Green One Pot Solvent-Free Synthesis of Pyrano[2,3-c]-Pyrazoles and Pyrazolo[1,5-a]Pyrimidines
Abstract
:Introduction
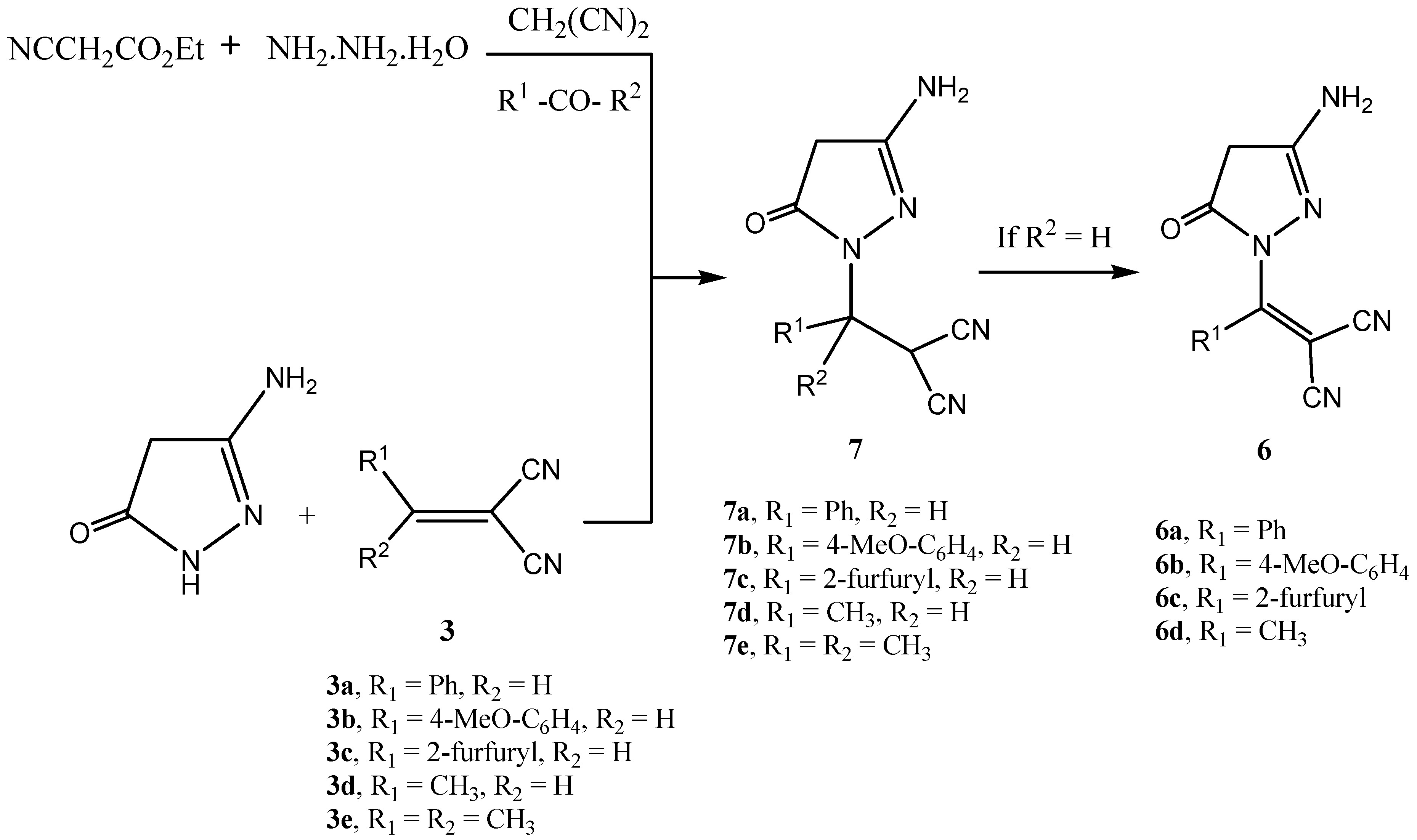
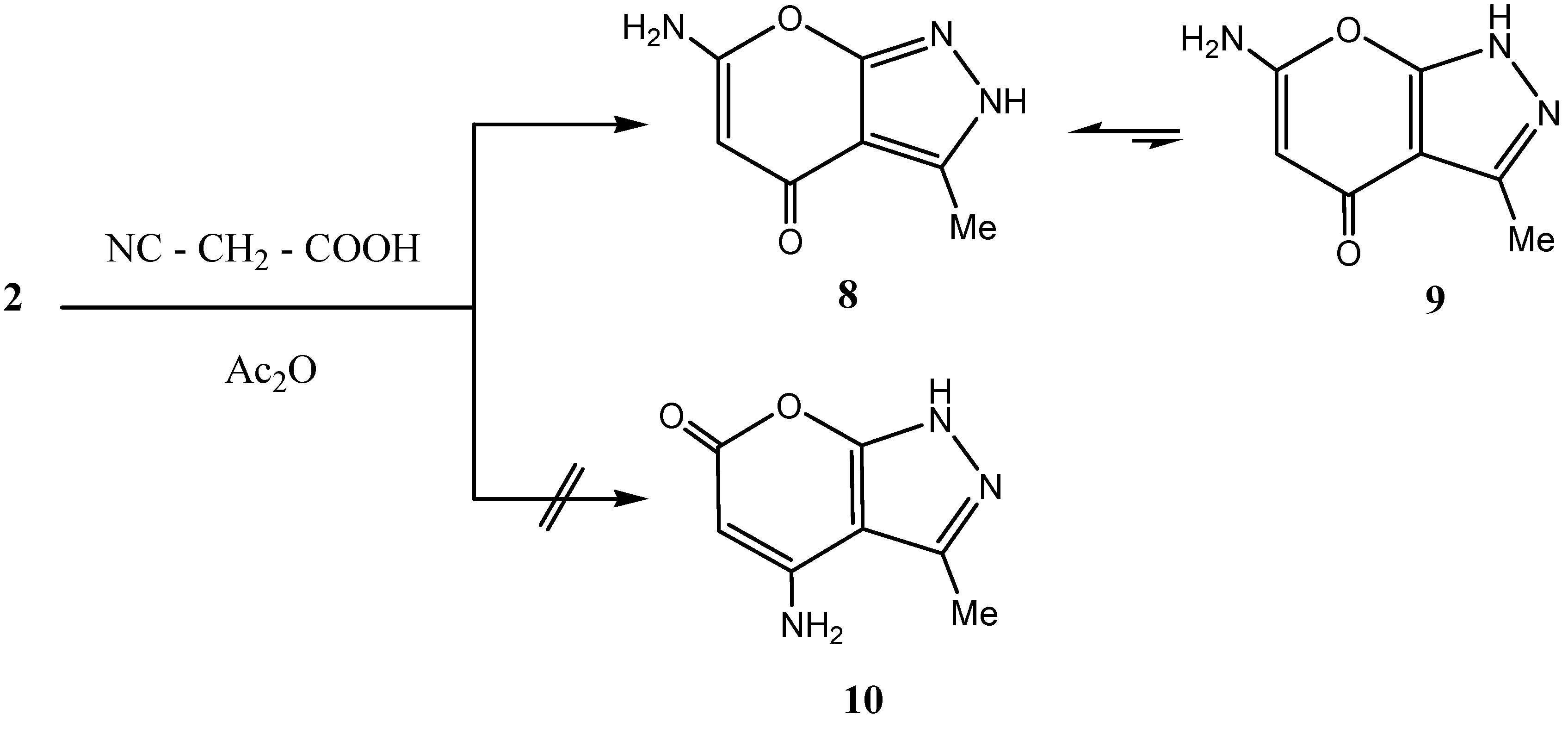
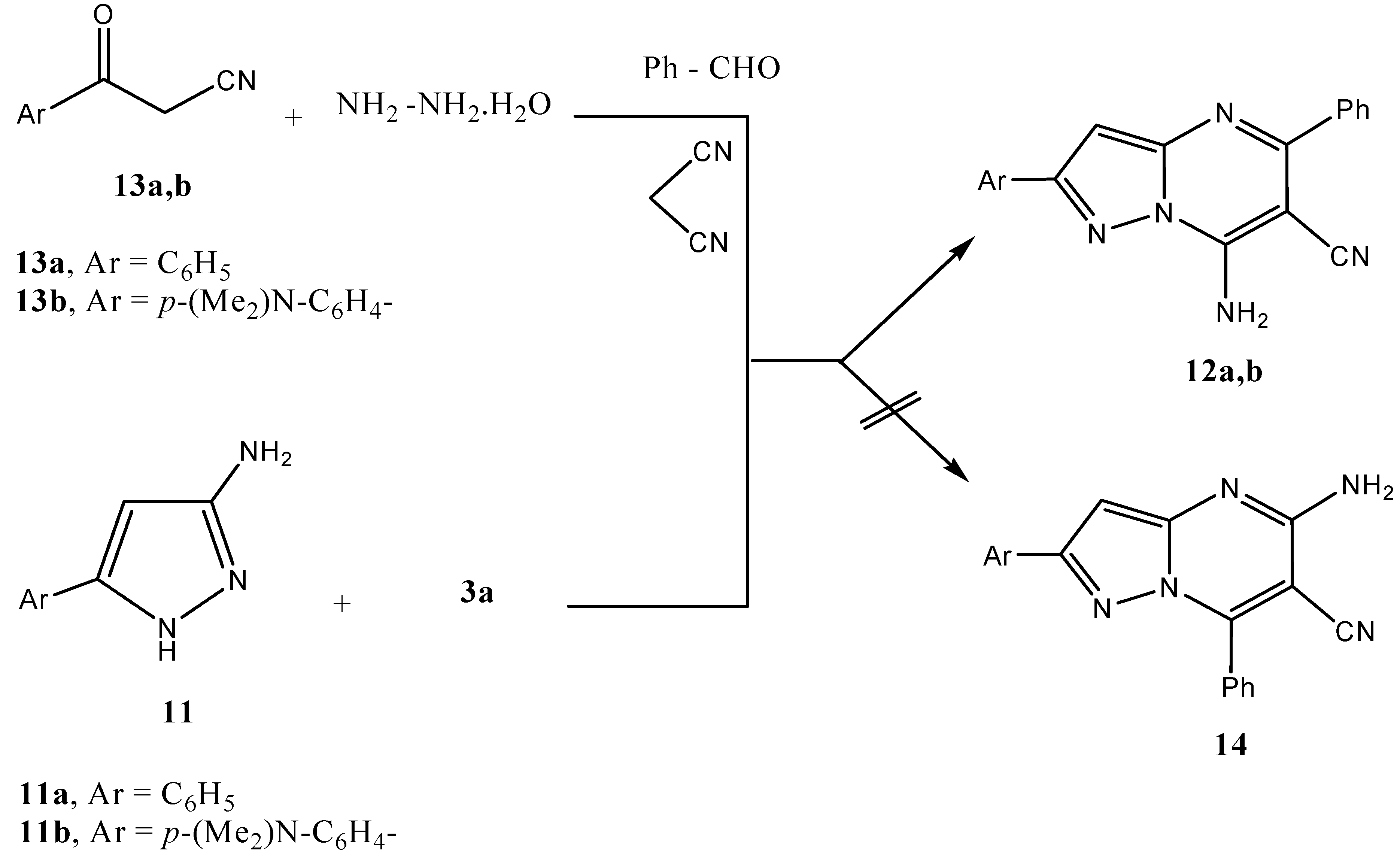
Result and Discussion

Experimental
General
Synthesis of pyranopyrazoles 4a-e
Method A
Method B
Reaction of 3-amino-5-pyrazolone with arylidene-malononitriles 3
Method A
Method B
Cyanoacetylation of methylpyrazolone 2
Synthesis of pyrazolopyrimidines 12a,b
Conclusions
Supplementary Materials
Supplementary File 1Acknowledgements
References
- Otto, H.H. Synthesis of Some 4H-Pyrano[2.3-c]pyrazoles. Arch. Pharm. 1974, 307, 444–447. [Google Scholar] [CrossRef]
- Abdou, S.; Fahmy, S.M.; Sadek, K.U.; Elnagdi, M.H. Activated nitriles in heterocyclic synthesis: A novel synthesis of pyrano[2,3-c]pyrazoles. Heterocycles 1981, 16, 2177–2180. [Google Scholar] [CrossRef]
- Shestopalov, A.M.; Emel'yanova, Y.M.; Shestopalov, A.A.; Rodinovskaya, L.A.; Niazimbetova, Z.I.; Evans, D.H. Cross-condensation of derivatives of cyanoacetic acid and carbonyl compounds. Part 1: Single-stage synthesis of 1'-substituted 6-amino-spiro-4-(piperidine-4')-2H,4H-pyrano[2,3-c]pyrazole-5-carbonitriles. Tetrahedron 2003, 59, 7491–7496. [Google Scholar] [CrossRef]
