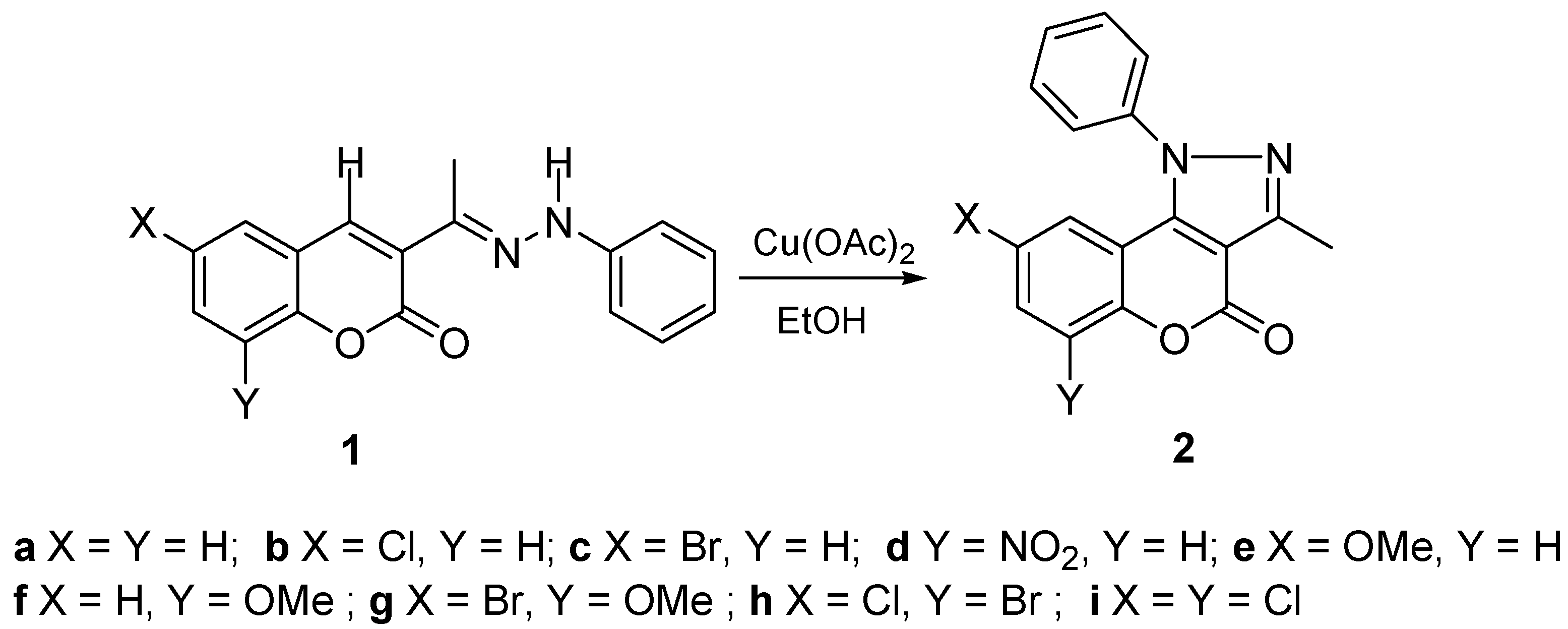
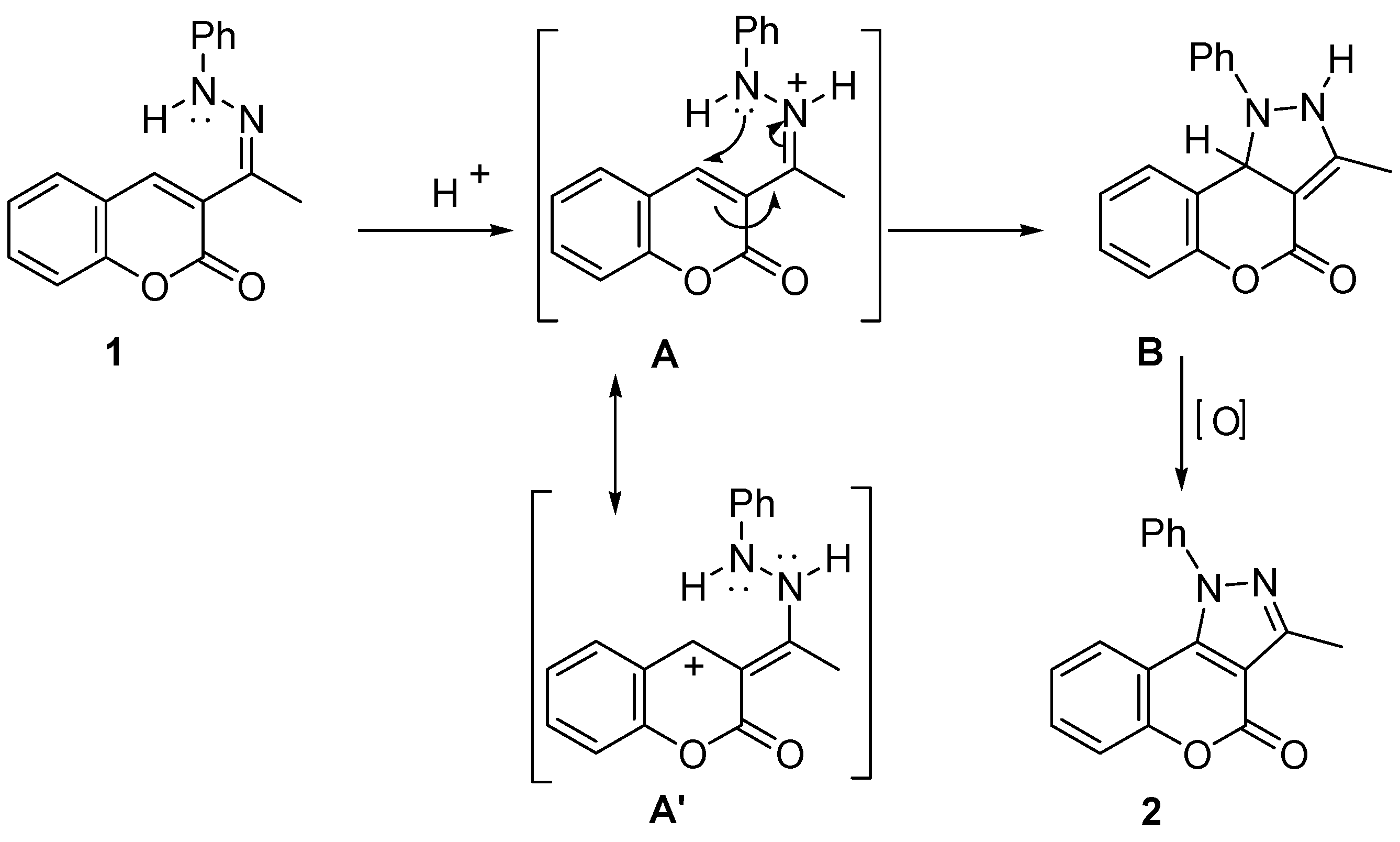
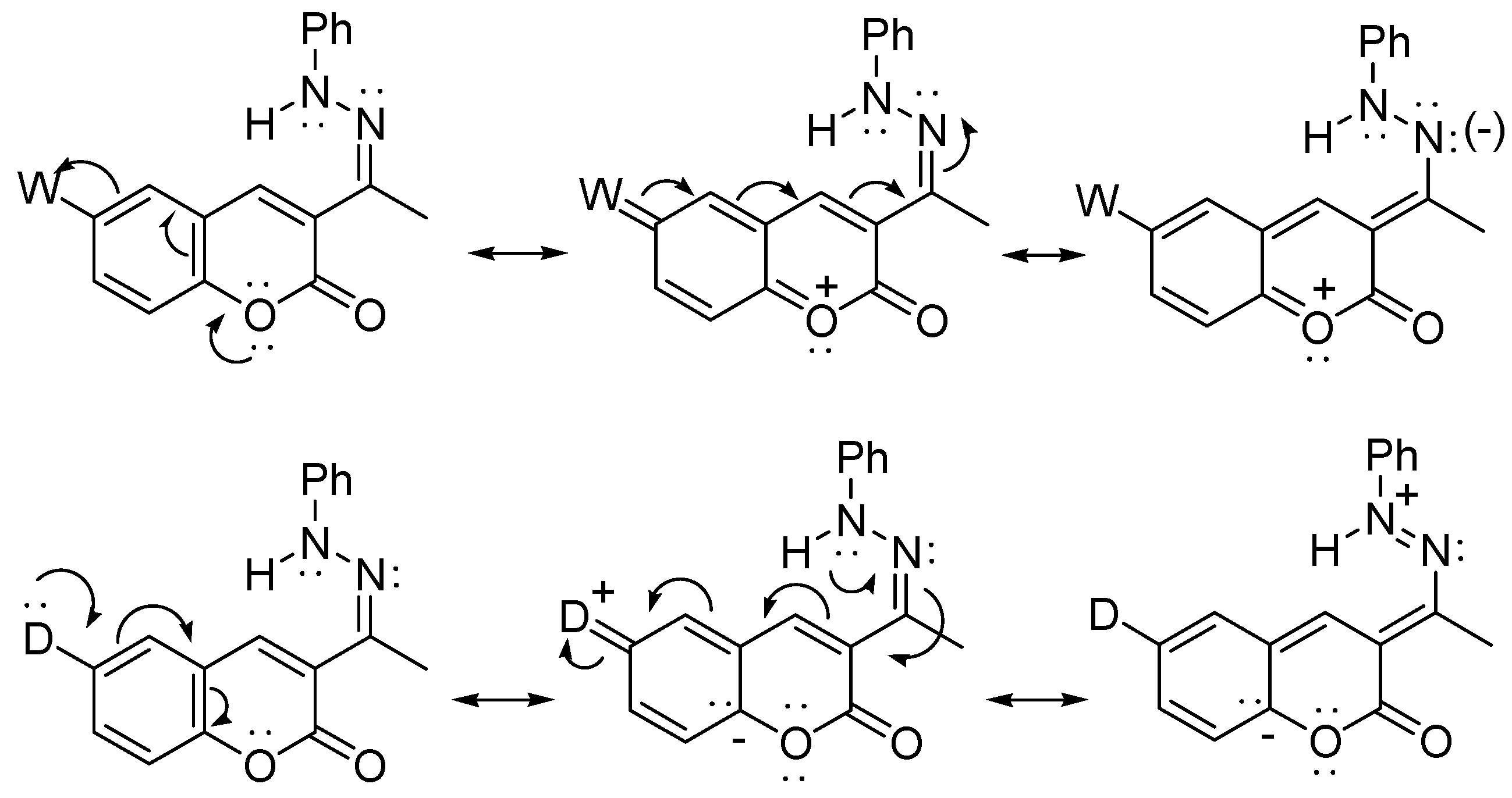
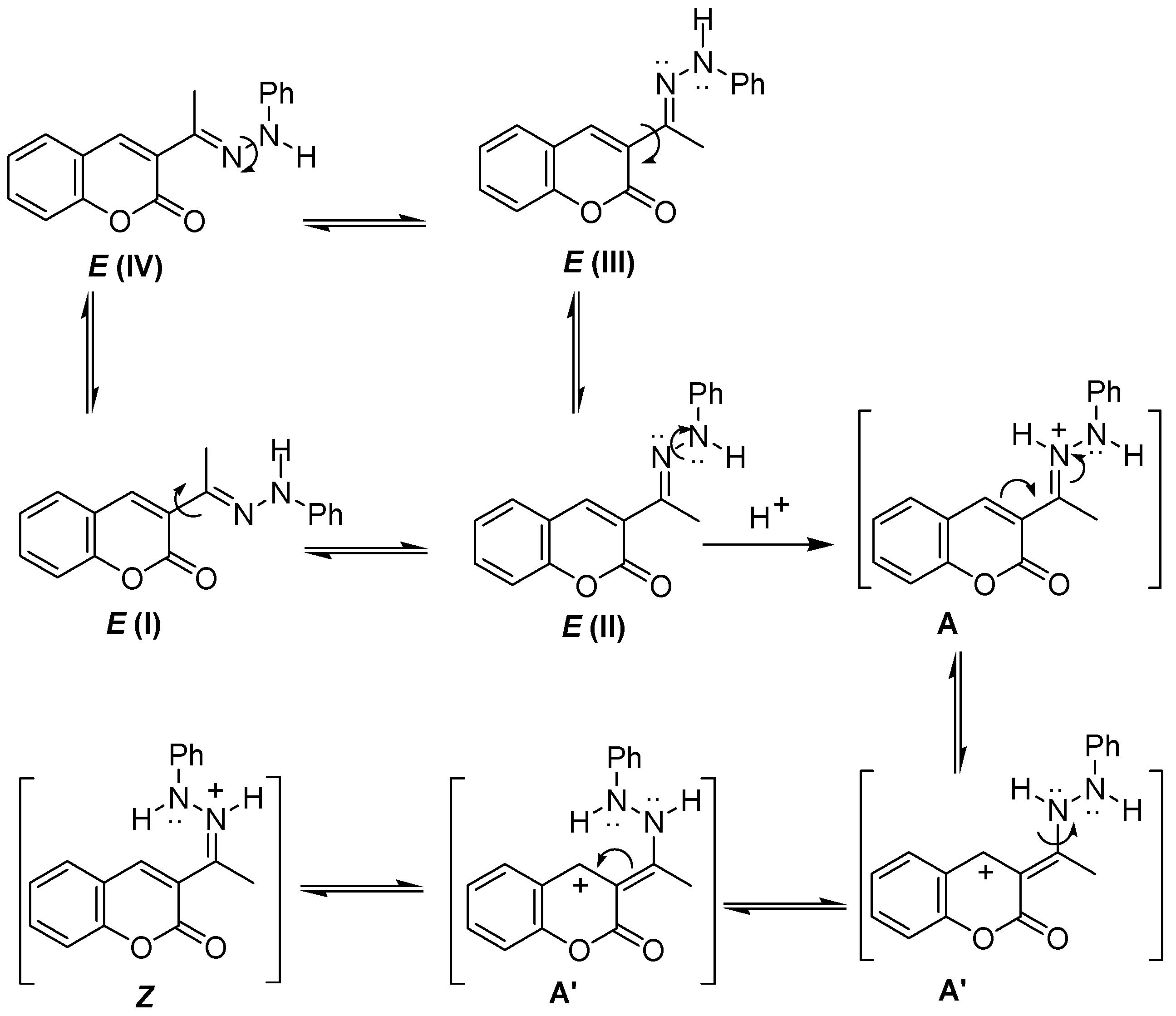
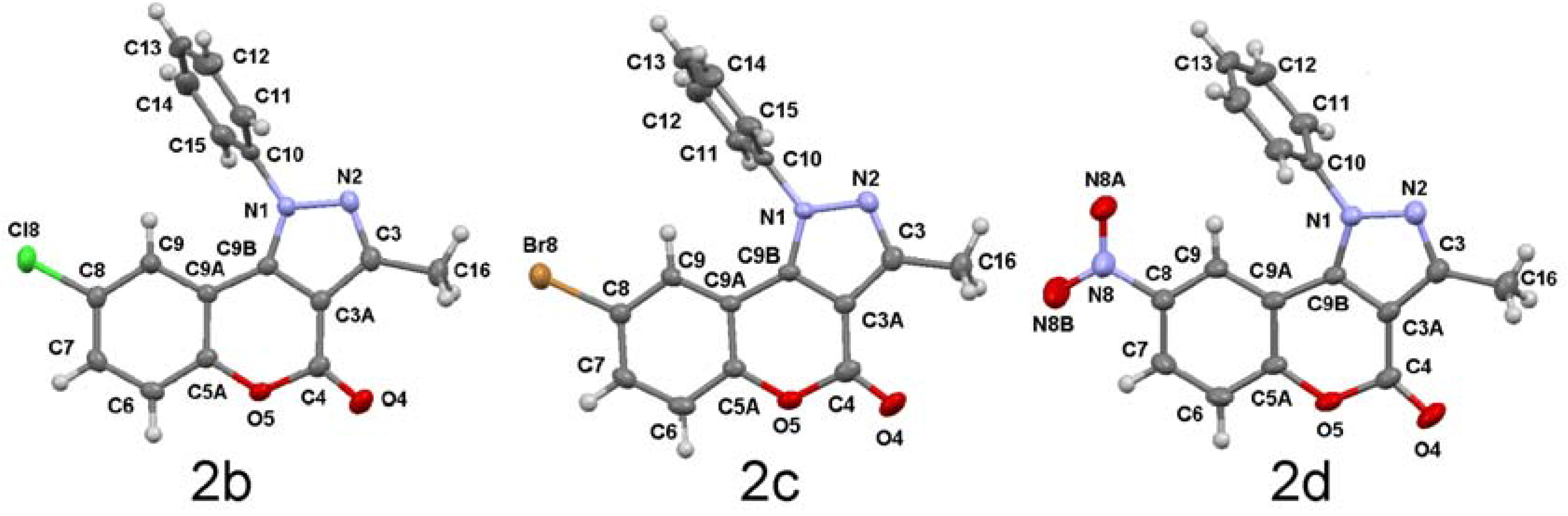
