Taibbi, M.; El-Dine, A.S. Synthesis of 1,3,4-thiadiazoles, imidazo-(1,3,4-thiadiazole) and thiadiazole-pyrimidines derived from benzimidazole as potential antimicrobial agents. Farmaco 1990, 45, 1341–1349. [Google Scholar]
- Klein, O.; Chin, N.X.; Huang, H.B.; Neu, H.C. In vitro activity of SCE-2787, a new cephalosporin with potent activity against pseudomonas aeruginosa and members of the Enterobacteriacae family. Antimicrob. Agents Chemother. 1994, 38, 2896–2901. [Google Scholar] [CrossRef]
- Kishi, M.; Ishitobi, H.; Nagata, W.; Tsuii, T. Synthesis of 3-(1-methyl-1,2,3,4-tetrazole-5-yl) and 2-methyl-1,3,4-thiadiazole-5-yloxymethyl)-3-cephem derivatives. Heterocycles 1979, 13, 197–202. [Google Scholar] [CrossRef]
- Iwamatsu, K.; Atsumi, K.; Hatanka, M. Synthetic cephalosporins. VII. Synthesis and antibacterial activity of 7-((z)-2-(2-Aminothiazole-4-yl)-2-(3-(3-hydroxy-4-pyridine-1-yl)-3-carboxypropoxyimino) acetamido)-(1,2,3-thiadiazole-5-yl) thiomethyl-3-cephem-4-carboxylic acid and related compounds. Chem. Pharm. Bull. 1990, 38, 3476–3479. [Google Scholar] [CrossRef]
- Janice, P.-D.; Susan, E.D.; Stjepan, K.; John, L.O.; Fred, T.C. Oral absorption of cephalosporin antibiotics 2. Expanded SAR of 7-(arylacetamido)-3-substituted cephalosporins. J. Med. Chem. 1988, 31, 1993–1997. [Google Scholar] [CrossRef]
- Mazzone, G.; Bonina, F.; Pullisi, G.; Reina, R.; Arrigo, C.; Cosentino, C.; Blandino, G. Synthesis and biological evaluation of some 5-aryl-1,2-amino-1,3,4-oxa(thia) diazoles. Farmaco 1982, 37, 685–700. [Google Scholar]
- Mamolo, M.G.; Falogioni, V.; Zanpieir, D.; Vio, L.; Banfi, F. Synthesis and antimycobacterial activity of 5-(pyridine-2-yl)-1,3,4-thiadiazole-2-yl) thioacetic acid arylidine-hydrazine derivatives. Farmaco 2001, 56, 587–592. [Google Scholar] [CrossRef]
- Koji, I.; Tadashi, K.; Kyoji, M.; Yoshio, H.; Hiromu, N.; Kiyoshi, M.; Yasuo, K.; Hideaki, M.; Tadashi, Y. Synthesis and SAR of 7B-(z)-2-(2-aminothiazole-4-yl)-3-(substituted)-2-propenoylamino)-3-cephems with C3 substitutions. J. Antibiot. 1994, 47, 466–476. [Google Scholar] [CrossRef]
- Cecil, R. The role of sulfur in proteins. In The Proteins, 2nd; Neurath, H., Ed.; Academic Press: New York, NY, USA, 1963; Volume 1, p. 379. [Google Scholar]
- Boyer, P.D. Sulfhydryl and disulfide groups of enzymes. In The Enzymes, 2nd; Boyer, P.D., Hardy, H., Myrback, K., Eds.; Academic Press: New York, NY, USA, 1959; Volume 1, pp. 511–588. [Google Scholar]
- Anderson, M.; Holmgren, A.; Spyrou, G. NK-lysine, a disulfide-containing effectors peptide of T-lymphocytes, is reduced and inactivated by human thioredoxin reductase. Implication for a protective mechanism against NK-lysine cytotoxicity. J. Biol. Chem. 1996, 271, 10116–10120. [Google Scholar] [CrossRef]
- Nagrajan, R.; Neuss, N.; Marsh, M.M. Crystal and molecular structure of LLS 88 alpha, an antiviral epidithiapiperazinedione derivatives from Aspergillus terrus. J. Am. Chem. Soc. 1968, 90, 6518–6519. [Google Scholar] [CrossRef]
- Kishi, Y.; Fukuyama, T.; Nakatsuka, S. New method for the synthesis of epidithiodiketopiperazines. J. Am. Chem. Soc. 1973, 95, 6490–6492. [Google Scholar] [CrossRef]
- Fuloria, N.K.; Singh, V.; Shaharyar, M.; Ali, M. Synthesis, characterization and biological studies of new Schiff bases and azetidinones derived from propionic acid derivatives. Asian J. Chem. 2008, 20, 6457–6462. [Google Scholar]
- Singh, N.B.; Singh, H.J. Synthesis of 2-arylideneamino-5-aryl-1,3,4-thiadiazoles as potential fungicides. J. Indian Chem. Soc. 1975, 52, 1200. [Google Scholar]