- Sharanin, Y.A.; Sharanina, L.G.; Puzanova, V.V. Nitrile cyclization reactions. VII. Synthesis of 6-amino-4-aryl-3-methyl-5-cyano-1H,4H-pyrazolo[3,4-b]pyrans. Zh. Org. Khim. 1983, 19, 2609. [Google Scholar]
- El-Tamany, E.S.; El-Shahed, F.A.; Mohamed, B.H. Synthesis and biological activity of some pyrazole derivatives. J. Serb. Chem. Soc. 1999, 64, 9–18. [Google Scholar]
- Girgis, N.S.; Elgemeie, G.A.M.; Nawar, G.A.M.; Elnagdi, M.H. α, β -Unsaturated nitriles in heterocyclic synthesis: The reaction of β -(2-furanyl)- and β -(2-thienyl)acrylonitrile with active methylene reagents. Liebigs. Ann. Chem. 1983, 1468–1475. [Google Scholar]
- Shestopalov, A.M.; Yakubov, A.P.; Tsyganov, D.V.; Emel'yanova, Y.M.; Nesterov, V.N. Synthesis of substituted 6-amino-4-aryl-5-cyano-2H,4H-pyrano[2,3-c]pyrazoles. Crystal and molecular structure of 6-amino-5-cyano-3-methyl-4-(2',4',6'-triethylphenyl)-2H,4H-pyrano[2,3-c]pyrazole. Chem. Heterocycl. Compds. 2002, 38, 1180–1189. [Google Scholar] [CrossRef]
- Redkin, R.G.; Shemchuk, L.A.; Chernykh, V.P.; Shishkin, O.V.; Shishkina, S.V. Synthesis and molecular structure of spirocyclic 2-oxindole derivatives of 2-amino-4H-pyran condensed with the pyrazolic nucleus. Tetrahedron 2007, 63, 11444–11450. [Google Scholar] [CrossRef]