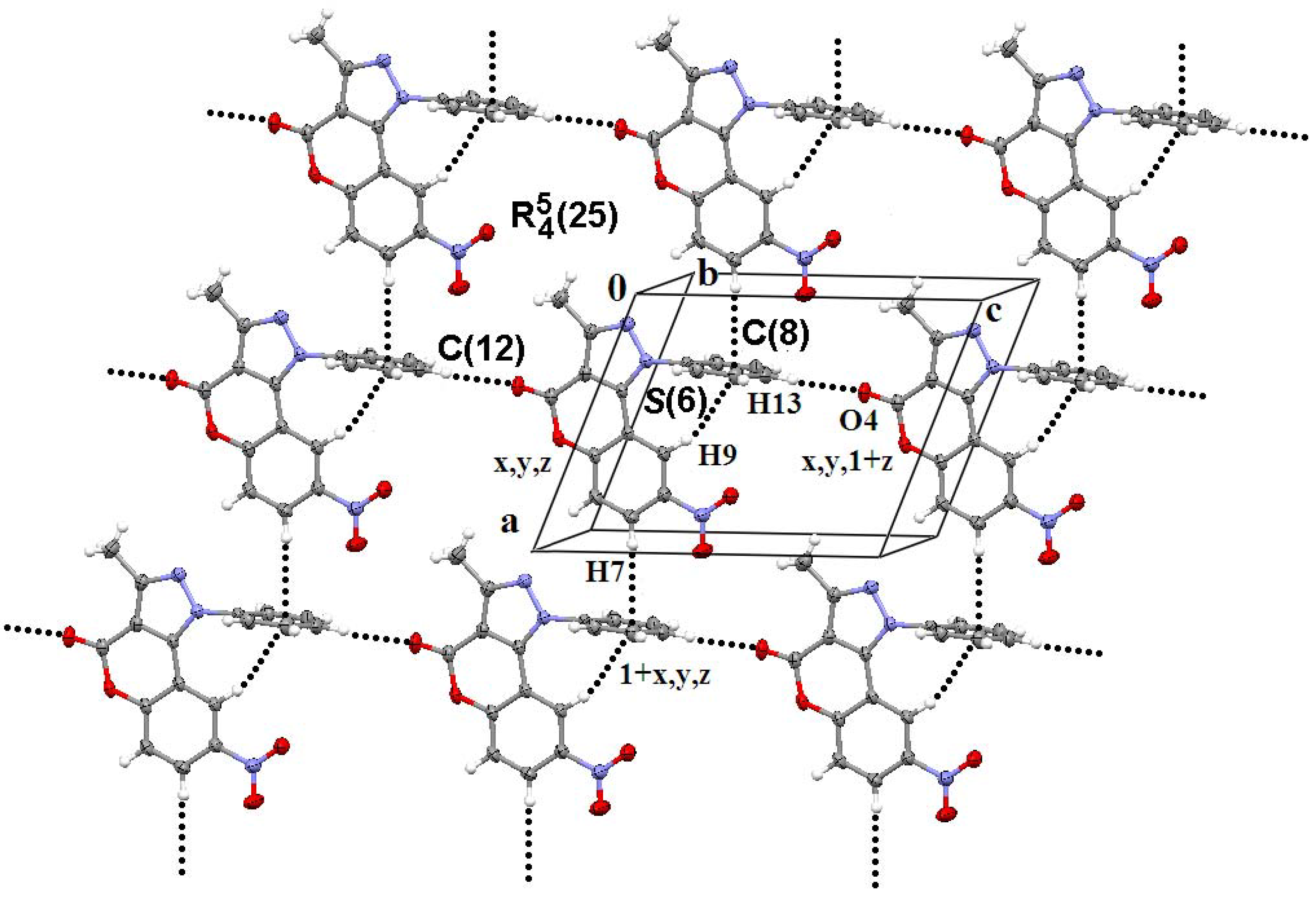
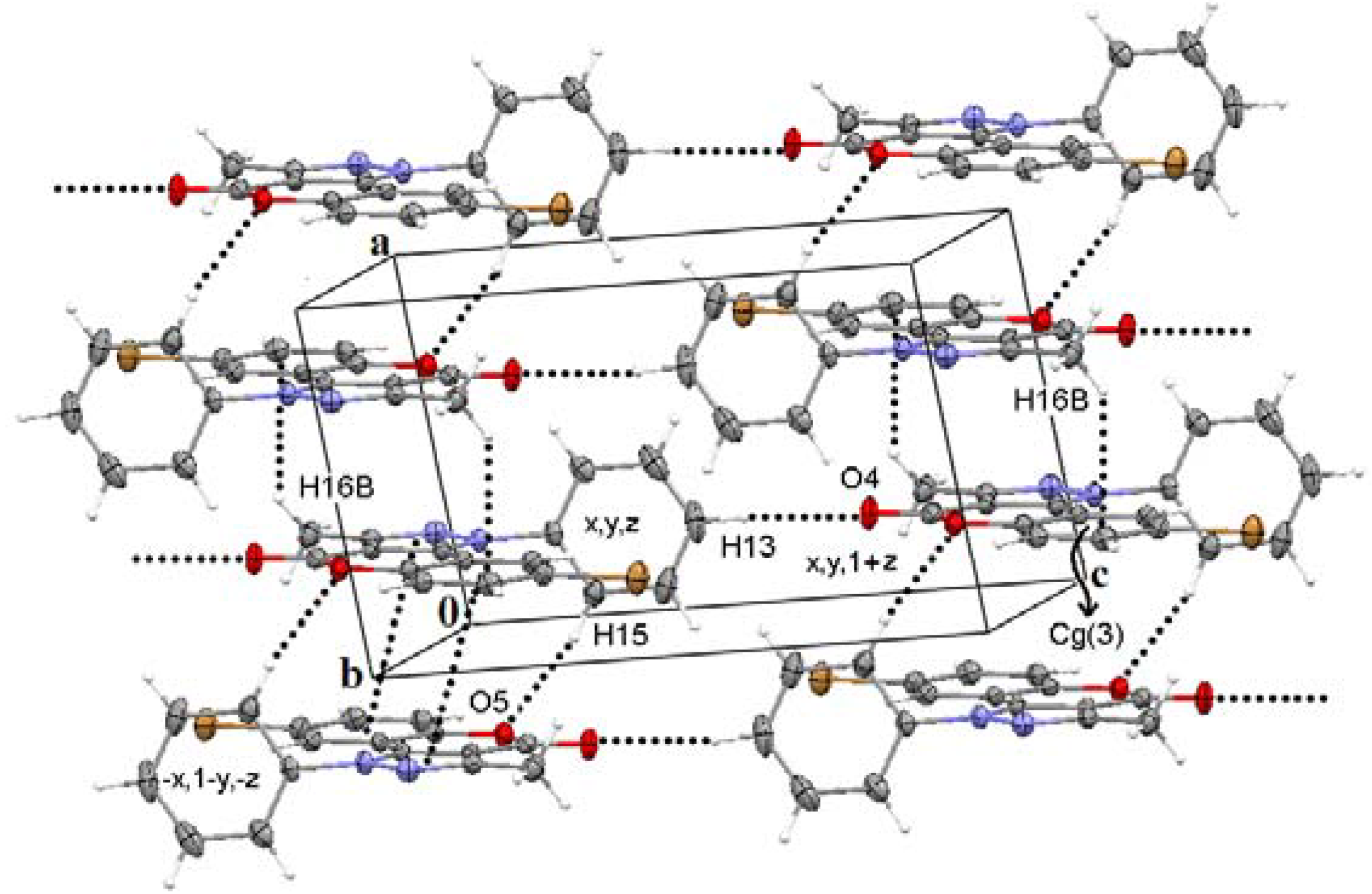
3.3. General Methods of Synthesis
6-Substituted-3-[1-(phenylhydrazono)-ethyl]-chromen-2-ones
1a-i. were synthesized from phenyl-hydrazine and 0.5 g of the corresponding coumarins, following standard procedures. The syntheses of compounds
2a [
15,
17],
2b [
15],
2c,
2d [
23] have been reported elsewhere, albeit with lack of some spectroscopic data, thus for completeness purposes they are included but elemental analysis was performed only to the new compounds
2e-f.