- Wadher, S.J.; Puranik, M.P.; Karande, N.A.; Yede, P.G. Synthesis and biological evaluation of Schiff bases of dapsone and their derivatives as antimicrobials. Int. J. PharmTech Res. 2009, 1, 22–33. [Google Scholar]
- Hunashal, R.H.; Ronad, P.P.; Maddi, V.; Darbhamulla, S.; Kamdod, M. Synthesis and biological activities of N-(4-(substituted benzylideneamino)-3-(2,4-dichlorophenoxymethyl)-1,2,4-triazole-5yl))-isonicontinyl hydrazides. Int. J. Drug Des. Discov. 2010, 1, 107–113. [Google Scholar]
- Nursen, S.; Seza, A.; Elif, L.; Iffet, S. Antimicrobial activity of some new amino acid Schiff bases. G.U.J. Sci. 2003, 16, 283–288. [Google Scholar]
- Bektas, H.; Karaali, N.; Sahin, D.; Demirbas, A.; Karaoglu, S.A.; Demirbas, N. Synthesis and antimicrobial activities of some new 1,2,4-triazole derivatives. Molecules 2010, 15, 2427–2438. [Google Scholar] [CrossRef]
- Nam, S.C.; Goo, N.K.; Cyril, P. Synthesis of 5-aroylamino-3H-1,3,4-thiadiazole-2-thiones and their tautomerism. J. Heterocycl. Chem. 1993, 30, 397–401. [Google Scholar] [CrossRef]
- Tanaka, S.K.; Summerill, R.A.; Minassion, R.F.; Bush, K.; Visinic, D.A.; Bonner, D.P.; Sykes, R.S. In vitro evaluation of tigemonam, a novel oral Monobactam. Antimicrob. Agents Chemother. 1987, 31, 219–225. [Google Scholar] [CrossRef]
- Vogel, A.I. Vogel's Textbook of Practical Organic Chemistry, 4th ed; Longman Scientific & Technical-Longman Group: Suffolk, UK, 1978; pp. 584–585. [Google Scholar]
- Perren, Y.G.; Minor, M.F.; Holdrege, C.T.; Gottstein, W.J.; Godfrey, J.; Crast, L.B.; Babel, R.B.; Cheney, L.C. Derivatives of 6-aminopenicillanic acid. Partially synthetic penicillins prepared from α-aryloxyalkanoic acids. J. Am. Chem. Soc. 1960, 82, 3934–3938. [Google Scholar] [CrossRef]
- Guha, P.C. Constitution of the so-called dithio-urazole of martin Freud. J. Am. Chem. Soc. 1922, 44, 1502–1510. [Google Scholar] [CrossRef]
- Wilson, J.M.; Bayer, R.J.; Hupe, D.J. Structure-reactivity correlation for thiol- disulfide interchange reaction. J. Am. Chem. Soc. 1977, 99, 7922–7926. [Google Scholar] [CrossRef]
- McAlan, D.T.; Cullum, T.V.; Dean, R.A.; Filder, F.A. The preparation and properties of sulfur compounds related to petroleum. I. The dialkylsulfide and disulfides. J. Am. Chem. Soc. 1951, 73, 3627–3632. [Google Scholar] [CrossRef]
- Barry, A.L. The Antimicrobial Susceptibility Test: Principle and Practices; Illus Lea and Febiger: Philadelphia, PA, USA, 1976; p. 180. [Google Scholar]
- Coyle, M.B. Manual of Antimicrobial Susceptibility Testing, 1st ed; American society for Microbiology: Washington, DC, USA, 2005; pp. 109–111. [Google Scholar]
- Sample Availability: Samples of the titled compounds are available from the author.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alwan, S.M. Synthesis and Preliminary Antimicrobial Activities of New Arylideneamino-1,3,4-thiadiazole-(thio/dithio)-acetamido Cephalosporanic Acids. Molecules 2012, 17, 1025-1038. https://doi.org/10.3390/molecules17011025
Alwan SM. Synthesis and Preliminary Antimicrobial Activities of New Arylideneamino-1,3,4-thiadiazole-(thio/dithio)-acetamido Cephalosporanic Acids. Molecules. 2012; 17(1):1025-1038. https://doi.org/10.3390/molecules17011025
Chicago/Turabian StyleAlwan, Shakir Mahmood. 2012. "Synthesis and Preliminary Antimicrobial Activities of New Arylideneamino-1,3,4-thiadiazole-(thio/dithio)-acetamido Cephalosporanic Acids" Molecules 17, no. 1: 1025-1038. https://doi.org/10.3390/molecules17011025





