Synthesis and Anti-Hyperlipidemic Evaluation of N‑(Benzoylphenyl)-5-fluoro-1H-indole-2-carboxamide Derivatives in Triton WR-1339-Induced Hyperlipidemic Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Studies

2.2. Pharmacological Studies
2.2.1. Induction of Hyperlipidemia by Triton WR-1339
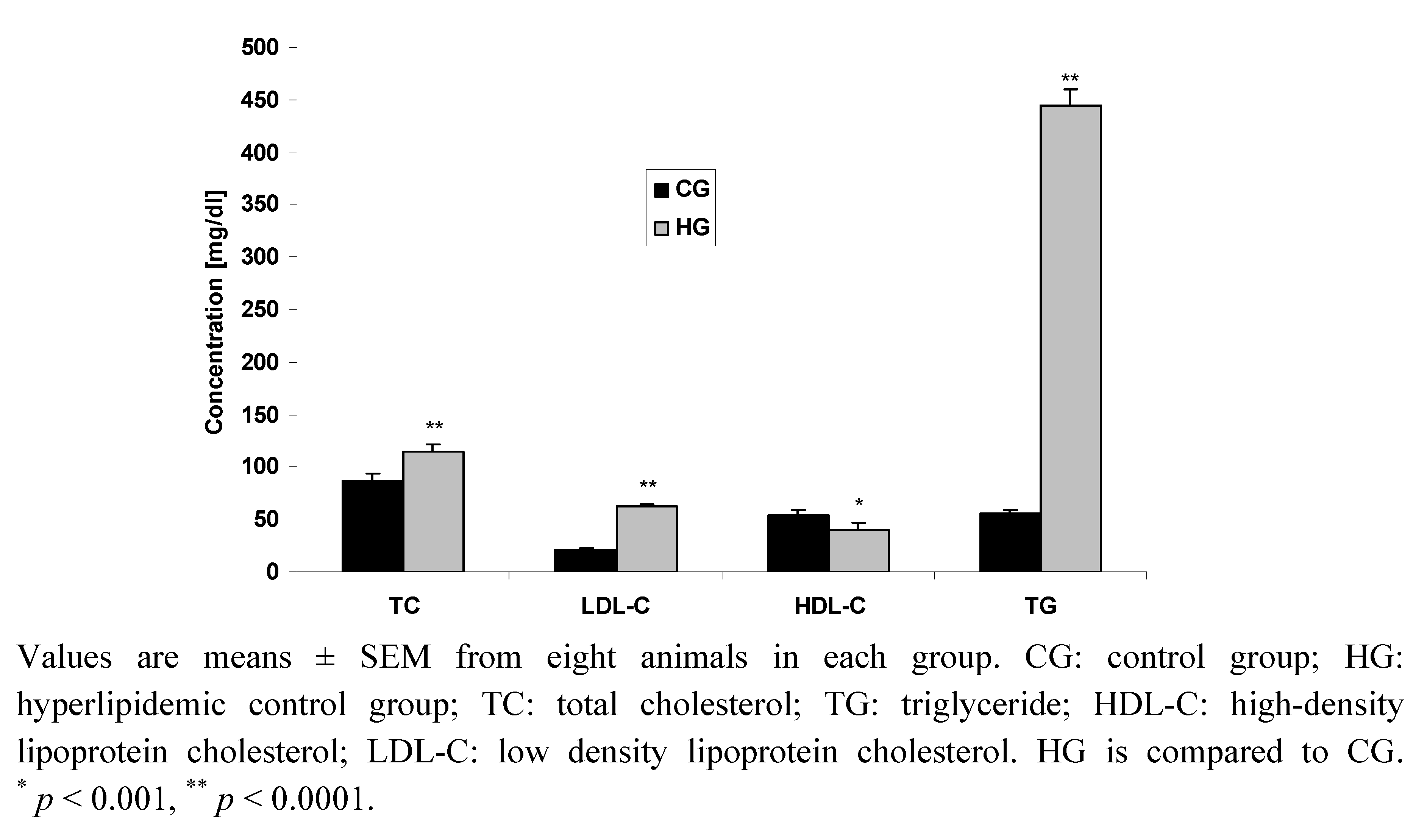
2.2.2. Effect of Compounds 1, 2, 3 and Bezafibrate on Rat Plasma Lipid Profile
| Lipid profile | TC (mg/dL) | TG (mg/dL) | HDL-C(mg/dL) | LDL-C (mg/dL) |
|---|---|---|---|---|
| CG | 86.2 ± 7.2 | 55.2 ± 4.5 | 54.6 ± 4.1 | 20.5 ± 1.9 |
| HG | 114.2 ± 6.6 | 445.0 ± 14.5 | 39.5 ± 6.7 | 63.2 ± 1.6 |
| C 1 | 134.3 ± 12.2 | 533.2 ± 16.3 | 42.2 ± 5.7 | 65.8 ± 1.3 |
| C 2 | 124.0 ± 4.9 | 46.0 ± 5.4 b | 73.6 ± 2.3 b | 41.2 ± 2.6 b |
| C 3 | 114.0 ± 0.98 | 75.3 ± 7.0 b | 60.5 ±10.3 a | 38.5 ± 2.3 b |
| BF | 125.3 ± 4.0 | 95.6 ± 5.0 b | 50.2 ± 3.7 a | 56.0 ± 3.1 |
3. Experimental
3.1. General
3.2. Animals and Treatments
3.3. Triton model of Hyperlipidemia
3.4. Pharmacological Experimental Design
3.5. Statistical Analysis
4. Conclusions
- Samples Availability: Samples of the compounds are available from the authors.