3-[1-(Phenylhydrazono)-ethyl]-chromen-2-one (1a). Obtained from 3-acetyl-2H-1-benzopyran-2-one (0.5 g, 2.66 mmol) and phenylhydrazine (0.26 mL, 2.66 mmol) as an orange solid in 85% yield (0.633 g, 2.26 mmol), mp = 193–196 °C, IR νneat (cm−1): 3295 (N-H), 1695 (OC=O), 1596 (C=O), 1255, 1155 (C-O). 1H-NMR (DMSO-d6) δ: 9.43 (s, 1H, NH), 8.16 (s, 1H, H-4), 7.81 (d, 1H, H-5, 3J = 7.7), 7.57 (dd, 1H, H-7, 3J = 8.0, 7.5), 7.38 (d, 1H, H-8, 3J = 8.3, 3J = 8.3), 7.33 (t, 1H, H-6, 3J = 8.0, 7.6,4J = 2.2), 6.74–7.24 (m, 5H, Ph), 2.20 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ: 160.2 (C-2), 153.6 (C-9), 146.2 (C-11), 139.8 (C-4), 139.2 (Ci), 132.2 (C-7), 129.5 (C-5), 129.5 (Cm), 127.9 (C-3), 125.2 (C-6), 120.0 (Cp), 116.4 (C-8), 119.9 (C-10), 113.7 (Co), 15.8 (Me). Anal. Calcd for C17H14N2O2; C, 73.37; H, 5.07; N, 10.12. Found: C, 73.27; H, 4.91; N, 10.12. m/z = 277.1 (M, 22%), 77 (20%).
6-Chloro-3-[1-(phenylhydrazono)-ethyl]-chromen-2-one (1b). Obtained from 3-acetyl-6-cloro-2H-1-benzopyran-2-one (0.5 g, 2.22 mmol) and phenylhydrazine (0.22 mL, 2.22 mmol) as an orange solid in 82% yield (0.578 g, 1.83 mmol), mp = 184–188 °C, IR νneat (cm−1): 3300 (N-H), 1703 (OC=O), 1598 (C=O), 1251, 1155 (C-O), 810 (C-Cl). 1H-NMR (DMSO-d6) δ: 9.49 (s, 1H, NH), 8.17 (s, 1H, H-4), 7.97 (d, 1H, H-5, 4J = 2.3), 7.57 (dd, 1H, H-7, 3J = 8.8, 4J = 2.3), 7.43 (d, 1H, H-8, 3J = 8.8), 6.75–7.25 (m, 5H, Ph), 2.20 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ: 159.2 (C-2), 151.6 (C-9), 145.4 (C-11), 138.1 (Ci), 137.8 (C-4), 130.9 (C-7), 129.0 (C-6), 128.8 (Cm), 128.2 (C-3), 127.7 (C-5), 120.8 (Cp), 119.5 (C-10), 117.8 (C-8), 113.1 (Co), 15.0 (Me). m/z = 312 (M, 30%), 313 (8%), 240 (8%), 77 (28%).
6-Bromo-3-[1-(phenylhydrazono)-ethyl]-chromen-2-one (1c). Obtained from 3-acetyl-6-bromo-2H-1-benzopyran-2-one (0.5 g, 1.87 mmol) and phenylhydrazine (0.18 mL, 1.87 mmol) as an orange solid in 67% yield, (0.451 g, 1.25 mmol), mp = 184–186 °C, IR νneat (cm−1): 3301 (N-H), 1704 (OC=O), 1597 (C=O), 1250, 1158 (C-O), 681 (C-Br). 1H-NMR (DMSO-d6) δ: 9.48 (s, 1H, NH), 8.15 (s, 1H, H-4), 8.08 (d, 1H, H-5, 4J = 2.3), 7.68 (dd, 1H, H-7, 3J = 8.8, 4J = 2.3), 7.35 (d, 1H, H-8,3J = 8.8), 6.75–7.23 (m, 5H, Ph), 2.17 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ: 159.1 (C-2), 152.0 (C-9), 145.4 (C-11), 138.1 (Ci), 137.7 (C-4), 133.8 (C-7), 130.8 (C-5), 128.9 (Cm), 128.3 (C-3), 121.3 (C-6), 119.5 (Cp), 118.1 (C-8), 116.1 (C-10), 113.1 (Co), 15.0 (Me). m/z = 356 (M, 100%), 358 (30%), 357 (20%), 278 (5%), 77 (27%).
6-Nitro-3-[1-(phenylhydrazono)-ethyl]-chromen-2-one (1d). Obtained from 3-acetyl-6-nitro-2H-1-benzopyran-2-one (0.5 g, 2.14 mmol) and phenylhydrazine (0.21 mL, 2.14 mmol) as an orange solid in 53% yield (0.370 g, 1.14 mmol), mp = 204–206 °C, IR νneat (cm−1): 3328 (N-H), 1726 (OC=O), 1604 (C=O), 1516, 1340 (C-NO2), 1239, 1113 (C-O). 1H-NMR (DMSO-d6) δ: 9.55 (s, 1H, NH), 8.84 (d, 1H, H-5, 4J = 2.6), 8.35 (dd, 1H, H-7, 3J = 9.1, 4J = 2.6), 8.40 (s, 1H, H-4), 7.60 (d, 1H, H-8, 3J = 9.1), 6.76-7.78 (5H, -Ph), 2.20 (s, 3H, CH3). 13C-NMR (DMSO-d6) δ: 159.2 (C-2), 157.1 (C-9), 144.4 (C-11), 144.2 (C-6), 137.7 (C-4), 137.5 (Ci), 130.6 (C-3), 129.6 (Cm), 126.1 (C-7), 124.2 (C-5), 121.5 (Cp), 119.9 (C-10), 117.7 (C-8), 113.6 (Co), 13.7 (Me). m/z = 322 (M, 20%), 246 (5%), 77 (15%).
