Synthesis, Photophysical and Electrochemical Properties of a Mixed Bipyridyl-Phenanthrolyl Ligand Ru(II) Heteroleptic Complex Having trans-2-Methyl-2-butenoic Acid Functionalities
Abstract
:1. Introduction
2. Results and Discussion
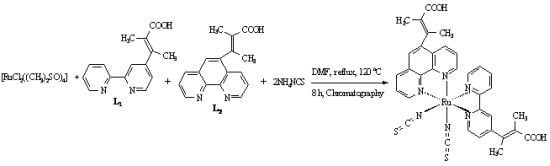
2.1. Chemistry

2.2. Infrared Spectra Studies of the Ligands and Complex

2.3. 1H and 13C-NMR Spectroscopic Studies

2.4. Electronic Absorption and Emission Spectra
2.4.1. Electronic Absorption Spectroscopy

2.4.2. Emission Study

2.5. Electrochemical Study


3. Experimental
3.1. Materials and General Physical Measurements
3.2. Synthesis of 4-(trans-2-Methyl-2-butenoic acid)-2,2'-bipyridine (L1)
3.3. Synthesis of 5-(trans-2-Methyl-2-butenoic acid)-1,10-phenanthroline (L2)
3.4. Synthesis of cis-Dithiocyanato-4-(trans-2-methyl-2-butenoic acid)-2,2'-bipyridyl-5-(trans-2-methyl-2-butenoic acid)-1,10-phenanthrolyl-ruthenium(II) complex [RuL1L2(NCS)2]
3.5. Synthesis of cis-Dithiocyanato-bis-4-(trans-2-methyl-2-butenoic acid)-2,2'-bipyridyl)-ruthenium(II) complex [Ru(L1)2(NCS)2]
3.6. Synthesis of cis-Dithiocyanato-bis-5-(trans-2-methyl-2-butenoic acid)-1,10-phenanthrolyl-ruthenium(II) complex [Ru(L2)2(NCS)2]
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Conflict of Interest
References
- Bazani, V.; Scandola, F. Supramolecular Photochemistry; Ellis Horwood: Chichester, UK, 1991. [Google Scholar]
- Demeunynck, M.; Bailly, C.; Wilson, W.D. DNA and RNA Binders: From Small Molecules to Drugs; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Gielen, M.; Tiekink, E.R.T. Metallotherapeutic Drugs and Metal-Based Diagnostic Agents: The Use of Metals in Medicine; John Wiley & Sons Ltd.: Chichester, UK, 2005. [Google Scholar]
- Kalyanasundaram, K.; Grätzel, M. Applications of functionalized transition metal complexes in photonic and optoelectronic devices. Coord. Chem. Rev. 1998, 77, 347–414. [Google Scholar] [CrossRef]
- Wang, P.; Zakeeruddin, S.M.; Moser, J.E.; Humphry-Baker, R.; Comte, P.; Aranyos, V.; Hagfeldt, A.; Nazeeruddin, M.K.; Gratzel, M. Stable new sensitizer with improved light harvesting for nanocrystalline dye-sensitized solar cells. Adv. Mater. 2004, 16, 1806–1811. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wu, S.J.; Wu, C.G.; Chen, J.G.; Ho, K.C. A Ruthenium complex with super-high light harvesting capacity for dye-sensitized solar cells. Angew. Chem. Int. Ed. 2006, 45, 5822–5825. [Google Scholar] [CrossRef]
- Leroy-Lhez, S.; Belin, C.; D’aleo, A.; Williams, R.M.; De Cola, L.; Fages, F. Extending excited-state lifetimes by interchromophoric triplet-state equilibration in a pyrene-Ru(II) diimine dyad system. Supramol. Chem. 2003, 15, 627–637. [Google Scholar] [CrossRef]
- Juris, A.; Balzani, V.; Barigelletti, F.; Campagna, S.; Belser, P.; Von Zelewsky, A.V. Ruthenium(II) polypyridine complexes: Photophysics, photochemistry, electrochemistry, and chemiluminescence. Coord. Chem. Rev. 1988, 84, 85–277. [Google Scholar]
- Balzani, V.; Juris, A.; Venturi, M.; Campagna, S.; Serroni, S. Luminescent and redox-active polynuclear transition metal complexes. Chem. Rev. 1996, 96, 756–788. [Google Scholar]
- De Cola, L.; Belser, P. Photoinduced energy and electron transfer processes in rigidly bridged dinuclear Ru/Os complexes. Coord. Chem. Rev. 1998, 177, 301–346. [Google Scholar] [CrossRef]
- Kaes, C.; Katz, A.; Hosseini, M.W. Bipyridine: The most widely used ligand. A review of molecules comprising at least two 2,2'-bipyridine units. Chem. Rev. 2000, 100, 3553–3590. [Google Scholar] [CrossRef]
- Chao, H.; Ji, L.N. DNA interactions with ruthenium(II) polypyridine complexes containing asymmetric ligands. Bioinorg. Chem. Appl. 2005, 3, 15–28. [Google Scholar] [CrossRef]
- Vos, J.G.; Kelly, J.M. Ruthenium polypyridyl chemistry; from basic research to applications and back again. Dalton Trans. 2006, 4869–4883. [Google Scholar]
- Tyson, D.S.; Luman, C.R.; Castellano, F.N. Photodriven electron and energy transfer from a light-harvesting metallodendrimer. Inorg. Chem. 2002, 41, 3578–3586. [Google Scholar]
- Adeloye, A.O.; Ajibade, P.A. A high molar extinction coefficient mono-anthracenyl bipyridyl heteroleptic ruthenium(II) complex: Synthesis, photophysical and electrochemical properties. Molecules 2011, 16, 4615–4631. [Google Scholar] [CrossRef]