- Rodinovskaya, L.; Shestopalov, A.; Gromova, A.; Shestopalov, A. Substituted 4-(3-cyanopyridin-2-ylthio)acetoacetates: New convenient reagents for the synthesis of heterocycles. Synthesis 2006, 2357–2370. [Google Scholar]
- Rodinovskaya, L.A.; Gromova, A.V.; Shestopalov, A.M.; Nesterov, V.N. Synthesis of 6-amino-4-aryl-5-cyano-3-(3-cyanopyridin-2-ylthiomethyl)-2,4-dihydropyrano[2,3-c]pyrazoles and their hydrogenated analogs. Molecular structure of 6-amino-5-cyano-3-(3-cyano-4,6-dimethylpyridin-2-ylthiomethyl)-4-(2-nitrophenyl)-2,4-dihydropyrano[2,3-c]pyrazole. Russ. Chem. Bull. 2003, 52, 2207–2213. [Google Scholar] [CrossRef]
- Metwally, N.H.; Abdelrazek, F.M.; Sobhy, N.A. About the reaction of α -substituted cinnamonitriles with 3-substituted and 1,3-disubstituted pyrazolin-5-ones. Afinidad 2005, 62, 616. [Google Scholar]
- Abdelrazek, F.M.; Metz, P.; Metwally, N.H.; El-Mahrouky, S.F. Synthesis and molluscicidal activity of new cinnoline and pyrano [2,3-c]pyrazole derivatives. Arch. Pharm. Chem. Life Sci. 2006, 339, 456–460. [Google Scholar] [CrossRef]
- Lehmann, F.; Holm, M.; Laufer, S. Three-component combinatorial synthesis of novel dihydropyrano[2,3-c]pyrazoles. J. Comb. Chem. 2008, 10, 364–367. [Google Scholar] [CrossRef]