6-Methoxy-3-[1-(phenylhydrazono)-ethyl]-chromen-2-one (1e). Obtained from 3-acetyl-6-methoxy-2H-1-benzopyran-2-one (0.5 g, 2.29 mmol) and phenylhydrazine (0.23 mL, 2.29 mmol) as an orange solid in 72% yield (0.512 g, 1.65 mmol), mp = 147–149 °C, IR νneat (cm−1): 3303 (N-H), 1698 (OC=O), 1574 (C=O), 1243, 1134 (C-O). 1H-NMR δ: 7.98 (s, 1H, H-4), 7.63 (s, 1H, NH), 7.24 (d, 1H, H-8, 3J = 8.1), 7.05 (dd, 1H, H-7, 3J = 9.1, 4J = 2.1), 6.86–7.30 (m, 5H,-Ph), 6.97 (d, 1H, H-5,4J = 2.4), 2.28 (s, 3H, CH3). 13C-NMR δ: 160.9 (C-2), 156.3 (C-6), 148.4 (C-9), 144.8 (C-11), 139.7 (C-4), 139.3 (Ci), 129.5 (Cm), 127.9 (C-3), 120.9 (Cp), 120.1 (C-10), 119.7 (C-7), 117.6 (C-8), 110.2 (C-5), 113.5 (Co), 14.1 (Me). m/z = 307 (M, 24%), 230 (5%), 77 (15%).
8-Methoxy-3-[1-(phenylhydrazono)-ethyl]-chromen-2-one (1f). Obtained from 3-acetyl-8-methoxy-2H-1-benzopyran-2-one (0.5 g, 2.29 mmol) and phenylhydrazine (0.23 mL, 2.29 mmol) as an orange solid in 91% yield (0.647 g, 2.09 mmol), mp = 152–156 °C, IR νneat (cm−1): 3306 (N-H), 1700 (OC=O), 1601 (C=O), 1263, 1160 (C-O). 1H-NMR δ: 8.02 (s, 1H, H-4), 7.59 (s, 1H, NH), 7.28 (d, 1H, H-7, 3J = 7.7), 7.17 (t, 1H, H-6, 3J = 7.7), 7.06 (d, 1H, H-5, 3J = 7.7), 6.87-7.36 (m, 5H, Ph), 2.29 (s, 3H, CH3). 13C-NMR δ: 160.2 (C-2), 147.1 (C-8), 144.7 (C-11), 140.0 (C-4), 143.5 (C-9), 139.3 (Ci), 129.5 (Cm), 127.9 (C-3), 124.6 (C-5), 120.9 (C-6), 120.4 (C-10), 120.0 (Cp), 113.5 (Co), 113.4 (C-7), 14.1 (Me). m/z = 306.2 (M, 100%), 230 (5%), 77 (17%).
6-Bromo-8-methoxy-3-[1-(phenylhydrazono)-ethyl]-chromen-2-one (1g). Obtained from 3-acetyl-6-bromo-8-methoxy-2H-1-benzopyran-2-one (0.5 g, 1.68 mmol) and phenylhydrazine (0.16 mL, 1.68 mmol) as an orange solid in 74% (0.485 g, 1.25 mmol), mp = 185–188 °C, IR νneat (cm−1) 3312 (N-H), 1713 (OC=O), 1599 (C=N), 1258 (C-O). 1H-NMR δ: 7.95 (s, 1H, H-4), 7.58 (s, 1H, NH), 7.34 (s, 1H, H-5), 7.24 (s, 1H, H-7), 6.84–7.29 (m, 5H, Ph), 2.28 (s, 3H, CH3), 3.96 (s, 3H, OMe). 13C-NMR δ: 156.3 (C-2), 151.6 (C-8), 147.8 (C-9), 144.5 (C-11), 138.5 (C-13), 138.3 (C-4), 129.5 (Cm), 128.8 (C-3), 121.1 (Cp), 122.0 (C-5), 121.4 (C-6), 116.9 (C-10), 116.5 (C-7), 113.5 (Co), 56.7 (MeO–), 13.8 (Me). m/z = 386 (M, 100%), 308 (5%), 77 (20%).
8-Bromo-6-chloro-3-[1-(phenylhydrazono)-ethyl]-chromen-2-one (1h). Obtained from 3-acetyl-8-bromo-6-chloro-2H-1-benzopyran-2-one (0.5 g, 1.66 mmol) and phenylhydrazine (0.16 mL, 1.66 mmol) as an orange solid in 84% yield (0.548 g, 1.39 mmol), mp = 199–201 °C, IR νneat (cm−1) 3312 (N-H), 1707 (OC=O), 1530 (C=N), 1248 (C-O). 1H-NMR δ: 7.95 (s, 1H, H-4), 7.62 (s, 1H, NH), 7.71 (d, 1H, H-7, 4J = 2.4), 7.51 (d, 1H, H-5, 4J = 2.4), 6.90-7.29 (m, 5H, Ph.), 2.29 (s, 3H, CH3). 13C-NMR δ:159.2 (C-2), 144.3 (C-11), 140.2 (C-9), 137.8.5 (C-13), 137.6 (C-4), 134.2 (C-7), 130.1 (C-6), 129.6 (C-14), 129.2 (C-3), 126.9 (C-5), 121.6 (C-10), 113.5 (C-15), 110.7 (C-8), 13.8 (Me). m/z = 390.1 (M, 100%), 391.1 (30%), 392.0 (25%), 315 (5%), 76.9 (30%).
6,8-Dichloro-3-[1-(phenylhydrazono)-ethyl]-chromen-2-one (1i). Obtained from 3-acetyl-6,8-dichloro-2H-1-benzopyran-2-one (0.5 g, 1.95 mmol) and phenylhydrazine (0.19 mL, 1.95 mmol) as an orange solid in 82% yield (0.557 mg, 1.60 mmol), mp = 196–198 °C, IR νneat (cm−1) 3311 (N-H), 1709 (OC=O), 1533 (C=N), 1162 (C-O). 1H-NMR δ: 7.96 (s, 1H, H-4), 7.62 (s, 1H, NH), 7.55 (d, 1H, H-7,4J = 2.2), 7.41 (2, 1H, H-5, 4J = 2.2), 6.94–7.30 (m, 5H, Ph), 2.29 (s, 3H, CH3). 13C-NMR δ: 159.0 (C-2), 151.3 (C-9), 144.3 (C-11), 138.8 (C-13), 137.6 (C-4), 134.2 (C-6), 131.3 (C-7), 129.8 (Cm), 129.7 (C-3), 124.2 (C-8), 121.6 (C-10), 121.3 (Cp), 113.6 (Co), 13.8 (Me). m/z = 347 (M, 20%), 346.3 (55%), 274 (8%), 77 (30%).