References
- McKenney, J.M. Dyslipiemias. In Applied Therapeutics, 7th; Koda-Kimble, M.A., Young, L.Y., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2001; pp. 11–1. [Google Scholar]
- Libby, P.; Schoenbeck, U.; Mach, F.; Selwyn, A.P.; Ganz, P. Current concepts in cardiovascular pathology: The role of LDL cholesterol in plaque rupture and stabilization. Am. J. Med. 1998, 104, 18S–27S. [Google Scholar] [CrossRef]
- Martin, M.J.; Hulley, S.B.; Browner, W.S.; Kuller, L.H.; Wentworth, D. Serum cholesterol, blood pressure, and mortality: implications from a cohort of 361,662 men. Lancet 1986, 2, 933–936. [Google Scholar]
- West, K.M.; Ahuja, M.S.; Bennet, P.H. The role of circulating glucose and triglyceride concentrations and their interactions with other "risk factors" as determinants of arterial disease in nine diabetic population samples from the WHO multinational study. Diabetes Care 1983, 6, 361–369. [Google Scholar]
- Otway, S.; Robinson, D.S. The effect of the nonionic detergent (Triton) on the removal of triglyceride fatty acids from the blood of the rats. J. Physiol. 1967, 190, 309–319. [Google Scholar]
- Schurr, P.E.; Schultz, J.R.; Parkinson, T.M. Triton-induced hyperlipidemia in rats as an animal model for screening hypolipidemic drugs. Lipids 1972, 7, 69–74. [Google Scholar]
- Hayashi, H.; Niinobe, S.; Matsumoto, Y.; Suga, T. Effects of Triton WR-1339 on lipoprotein lipolytic activity and lipid content of rat liver lysosomes. J. Biochem. 1981, 89, 573–579. [Google Scholar]
- Frick, M.H.; Elo, O.; Haapa, K.; Heinonen, O.P.; Heinsalmi, P.; Helo, P.; Huttunen, J.K.; Kaitaniemi, P.; Koskinen, P.; Manninen, V.; Mäenpää, H.; Mälkönen, M.; Mänttäri, M.; Norola, S.; Pasternack, A.; Pikkarainen, J.; Romo, M.; Sjöblom, T.; Nikkilä, E.A. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. New Engl. J. Med. 1987, 317, 1237–1245. [Google Scholar]
- Rubins, H.B.; Robins, S.J.; Collins, D.; Fye, C.L.; Anderson, J.W.; Elam, M.B.; Faas, F.H.; Linares, E.; Schaefer, E.J.; Schectman, G.; Wilt, T.J.; Wittes, J. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. New Engl. J. Med. 1999, 341, 410–418. [Google Scholar]
- Schoonjans, K.; Staels, B.; Auwerx, J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J. Lipid Res. 1996, 37, 907–925. [Google Scholar]
- Al-Qirim, T.; Shahwan, M.; Shattat, G.; Al-Hiari, Y.; Abu Sheikha, G.; Zaidi, S. Pharmacological evaluation of novel indole-2-carboxamides as potent lipid-lowering agents in Triton-WR1339-induced hyperlipidemic rats. Z. Naturforsch. 2009, 64c, 619–625. [Google Scholar]
- Bosies, E.; Heerdt, R.; Kuknle, H.F.; Schmidt, F.H.; Stach, H. Hypoglycemically and hypolipidemically active derivatives of phenylalkane carboxylic acids. U.S. Patent 4,113,871, 4 August 1976. [Google Scholar]
- Dasseux, J.; Oniciu, C.D. Ketone compounds and compositions for cholesterol management and related uses. U.S. Patent 20,100,137,444, 26 June 2009. [Google Scholar]
- Kopin, A.S.; Carey, M.; Wang, D. Methods of altering intestinal motility and absorption of hydrophobic compounds through the use of agonists and/or antagonists of the cholecystokinin–1 receptor. U.S. Patent 224,869, 13 September 2005. [Google Scholar]
- Sher, P.M.; Ellsworth, B.A. Triglyceride and triglyceride-like prodrugs of glycogen phosphorylase inhibiting compounds. U.S. Patent 7098235, 13 November 2003. [Google Scholar]
- Robichaud, L.J.; Stewart, S.F.; Adolphson, R.L. CI-922 - a novel, potent antiallergic compound. I. Inhibition of mediator release in vitro. Int. J. Immunopharmacol. 1987, 19, 41–49. [Google Scholar]