- Al-Matar, H.M.; Khalil, K.D.; Meier, H.; Kolshorn, H.; Elnagdi, M.H. Chitosan as heterogeneous catalyst in Michael additions: The reaction of cinnamonitriles with active methylene moieties and phenols. Arkivoc 2008, xvi, 288–301. [Google Scholar]
- Vasuki, G.; Kumaravel, K. Rapid four-component reactions in water: Synthesis of pyranopyrazoles. Tetrahedron Lett. 2008, 49, 5636–5638. [Google Scholar] [CrossRef]
- Gogoi, S.; Zhao, C. Organocatalyzed enantioselective synthesis of 6-amino-5-cyanodihydropyrano[2,3-c]pyrazoles. Tetrahedron Lett. 2009, 50, 2252–2255. [Google Scholar]
- Litvinov, Y.M.; Shestopalov, A.A.; Rodinovskaya, L.A.; Shestopalov, A.M. New Convenient Four-Component Synthesis of 6-Amino-2,4-dihydropyrano[2,3-c]pyrazol-5-carbonitriles and One-Pot Synthesis of 6′-Aminospiro[(3H)-indol-3,4′-pyrano[2,3-c]pyrazol]-(1H)-2-on-5′-carbonitriles. J. Comb. Chem. 2009, 11, 914–919. [Google Scholar]
- Nagarajan, A.S.; Reddy, B.S.R. Synthesis of substituted pyranopyrazoles under neat conditions via a multicomponent reaction. Synlett 2009, 12, 2002–2004. [Google Scholar]
- Khalil, K.D.; Al-Matar, H.M.; Al-Dorri, D.M.; Elnagdi, M.H. Studies with enaminones and enaminonitriles: Synthesis of 3-aroyl- and 3-heteroaroylpyrazolo[1,5-a]pyrimidines. Tetrahedron 2009, 65, 9421–9427. [Google Scholar] [CrossRef]