3-Methyl-1-phenyl-1H-chromeno[4,3-c]pyrazol-4-one (2a). Cu(CH3COO)2·H2O (0.025 g, 0.125 mmol) was dissolved in ethyl alcohol (20 mL) and added to a solution of 1a (0.500 g, 1.78 mmol) and ethyl alcohol (30 mL). The mixture was refluxed during 3 h, the resulting solid was filtered, washed with cold ethyl alcohol (5 mL) and several times with distilled water, air dried and recrystallized from ethyl acetate to obtain 0.372 mg (1.34 mmol) of 2a as a white powder in 76% yield, mp = 227–230 °C, IR νneat (cm−1): 1726 (OC=O), 1272, 1202 (C-O). 1H-NMR δ: 7.44 (t, 1H, H-7, 3J = 8.6, 4J = 1.6 Hz), 7.40 (d, 1H, H-6, 3J = 8.1), 7.09 (d, 1H, H-9, 3J = 7.9), 7.02 (t, 1H, H-8, 3J = 7.9, 4J = 1.6), 7.52–7.62 (m, 5H, Ph), 2.67 (s, 3H, CH3). 13C-NMR δ: 158.3 (C-4), 153.4 (C-5a), 151.0 (C-3), 141.9 (C-9b), 139.5 (Ci), 131.3 (C-7), 130.4 (Cp), 130.1 (Co), 127.0 (Cm), 124.1 (C-8), 122.6 (C-9), 118.2 (C-6), 112.0 (C-9a), 106.5 (C-3a), 13.1 (Me). m/z = 276.2 (M, 100%), 247.3 (5%), 206.2 (14%), 77.0 (16%).
8-Chloro-3-methyl-1-phenyl-1H-chromeno[4,3-c]pyrazol-4-one (2b). Obtained as described for 2a starting from 1b (0.500 g, 1.59 mmol) to give 2b (0.343 g, 1.10 mmol, 69% yield) as a pale yellow powder, mp = 280–283 °C, IR νneat (cm−1): 1743 (OC=O), 1204 (C-O), 814 (C-Cl). 1H-NMR δ: 7.58 (dd, 1H, H-7 3J = 8.8, 4J = 1.9), 7.35 (d, 1H, H-6 3J = 8.8), 7.03 (d, 1H, H-9 4J = 1.9), 7.38–7.65 (m, 5H, Ph), 2.68 (s, 3H, CH3). 13C-NMR δ: 157.6 (C-4), 151.8 (C-5a), 151.2 (C-3), 140.7 (C-9b), 139.0 (Ci), 131.2 (C-7), 130.8 (C-p), 130.2 (C-o), 129.5 (C-8), 126.9 (C-m), 122.3 (C-9), 119.6 (C-6), 113.1 (C-9a), 106.8 (C-3a), 13.1 (Me). m/z = 310.2 (M, 100%), 311.0 (70%), 309.3 (45%), 275.3 (5%), 77 (22%).
8-Bromo-3-methyl-1-phenyl-1H-chromeno[4,3-c]pyrazol-4-one (2c). Obtained as described for 2a starting from 1c (0.500 g, 1.39 mmol) to afford 2c (0.388 g, 1.09 mmol, 78% yield) as a white powder, mp = 278–280 °C, IR νneat (cm−1): 1742 (OC=O), 1266, 1203 (C-O). 1H-NMR δ: 7.52 (dd, 1H, H-7), 7.28 (d, 1H, H-6, 3J = 8.9), 7.16 (d, 1H, H-9 4J = 2.4), 7.54–7.78 (m, 5H, Ph), 2.67 (s, 3H, CH3). 13C-NMR δ: 157.6 (C-4), 152.3 (C-5a), 151.2 (C-3), 140.6 (C-9b), 139.0 (Ci), 134.0 (C-7), 130.8 (Cp), 130.3 (Co), 126.9 (Cm), 125.3 (C-9), 119.9 (C-8), 116.8 (C-6), 113.7 (C-9a), 106.8 (C-3a), 13.1 (Me). m/z = 354.3 (M, 80%), 356.1 (100%), 356.9 (35%), 358.0 (5%), 274.3 (5%), 77 (25).
3-Methyl-8-nitro-1-phenyl-1H-chromeno[4,3-c]pyrazol-4-one (2d). Obtained as described for 2a starting from 1d (0.500 g, 1.54 mmol) to give 2d (0.412 g, 1.28 mmol, 83% yield) as a pale yellow powder, mp = 248–254 °C, IR νneat (cm−1): 1756 (OC=O), 1259, 1207 (C-O), 1519 (C-NO2). 1H-NMR δ: 8.31 (dd, 1H, H-7, 3J = 9.1, 4J = 2.6), 8.02 (d, 1H, H-9 4J = 2.6), 7.55 (d, 1H, H-6, 3J = 9.1), 7.56–7.72 (m, 5H, Ph), 2.71 (s, 3H, CH3). 13C-NMR δ: 156.9 (C-4), 156.6 (C-5a), 151.5 (C-3), 143.6 (C-9b), 140.2 (C-8), 138.6 (Ci), 131.2 (Cp), 130.6 (Co), 126.7 (Cm), 126.0 (C-7), 119.3 (C-9), 118.8 (C-6), 112.4 (C-9a), 106.8 (C-3a), 13.1 (Me). m/z = 321.0 (M, 100%), 320.2 (25%), 322.9 (5%), 275.3 (10%), 77 (21%).