- Adeloye, A.O.; Ajibade, P.A. Synthesis and characterization of a Ru(II) complex with functionalized phenanthroline ligands having single-double linked anthracenyl and 1-methoxy-1-buten-3-yne moieties. Molecules 2010, 15, 7570–7581. [Google Scholar]
- Adeloye, A.O.; Ajibade, P.A. Synthesis and characterization of a heteroletic Ru(II) complex of phenanthroline containing Oligo-anthracenyl carboxylic acid moieties. Int. J. Mol. Sci. 2010, 11, 3158–3176. [Google Scholar] [CrossRef]
- Vyas, P.; Bhatt, A.K.; Ramachandraiah, G.; Bedekar, A.V. Environmentally benign chlorination and bromination of aromatic amines, hydrocarbons and naphthols. Tetrahedron Lett. 2003, 44, 4085–4088. [Google Scholar] [CrossRef]
- Evans, I.P.; Spencer, A.; Wilkinson, G. Dichlorotetrakis(dimethyl sulphoxide) ruthenium(II) and its use as a source material for some new Ruthenium(II) complexes. J. Chem. Soc. Dalton 1973, 204–208. [Google Scholar]
- Mitsopoulou, C.A.; Veroni, I.; Philippopoulos, A.I.; Falaras, P. Synthesis, characterization and sensitization properties of two novel mono and bis carboxyl-dipyrido-phenazine ruthenium(II) charge transfer complexes. J. Photochem. Photobiol. A: Chem. 2007, 191, 6–12. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Duffy, N.W.; Dobson, K.D.; Gordon, K.C.; Robinson, B.H.; McQuillan, A.J. In situ infrared spectroscopic analysis of the adsorption of ruthenium(II) bipyridyl dicarboxylic acid photosensitisers to TiO2 in aqueous solutions. Chem. Phys. Lett. 1997, 266, 451–455. [Google Scholar] [CrossRef]
- Sun, Y.; Onicha, A.C.; Myahkostupov, M.; Castellano, F.N. Viable alternative to N719 for dye-sensitized solar cells. Appl. Mater. Interfaces 2010, 2, 2039–2045. [Google Scholar] [CrossRef]
- Qu, J.Q.; Zhang, J.Y.; Grimsdale, A.C.; Mullen, K. Dendronized perylene diimide emitters: Synthesis, luminescence and electron and energy transfer studies. Macromolecules 2004, 37, 8297–8306. [Google Scholar] [CrossRef]
- Xu, B.; Holdcroft, S. Molecular control of luminescence from poly-(3-hexylthiophenes). Macromolecules 1993, 26, 4457–4460. [Google Scholar] [CrossRef]
- Crosby, G.A.; Watts, R.J.; Carstens, D.H.W. Inversion of excited state of transition-metal complexes. Science 1970, 170, 1195–1196. [Google Scholar]
- Henderson, L.J., Jr.; Fronczek, F.R.; Cherry, W.R. Selective perturbation of ligand field excited states in polypyridine ruthenium(II) complexes. J. Am. Chem. Soc. 1984, 106, 5876–5879. [Google Scholar]
- Staniszewski, A.; Heuer, W.B.; Meyer, G.J. High-extinction ruthenium compounds for sunlight harvesting and hole transport. Inorg. Chem. 2008, 47, 7062–7064. [Google Scholar]
- Nalwa, H.S.; Shirk, J.S. Phthalocyanines, Properties and Applications; Leznoff, C.C., Lever, A.B.P., Eds.; VCH: New York, NY, USA, 1996; Volume 4, pp. 79–182. [Google Scholar]
- Roundhill, D.M. Photochemistry and Photophysics of Metal Complexes; Plenum Address: New York, NY, USA, 1993. [Google Scholar]
- Nazeeruddin, Md.K.; Kay, A.; Rodicio, I.; Humphry-Baker, R.; Muller, E.; Liska, P.; Vlachopoulos, N.; Grätzel, M. Conversion of light to electricity by cis-X2(dcbpy)2Ru(II) CT sensitizers on nanocrystalline TiO2 electrodes. J. Am. Chem. Soc. 1993, 115, 6382–6390. [Google Scholar]
- Samples Availability: Samples of the compounds L1, L2 and [RuL1L2(NCS)2] are available from the author.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Adeloye, A.O. Synthesis, Photophysical and Electrochemical Properties of a Mixed Bipyridyl-Phenanthrolyl Ligand Ru(II) Heteroleptic Complex Having trans-2-Methyl-2-butenoic Acid Functionalities. Molecules 2011, 16, 8353-8367. https://doi.org/10.3390/molecules16108353
Adeloye AO. Synthesis, Photophysical and Electrochemical Properties of a Mixed Bipyridyl-Phenanthrolyl Ligand Ru(II) Heteroleptic Complex Having trans-2-Methyl-2-butenoic Acid Functionalities. Molecules. 2011; 16(10):8353-8367. https://doi.org/10.3390/molecules16108353
Chicago/Turabian StyleAdeloye, Adewale O. 2011. "Synthesis, Photophysical and Electrochemical Properties of a Mixed Bipyridyl-Phenanthrolyl Ligand Ru(II) Heteroleptic Complex Having trans-2-Methyl-2-butenoic Acid Functionalities" Molecules 16, no. 10: 8353-8367. https://doi.org/10.3390/molecules16108353