- Anwar, H.W.; Fleita, D.H.; Kolshorn, H.; Meier, H.; Elnagdi, M.H. 2H-3-Pyrazolamines as precursors for the synthesis of polyfunctionally substituted pyrazolo[1,5-a]pyrimidines. ARKIVOC 2006, xv, 133–141. [Google Scholar]
- Abdel Galil, F.M.; Abdel Motaleb, R.M.; Elnagdi, M.H. Nitriles in heterocyclic synthesis: The reaction of acetophenonylidenemalononitrile with some active methylene reagents and acrylonitrile derivatives. Anales de Quimica, Serie C: Quimica Organica y Bioquimica 1988, 84, 19–21. [Google Scholar]
- Sample Availability: Samples of the compounds 4, 6, 8 and 12a,b are available from the authors.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Matar, H.M.; Khalil, K.D.; Adam, A.Y.; Elnagdi, M.H. Green One Pot Solvent-Free Synthesis of Pyrano[2,3-c]-Pyrazoles and Pyrazolo[1,5-a]Pyrimidines. Molecules 2010, 15, 6619-6629. https://doi.org/10.3390/molecules15096619
Al-Matar HM, Khalil KD, Adam AY, Elnagdi MH. Green One Pot Solvent-Free Synthesis of Pyrano[2,3-c]-Pyrazoles and Pyrazolo[1,5-a]Pyrimidines. Molecules. 2010; 15(9):6619-6629. https://doi.org/10.3390/molecules15096619
Chicago/Turabian StyleAl-Matar, Hamad M., Khaled D. Khalil, Aisha Y. Adam, and Mohamed H. Elnagdi. 2010. "Green One Pot Solvent-Free Synthesis of Pyrano[2,3-c]-Pyrazoles and Pyrazolo[1,5-a]Pyrimidines" Molecules 15, no. 9: 6619-6629. https://doi.org/10.3390/molecules15096619