- Olgen, S.; Coban, T. Synthesis and antioxidant properties of novel N-substituted indole-2-carboxamide and indole-3-acetamide derivatives. Arch. Pharm. 2002, 335, 331–338. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Z.; Luo, X.; Shen, J.; Liu, H.; Shen, X.; Chen, K.; Jiang, H. Inhibitory mode of indole-2-carboxamide derivatives against HLGPa: Molecular docking and 3D-QSAR analyses. Bioorg. Med. Chem. 2004, 15, 4147–4157. [Google Scholar]
- Lauk, L.; Galati, E.M.; Forestieri, A.M.; Kirjavainen, S.; Trovato, A. Mucuma pruriens infusion lowers cholesterol and total lipid plasma levels in the rats. Phytother. Res. 1989, 3, 263–264. [Google Scholar] [CrossRef]
- Khanna, A.K.; Chauder, R.; Chandan, S.; Srivastava, A.K.; Kapoor, N.K. Hypolipidemic activity of Achyranthus aspera linn in normal and triton induces htperlipemic rats. Indian J. Exp. Biol. 1992, 30, 128–130. [Google Scholar]
- Yamamoto, K.; Byrne, R.; Edelstein, C.; Shen, B.; Scanu, A.M. In vitro effect of Triton WR-1339 on canine plasma high density lipoproteins. J. Lipid Res. 1984, 25, 770–779. [Google Scholar]
- Otway, S.; Robinson, D.S. The effect of the nonionic detergent (Triton) on the removal of triglyceride fatty acids from the blood of the rats. J. Physiol. 1967, 190, 309–319. [Google Scholar]
- Campillo, J.E.; Torres, M.D.; Dominguez, E.; Romero, A.; P´erez, C. Ficus carica leaf administration reduces hypertrygliceridaemia in streptozotocindiabetic rats. Diabetologia 1994, 37, A213. [Google Scholar]
- P´erez, C.; Canal, J.R.; Campello, J.E.; Adelaida, R.; Torres, M.D. Hypotriglyceridaemic activity of Ficus carica leaves in experimental hypertriglyceridaemic rats. Phytother. Res. 1999, 13, 188–191. [Google Scholar] [CrossRef]
- Malloy, M.J.; Kan, J.P. Medical management of hyperlipidemic states. Ad. Int. Med 1994, 39, 603–631. [Google Scholar]
- Anila, L.; Vijayalakshmi, N.R. Flavonoids from Emblica officinalis and Mangifera indica- effectiveness for dyslipidemia. J. Ethnopharmacol. 2002, 79, 81–87. [Google Scholar] [CrossRef]
- Staels, B.; Dallongville, J.; Auwerx, J.; Schoonjans, K.; Leitersdorf, E.; Fruchart, J.C. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 1998, 98, 2088–2093. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shattat, G.; Al-Qirim, R.; Al-Hiari, Y.; Sheikha, G.A.; Al-Qirim, T.; El-Huneidi, W.; Shahwan, M. Synthesis and Anti-Hyperlipidemic Evaluation of N‑(Benzoylphenyl)-5-fluoro-1H-indole-2-carboxamide Derivatives in Triton WR-1339-Induced Hyperlipidemic Rats. Molecules 2010, 15, 5840-5849. https://doi.org/10.3390/molecules15095840
Shattat G, Al-Qirim R, Al-Hiari Y, Sheikha GA, Al-Qirim T, El-Huneidi W, Shahwan M. Synthesis and Anti-Hyperlipidemic Evaluation of N‑(Benzoylphenyl)-5-fluoro-1H-indole-2-carboxamide Derivatives in Triton WR-1339-Induced Hyperlipidemic Rats. Molecules. 2010; 15(9):5840-5849. https://doi.org/10.3390/molecules15095840
Chicago/Turabian StyleShattat, Ghassan, Rania Al-Qirim, Yusuf Al-Hiari, Ghassan Abu Sheikha, Tariq Al-Qirim, Waseem El-Huneidi, and Moyad Shahwan. 2010. "Synthesis and Anti-Hyperlipidemic Evaluation of N‑(Benzoylphenyl)-5-fluoro-1H-indole-2-carboxamide Derivatives in Triton WR-1339-Induced Hyperlipidemic Rats" Molecules 15, no. 9: 5840-5849. https://doi.org/10.3390/molecules15095840
APA StyleShattat, G., Al-Qirim, R., Al-Hiari, Y., Sheikha, G. A., Al-Qirim, T., El-Huneidi, W., & Shahwan, M. (2010). Synthesis and Anti-Hyperlipidemic Evaluation of N‑(Benzoylphenyl)-5-fluoro-1H-indole-2-carboxamide Derivatives in Triton WR-1339-Induced Hyperlipidemic Rats. Molecules, 15(9), 5840-5849. https://doi.org/10.3390/molecules15095840