8-Methoxy-3-methyl-1-phenyl-1H-chromeno[4,3-c]pyrazol-4-one (2e). Obtained as described for 2a starting from 1e (0.500 g, 1.61 mmol) to obtain 2e (0.258 g, 0.84 mmol, 52% yield) as a white powder, mp = 232–234 °C, IR νneat (cm−1): 1734 (OC=O), 1238, 1203 (C-O). 1H-NMR δ: 7.32 (d, 1H, H-6,3J = 9.0), 6.98 (dd, 1H, H-7, 3J = 9.0, 4J = 3.1), 6.50 (d, 1H, H-9, 4J = 3.1), 7.63-7.54 (m, 5H, Ph), 2.68 (s, 3H, CH3). 13C-NMR δ: 158.4 (C-4), 155.6 (C-8), 151.0 (C-3), 147.9 (C-5a), 141.9 (C-9b), 139.5 (Ci), 130.5 (Co), 130.5 (Cp), 127.4 (Cm), 119.2 (C-7), 118.8 (C-6), 112.1 (C-9a), 106.7 (C-3a), 105.5 (C-9), 13.4 (Me). m/z = 306.1 (M, 100%), 291.3 (28%), 277 (3%), 77 (22%). Anal. Calcd. for C18H14N2O3; C, 70.58; H, 4.61; N, 9.14. Found: C, 70.22; H, 4.50; N, 9.00.
6-Methoxy-3-methyl-1-phenyl-1H-chromeno[4,3-c]pyrazol-4-one (2f). Obtained as described for 2a starting from 1f (0.500 g, 1.61 mmol) to give 2f (0.248 g, 0.806 mmol, 50% yield) as a white powder, mp = 238–240 °C, IR νneat (cm−1): 1743 (OC=O), 1273, 1207 (C-O). 1H-NMR δ: 7.02 (dd, 1H, H-7,3J = 8.2, 7.6), 6.97 (t, 1H, H-8, 3J = 7.6, 8.2), 6.65 (dd, 1H, H-9 3J = 7.6, 4J = 1.5), 7.54–7.62 (m, 5H, Ph), 2.69 (s, 3H, CH3). 13C-NMR δ: 157.6 (C-4), 151.0 (C-3), 148.4 (C-6), 143.3 (C-5a), 142.1 (C-9b), 139.6 (Ci), 130.4 (Cp), 130.0 (Co), 127.2 (Cm), 123.9 (C-8), 114.1 (C-9), 112.9 (C-7), 112.7 (C-9a), 106.6 (C-3a), 13.2 (Me). m/z = 306.1 (M, 100%), 291.3 (5%), 277 (20%), 77 (22%). Anal. Calcd. for C18H14N2O3; C, 70.58; H, 4.61; N, 9.14. Found: C, 70.83; H, 4.70; N, 9.00.
8-Bromo-3-methyl-6-methoxy-1H-chromeno[4,3-c]pyrazol-4-one (2g). Obtained as described for 2a starting from 1g (0.500 g, 1.28 mmol) to obtain 2g (0.393 g, 1.01 mmol, 79% yield) as a pale yellow powder, mp = 289–292 °C, IR νneat (cm−1): 1744 (OC=O), 1275, 1205 (C-O). 1H-NMR δ: 6.72 (s, 1H, H-9), 7.06 (s, 1H, H-6), 7.51-7.62 (m, 5H, Ph), 2.67 (s, 3H, CH3). 13C-NMR δ: 156.7 (C-4), 151.1 (C-5a), 148.9 (C-3), 142.3 (C-6), 140.1 (C-9b), 139.0 (C-10), 130.7 (C-9), 130.1 (C-11), 127.0 (C-12), 123.9 (C-5), 123.3 (C-8), 116.5 (C-13), 116.1 (C-7), 113.7 (C-9a), 106.8 (C-3a), 13.1 (Me). m/z = 384.5 (M, 80%), 386.2 (100%), 385.5 (25%), 357.5 (10%), 290.5 (10%), 77.0 (25%). Anal. Calcd. for C18H13N2O3Br; C, 56.13; H, 3.40; N, 7.27. Found: C, 55.88; H, 3.40; N, 7.20.
6-Bromo-8-Chloro-3-methyl-1H-chromeno[4,3-c]pyrazol-4-one (2h). Obtained as described for 2a starting from 1h (0.5 g, 1.27 mmol) to obtain 2h (0.249 g, 0.64 mmol, 50% yield) as a pale yellow powder, mp = 259–261 °C, IR νneat (cm−1): 1749 (OC=O), 1277, 1224 (C-O). 1H-NMR δ: 6.90 (s, 1H, H-9), 7.82 (s, 1H, H-7), 7.53-7.65 (m, 5H, Ph), 2.65 (s, 3H, CH3). 13C-NMR δ: 156.2 (C-4), 151.1 (C-5a), 148.2 (C-3), 140.2 (C-9b), 138.8 (C-10), 134.2 (C-7), 130.9 (C-9), 130.6 (C-11), 129.5 (C-8), 126.9 (C-12), 121.4 (C-13), 113.9 (C-6), 112.6 (C-9a), 106.8 (C-3a), 13.0 (Me). m/z = 390.0 (M, 100%), 389.5 (60%), 388.5 (62%), 310 (5%), 77(25%). Anal. Calcd. for C17H10N2O2BrCl; C, 52.40; H, 2.59; N, 7.19. Found: C, 52.70; H, 2.63; N, 7.00.
6,8-Dichloro-3-methyl-1H-chromeno[4,3-c]pyrazol-4-one (2i). Obtained as described for 2a starting from 1i (0.5 g, 1.43 mmol) to obtain 2i (0.259 g, 0.74 mmol, 52% yield) as a pale yellow powder, mp = 224–226 °C, IR νneat (cm−1): 1750 (OC=O), 1225 (C-O). 1H-NMR δ: 6.90 (s, 1H, H-9), 7.47 (s, 1H, H-7), 7.52-7.64 (m, 5H, Ph), 2.65 (s, 3H, CH3). 13C-NMR δ: 156.2 (C-4), 151.1 (C-5a), 147.8 (C-3), 140.2 (C-9b), 138.8 (C-10), 131.3 (C-7), 130.9 (C-9), 130.3 (C-11), 129.1 (C-8), 126.9 (C-12), 124.1 (C-6), 120.8 (C-13), 114.0 (C-9a), 106.8 (C-3a), 13.0 (Me). m/z = 344.5 (M, 100%), 346.2 (80%), 345.3 (68%), 308.5, 77 (22%). Anal. Calcd. for C17H10N2O2Cl2; C, 59.15; H, 2.92; N, 8.11. Found: C, 58.90; H, 2.89; N, 8.00.